Method Article
Behavioral Tasks for Examining Identity Recognition In Mice
In This Article
Summary
By pairing negatively or positively valenced experiences with individual social targets, we developed behavior tasks for examining the identity recognition of C57BL/6 mice. These tasks allow for the study of mechanisms of social memory of individual social targets in healthy and disease-related mouse models with impaired social cognition.
Abstract
Social animals, like rodents, are able to recognize and differentiate between the identity of familiar individuals. Recognizing the identity of familiar individuals is important for developing social structures such as hierarchy, kinship, and family. However, mechanisms underlying the recognition of social identity remain unclear. Most rodent studies of social recognition are based on the propensity of rodents to interact with a novel social target, a phenomenon known as social novelty. However, behavioral tasks for examining social novelty cannot reveal the recognition of familiar conspecifics based on their identities. Presented here are behavioral tasks allowing for the examination of identity recognition in C57BL/6 mice by associating two familiar mice with or without a valenced experience. Subjects had interactions with two mice either without (neutral) or with a valenced experience (negative or positive) and became familiar with these mice. The negatively valenced mouse was associated with shocks, while the positively valenced mouse was associated with a food reward. Following training, the recognition of the identity of these familiar mice can be revealed in a social discrimination test, which is represented as the preference for the positively valenced mouse and avoidance of the negatively valenced mouse compared to the neutral mouse. Behavioral tasks for identity recognition could be useful in probing social memory mechanisms and the pathophysiology of disorders with impaired social cognition, such as autism spectrum disorder or schizophrenia.
Introduction
Identity recognition, or the ability to identify familiar individuals based on prior experiences, is critical to the survival of social animals1. In rodents, social hierarchy, mate and offspring recognition, territorial defense, and the establishment and maintenance of groups are behaviors incumbent upon successful identity recognition2,3. The current understanding of social memory is based on rodent studies rooted in the ability to identify novel versus familiar conspecifics. These tasks have been useful for uncovering brain regions that are thought to mediate social memory, such as the hippocampal CA24 and ventral CA1 regions5. However, such studies remain limited in elucidating the mechanisms underlying identity recognition as they are restricted to the categorical recognition of social novelty.
Identity recognition has been demonstrated independently from social novelty via associative learning paradigms where valenced salient experiences are associated with one of two (or more) familiar social targets. These tasks are based on the notion that social interactions are often linked to emotional meaning, where negative experiences result in conflict-preventing behaviors, whereas playful or nurturing interactions are generally rewarding. The emotional affect attached to interactions is termed social valence. Male golden hamsters were able to discriminate between two familiar conspecific targets when one had previously attacked them6. Recognition memory of individual male Wistar rats was also important for the establishment of social rank7.
Recently, identity recognition has been examined in C57BL/6 mice, the most widely used mouse strain in neuroscience research, by associating individual C57BL/6 mice with or without appetitive stimuli8. This approach used head-fixed mice to undergo hundreds of training trials, which limited its use for examining naturalistic behaviors in freely moving mice. In other studies, identity recognition of C57BL/6 mice was supported by the preference of ethanol-administered conspecifics9,10. Nonetheless, the administration of substances could introduce confounds. Finally, few of these studies have been used to examine identity recognition in rodents of both sexes. An easily adoptable method that is applicable to both male and female C57BL/6 mice is a tool crucial to furthering the study of identity recognition.
Presented here are identity recognition paradigms that can be employed in both male and female mice. To study identity independent from the effects of novelty, two familiar social targets are used, and one of these targets is associated with a salient emotional experience that is presumed to inform interaction behavior. For the negative social valence association, an approach similar to social fear conditioning was adapted to elicit avoidance towards a specific social target11. If a negative social valence, such as an aversive foot shock, is associated with an individual, decreased interaction with the social target would be expected. Conversely, if a positive social valence, such as an appetitive reward, is associated with a specific individual, increased interaction with this mouse would be anticipated at subsequent testing. Taken together, the outlined behavioral tasks can be easily applied in most laboratories for the study of identity recognition in C57BL/6 mice and other mouse strains.
Protocol
Male adult C57BL/6 mice (8-10 weeks old; body weight: 18-25 g) were used in this study. Mice used as social targets were obtained from a different source (see Table of Materials) to ensure they were from different litters and previously unfamiliar to the subject mice. All protocols were approved by the Facility Animal Care Committee at the Douglas Research Centre and followed guidelines from the Canadian Council on Animal Care (protocol no.: DOUG-5935).
1. Materials (see Figure 1)
- Set up a large transparent cage (24 cm x 36 cm x 19.5 cm) to be the neutral context in the negative social valence experiment
- Use a black-walled, triangular shock box (length of each side = 46 cm; height = 29 cm) as the negative valence context. Make sure the floor of the shock box consists of metal grids connected to a Scrambled Grid Current Generator.
- Use a large transparent cage (26 cm x 47 cm x 21 cm) as the food habituation context in the positive social valence experiment.
- Use a large black-walled cage (24 cm x 36 cm x 19.5 cm) as the neutral context in the positive social valence experiment.
- Use a large white-walled cage (24 cm x 36 cm x 19.5 cm) with a food port as the positive social valence context. As the food port, use a plastic tube (0.5 cm diameter) that passes through one side of the wall and place a white plastic dish underneath the tube.
- Precut white easel pad papers to use as the floor of the neutral context (neutral training in both negative and positive social valence experiments) and positive social valence contexts. Cut the pad slightly smaller than the bottom surface of the context to prevent the subject mouse from burrowing under the paper. Replace the easel pads between subject mice.
- Use dustless precision pellets (20 mg, chocolate flavor) as a reward cue upon interaction with the positively valenced social target.
- Use a scale for weighing subject mice daily for the duration of the positive social valence experiments.
- Use a three-chamber box (81 cm x 23 cm x 23 cm), with openings in the middle chamber that connect to the left and the right chambers, for social discrimination testing. Use removable chamber doors to contain the subject mouse in the middle chamber between sessions, preventing handling stress.
- Use two identical rectangular plexiglass enclosures (10 cm x 5 cm x 30 cm) for both negative and positive social valence training.
- Use four circular wire cups (8 cm x 8 cm x 10 cm) for social discrimination testing. Keep two wire cups empty during session 1 of the social discrimination test. In session 2, place the social targets used in positive or negative social valence training in the other two wire cups.
NOTE: Label the wire cups (e.g., using tape) to differentiate between identical wire cups. Within an experiment, use the wire cups for the same purpose throughout the duration of the experiment. If a different social target must be placed inside the wire cup, clean the wire cup thoroughly with soap, water, and a hydrogen peroxide-based fast-acting disinfectant. - Place two glass bottles on top of the overturned wire cups during social discrimination testing to prevent the subject mice from climbing on top of the wire cups.
- Ensure that the enclosures in training have floors of a non-conductive material for negative social valence experiments. For example, use plastic or cardboard covered with electrical tape to ensure that the negatively valenced social target does not receive shocks during training.
- Use a silent timer to avoid external cues that might intervene with animal behaviors.
- Use a high-resolution (1,080 p/30 fps) webcam connected to a laptop computer for capturing mouse behavior during training and the social discrimination test.
- Use a white noise generator that generates static white noise at 60 dB measured at the center of the context.
NOTE: All behavioral procedures should be conducted with static white noise at 60 dB measured at the center of the context to mask environmental sounds that may affect animal behavior. - Use red light lamps for all behavioral procedures.
NOTE: This provides dark environmental conditions, which encourage exploration in mice and allow for the visualization of animal behavior in video recordings. - Clean enclosures, both empty and animal-containing, between subject mice with 70% ethanol, which is fast-drying. Clean training and testing contexts between subject mice with a hydrogen peroxide-based fast-acting disinfectant to eliminate potential influences on behavior that are due to olfactory cues from previous subject mice.
2. Behavioral procedures
- Negative social valence
- Context habituation
- Equip the experimental room with white noise and ambient red light for the duration of the experiment.
- Prepare the shock box by placing an empty plexiglass enclosure with a non-conductive floor at the center.
- Place the subject mouse in a corner of the shock box and allow the mouse to freely explore for 5 min.
- Return the subject mouse to its homecage.
- Repeat shock box habituation with the remaining mice.
- At least 1 h later, repeat the context habituation procedure (steps 2.1.1.1-2.1.1.5) in the neutral context.
- Negative valence training
- Begin negative valence training at least 1 h after the habituation to both contexts.
- Prepare the shock box by setting the current to 0.3 mA.
NOTE: The recommended current setting is based on the shock box we used and our experimental design (3 training days). This setting can be modified to fit with other experimental designs (e.g., stronger current may be used with designs of fewer training trials). - Place the negatively valenced social target in the plexiglass enclosure with a non-conductive bottom at the center of the shock box.
- Start video recording and place the subject mouse in a corner of the shock box.
- Start the timer and allow the subject mouse to freely explore.
- At 4 and 4.5 min, deliver a 1 s long electric shock.
NOTE: This allows for sufficient interaction time between the subject mouse and social target prior to experiencing aversive shocks. - Remove the subject mouse at 5 min and stop the recording and timer.
- Return the subject mouse to its homecage.
- Disinfect the shock box and repeat negative valence training with the remaining subject mice.
- Neutral training
- Begin neutral training at least 2 h after negative valence training.
- Place the neutral social target in the enclosure with a non-conductive bottom at the center of the context.
NOTE: A non-conductive bottom is used to ensure that the negative and neutral contexts have identical context cues. No shock is delivered in neutral training. - Start video recording and place the subject mouse in a corner of the neutral context.
- Start the timer and allow the subject mouse to freely explore.
- Remove the subject mouse at 5 min and stop the recording and timer.
- Return the subject mouse to its homecage.
- Order of training
- Day 1: After the habituation of both contexts (step 2.1.1), perform the neutral training (step 2.1.3) 2 h after the negative valence training (step 2.1.2).
- Day 2: Repeat training on day 2 with a reversed training order (steps 2.1.3 then 2.1.2; i.e., perform neutral training and then, negative valence training 2 h later).
- Day 3: Repeat training on day 3 with a reversed training order (steps 2.1.2 then 2.1.3; i.e., negative valence training followed by neutral training 2 h later).
- Context habituation
- Positive social valence
- Food deprivation
- Weigh each subject mouse daily for the duration of the experiment.
NOTE: If at any point, a subject mouse loses more than 15% of their original body weight, food deprivation must be stopped, and the mouse should be discarded from further experiments. - Provide 1 g of the regular mouse food per subject mouse in the homecage.
NOTE: Subject mice are group housed with 1 g of food available per mouse. For example, if four mice are group housed, then 4 g of food are placed in the homecage. - Repeat food deprivation daily for the duration of the positive social valence experiment (i.e., following each day of food pellet habituation, context habituation, and positive valence training).
- Weigh each subject mouse daily for the duration of the experiment.
- Sucrose food pellet habituation
- Begin food pellet habituation 12 h after the beginning of food deprivation.
- Equip the experimental room with white noise and ambient red light for the duration of the experiment.
- Place the subject mouse in a corner of the food habituation context.
- Start the timer and deliver 1 sucrose food pellet/min over the course of 10 min.
- Remove the subject at 10 min and return the mouse to its homecage.
- Record the number of sucrose food pellets consumed by the subject mouse.
- Repeat sucrose food pellet habituation for a total of 3 days.
NOTE: More habituation days can be added if mice do not consume any sucrose food pellets after 3 days. - Remove subjects that do not consume any sucrose food pellets during food pellet habituation from the study.
- Context habituation
- One day after sucrose food pellet habituation (day 4), prepare the positive valence context by placing an empty plexiglass enclosure at a corner of the context.
- Place the subject mouse in a corner of the positive valence context and allow the mouse to freely explore for 10 min.
- Return the subject mouse to its homecage.
- Repeat positive valence context habituation with the remaining mice.
- At least 1 h later, repeat the context habituation procedure in the neutral context.
- Positive valence training
- Begin positive valence training at least 1 h after the habituation to both contexts.
- Place the positively valenced social target in the enclosure at the center of the positive valence context.
- Start video recording and place the subject mouse in a corner of the positive valence context.
- Start the timer and allow the subject mouse to freely explore for 10 min.
- Upon each interaction bout with the social target that is >2 s, deliver 1 sucrose food pellet via the food port. Interaction is defined as periods where the subject mouse’s nose is <2 cm away from the periphery of the plexiglass enclosure. The subject’s head angle must be positioned towards the enclosure.
NOTE: Nose poking and sniffing may occur but are not necessary for sucrose food pellet delivery. - Remove the subject mouse at 10 min and stop the recording and timer.
- Return the subject mouse to its homecage.
- Record the number of sucrose food pellets delivered via the food port and the number of pellets consumed by the subject mouse.
- Replace the easel pad floor, disinfect the positive context, and repeat positive valence training with the remaining subject mice.
- Neutral training
- Begin neutral training at least 2 h after positive valence training.
- Place the neutral social target in the enclosure at a corner of the neutral context.
- Start video recording and place the subject mouse in a corner of the neutral context.
- Start the timer and allow the subject mouse to freely explore for 10 min.
- Remove the subject mouse at 10 min and stop the recording and timer.
- Return the subject mouse to its homecage.
- Replace the easel pad floor, disinfect the equipment, and repeat neutral training with the remaining subject mice.
- Order of training
- Days 1-3: Perform food pellet habituation (step 2.2.2).
- Day 4: After the habituation of both contexts (step 2.2.3), first perform positive valence training (step 2.2.4) followed by neutral training (step 2.2.5) 2 h later.
- Day 5: Repeat training on day 5 with a reversed training order (steps 2.2.5 then 2.2.4; i.e., neutral training followed by positive valence training 2 h later).
- Food deprivation
- Social discrimination test
- Equip the experimental room with white noise and ambient red light.
- Transport subjects to the experimental room and allow undisturbed habituation to the room for at least 1 h.
NOTE: Ensure that social targets remain outside of the experimental room during the habituation period. - In session 1, place two empty wire cups in the opposite corners of the left and right chambers of the three-chamber social discrimination box.
NOTE: Identical large weighted objects (e.g., glass bottles) are placed on top of the wire cups to ensure that the subject mouse does not climb on top of the wire cup. - Start the video recording, place the subject mouse in the center of the middle chamber, and start the timer.
NOTE: If possible, exit the room to minimize experimenter interference on subject mice. - After 8 min, return to the testing room and without touching the subject mouse, use the chamber doors to enclose the subject in the middle chamber.
- Replace the empty wire cups with an identical but different set of cups containing the social targets.
NOTE: The social targets are those used in negative or positive social valence training. - Remove the chamber doors and start the timer as the subject mouse explores in session 2.
- After 8 min, stop the recording and return the subject mouse and social targets to their respective homecages.
- Clean the cups with 70% ethanol and clean the arena with disinfectant.
- Repeat social discrimination testing (steps 2.3.3-2.3.9) with the remaining subject mice.
- Once half of the cohort has completed testing, counterbalance the wire cup location.
NOTE: Social targets should be moved with their respective wire cups to prevent conflicting olfactory cues. - Clean all wire cups with soap, water, and disinfectant. Clean the three-chamber social discrimination box with disinfectant.
3. Behavioral analysis
- Training
- Analyze the interaction time with each social target and score the number of interaction bouts.
NOTE: Analysis may be scored manually (animal head <2 cm away from the periphery of the enclosure and head angle of 180°) or using an automated program. - Record the number of food pellets consumed during positive social valence training to ensure that changes in social behaviors are not due to differences in motivation to consume food rewards.
- Analyze the interaction time with each social target and score the number of interaction bouts.
- Social discrimination test
- Measure the amount of time the subject mouse investigates each cup. An 8-min-long uninterrupted duration of the test begins once the experimenter leaves the room.
NOTE: Analysis may be scored manually (animal head <2 cm away from the periphery of the wire cup and head angle of 180°) or using an automated program. - Calculate pairwise analyses to better capture individual changes within test sessions. Additionally perform two-way repeated measures ANOVA (with the factors of session and cup) to detect between-session differences in exploration.
- Measure the amount of time the subject mouse investigates each cup. An 8-min-long uninterrupted duration of the test begins once the experimenter leaves the room.
Results
We have used the positive and negative social valence tasks to assess identity recognition in C57BL/6 mice (Figure 2). Compared to day 1 of sucrose food pellet habituation (H1, Figure 2B), most mice consumed all the given pellets by the last day of food habituation (H3). During training sessions (T1 and T2), the subject mice became familiar with two CD1 mice: neutral (Neu) and and positive valence-associated mouse (PV) by interacting with them separately in different contexts. Food rewards were given to the subject mice only after the interaction with mouse PV. During the social discrimination task, we found that trained mice preferred interacting with the PV mouse when compared to the neutral mouse (* p < 0.05, paired Student’s t-test). The intersession ratio of session 2 interaction time versus session 1 interaction also revealed a preference towards the PV mouse (* p < 0.05, paired Student’s t-test).
We also used the negative social valence task to examine identify recognition (Figure 2C). The subject mice became familiar with two CD1 mice: Neu and negative valence-associated mouse (NV) by interacting with them separately in different contexts. Two electric shocks were given near the end of the interaction session with the mouse NV, while no shocks were given after the interaction with the Neu mouse. We did not find differences in the interaction times between the subject mice and the Neu or the NV mice on each training day. However, we found that subject mice reduced interaction time with the NV mouse during the social discrimination test (* p < 0.05, paired Student’s t-test). The intersession ratio of session 2 interaction time versus session 1 interaction also revealed a preference towards the Neu mouse (* p < 0.05, paired Student’s t-test).

Figure 1: Apparatus for identity recognition tasks. (A) Neutral valence context for negative social valence testing. A white easel pad paper is placed at the bottom of the context. (B) Positive social valence context. Note the food port (arrow) in the middle bottom of the box. (C) Neutral valence context for positive social valence testing. (D) Food habituation context. A food delivery port is installed on the right hand side of the box, as shown in E (dashed circle) and F (enlarged). (G) Shock box. (H) Plexiglass enclosure. (I) Wire cup. (J) Three-chamber social discrimination box for the social discrimination test. Removable chamber doors for containing the subject mouse in the center chamber are shown in K (arrowheads). Please click here to view a larger version of this figure.
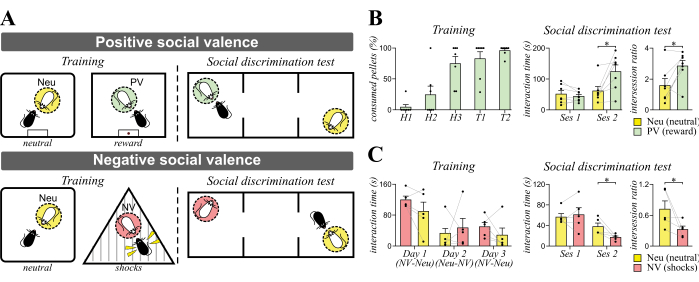
Figure 2: Design and expected results of identity recognition tasks. (A) Schematic diagrams of positive (top) and negative social valence versions of identity recognition tasks (bottom). C57BL/6 mice were trained to interact daily with the neutral (mouse Neu) and valence-associated social targets (mouse PV or NV). One day after training, subject mice were put in a three-chamber apparatus for the social discrimination test. We expect that after positive and negative social valence training, subject mice will display preference and avoidance towards positively valenced (green, mouse PV) and negatively valenced mice (red, mouse NV), respectively. (B) Representative data from a positive social valence training experiment. Subject mice were trained to interact with the positively valenced mouse (mouse PV) and neutral mouse (mouse Neu) daily for 2 days. The order of interaction was reversed on day 2. Left: Histograms show the percent of consumed sucrose food pellets during habituation (H1–3 that represent habituation days 1–3) and training (T1–2 that represent training days 1–2) with mouse PV. Right: Histograms show the increase in social interaction time with the positively valenced mouse PV during the social discrimination test. Intersession ratio (session 2 interaction time/session 1 interaction time) revealed the preference for the positively valenced mouse PV. * p < 0.05, paired Student’s t-test. (C) Representative data from a negative social valence training experiment. Subject mice were trained to interact with the negatively valenced mouse (mouse NV) and neutral mouse (mouse Neu) daily for 3 days. The order of interaction was reversed daily. Left: Histograms show the interaction time with mouse Neu and NV during different training days. Right: Histograms show the decrease of social interaction time with the negatively valenced mouse NV during the social discrimination test. Intersession ratio revealed the avoidance of the negatively valenced mouse NV. * p < 0.05, paired Student’s t-test. Abbreviations: Neu = neutral; PV = positive valence; NV = negative valence; H = habituation; T = training. Please click here to view a larger version of this figure.
Discussion
The identity recognition paradigms were designed to be easily implemented in laboratories with minimal specialized equipment and few training sessions within a week. Alternative valenced experiences may be utilized depending on available resources and the desired research question. For example, negatively valenced identity recognition following attacks from an aggressive social target was first described in golden hamsters and adapted to mice in our model6,12.
The valenced identity recognition paradigms can have wider applications within the field of social memory. Not only can these tasks be used to further study social memory or social valence mechanisms, but they can also aid in the study of disease models known to have social recognition impairments. Impairments in identity recognition underlie social behavior deficits in disorders such as schizophrenia13,14,15 and autism spectrum disorder16,17,18, where it has been estimated that one-third of adults with autism have difficulties in face individual identity recognition19. For example, Shank3 knockout mice and Df(16)A+/- are autism spectrum disorder and schizophrenia mouse models known to display impaired social cognition20,21,22. Our new paradigms may recapitulate social memory deficits and be adopted for the characterization of disease models and the identification of manipulations or treatments that improve social behavior deficits. Inanimate objects can also be studied in replacement of social targets to further parse out the mechanisms supporting object, compared to, social recognition12.
The social targets used in Figure 1 are same-sex CD1 strain mice, but we have achieved comparable valenced identity recognition using same-strain C57BL/6 targets. Thus, we have developed an easily adoptable method, which may be employed for the further study of social valence processing, social identity, and deficits of these social cognitive functions in animal models of brain diseases.
Identity recognition paradigms look at memory for specific individuals. In designing the experiments, one must take care to ensure that the training and testing sessions are entirely different and that the only cue held constant is the identity of the social targets. For example, the rooms where training and testing are conducted in, the enclosures that hold the social targets, and the cues associated with the training and testing contexts must all be different.
The four wire cups must be identical to each other, but as different as possible from the plexiglass enclosures. The wire cups and enclosures must be different in terms of material (e.g., plexiglass vs. metal), shape (e.g., rectangular vs. circular), and color (e.g., transparent vs. rose gold). This is done to ensure that the only cue informing exploratory behavior during testing is the identity of the social targets.
Context habituation is conducted on day 1 of training. This is done to encourage the exploration of social targets during training rather than the context, which will already be known to the subject mouse. Moreover, at least 2 h of waiting time is recommended between valenced and neutral training sessions. Providing the subject mice time between training sessions is thought to reduce generalization, as experiences allocated closer in time are more likely to be linked23. For this same reason, training is repeated, but counterbalanced in order, on subsequent days. The repetition is thought to encourage the further association of the experience with the respective social target. Ideally, more time between the training sessions may further allow the subject mice to learn to associate the social target with their respective valenced experience. For practical purposes, the waiting period was decided to be 2 h as it allowed for both neutral and valenced training sessions to take place in 1 day in a cohort of 8-12 mice. For example, training 12 mice may take approximately 2 h at which point the subject mouse that was trained first would be ready to begin the next training session. Of course, increasing the waiting period may improve subject mice performance but will be more time consuming for the experimenter.
Subject mice and social targets should arrive at the animal facility at least 7 days prior to the initiation of behavioral training to minimize the influence of transport stress. Social targets should be single-housed as olfactory cues from cagemates may impair identity recognition. The same two social targets can be used for a cohort of up to 12 subject mice. Importantly, the social targets must be returned to their homecage during each cleaning and setup period between subject mice. It is important to return the social targets to their homecages as prolonged confinement in the enclosures may alter their behavior and in turn influence the decision of subject mice to interact.
Subject mice and social targets can be conspecifics of the same or different strains. If same-strain social targets are used, it is critical that they are not of the same litter as the subject mice and social targets must be unfamiliar to each other. This can be ensured by ordering subject mice and social targets from different animal suppliers or ordering animals of slightly different ages (i.e., 8-week-old subject mice and 7-week-old social targets). If ear-tagging procedures are required, they should be conducted at least 24 h prior to the initiation of behavioral training. This is to minimize the influence of external stressors upon subject mouse behavior. For all behavioral training and testing, it is recommended to work with one subject mouse at a time. Although simultaneous training and testing of multiple subject mice may save time, it also increases olfactory cues in the experimental room that may influence the encoding or recall of identity recognition. As such, we recommend cohorts of 8-12 subject mice. For positive social valence experiments, it is recommended to handle the animals daily before training to minimize handling stress, which can discourage novel food consumption. It is also recommended to place 2-3 sucrose food pellets per mouse in the home cage for 3 days prior to habituation to facilitate the habituation process.
Although the presented data were scored manually, user-friendly and automated options for behavioral analysis exist and may be applied for the more efficient scoring of social interactions in training and testing videos. For example, DeepLabCut is a software employing user-trained neural networks for the markerless annotation of animal position and may be optimized for the precise identification of frames satisfying investigative behavior24.
Further control experiments can be employed to verify that successful identity recognition protocols have been achieved12. Reversing the training order should continue to elicit identity recognition and confirm that results are not due to the order of valenced and neutral training. Training may also be conducted as described, but testing can take place with the neutral and a novel social target, where increased interaction with the novel target would suggest that the subject mice have a social memory for the neutral target and have recognized that it is familiar. Similarly, testing with two novel subject mice can be done to confirm that avoidance or approach behaviors are, in fact, specific to the negatively and positively valenced social targets, respectively. Although it is presently untested whether the same subject mouse can undergo both negative and positive social valence training, we believe that it is possible if the experimenters have access to multiple social target strains or training contexts. Using the same strain of social targets for positive and negative valence experiments may complicate the association of a strain with a particular valence. If possible, using different social target strains may encourage exploration and avoid generalization between positive and negative experiences. Similarly, reusing the same training contexts would increase generalization between oppositely valenced experiences and would be minimized with the use of distinct contexts for training. If the experimental timeline permits, separating the positive and negative valence experiments by a few weeks would also be recommended.
In the case that there are difficulties establishing the identity recognition protocols, we suggest solutions to commonly encountered issues we have confronted. Group housing subject mice may function as extinction training as the valence associated with a social target becomes extinct following normal cagemate interaction25 but is necessary as social isolation impairs social memory and alters social behavior26,27. In fact, we found that single housing did not elicit identity recognition in male mice. However, single housing may be required if animals are recovering from stereotaxic surgeries or have intracranial implants that may be sensitive to detachment. In such cases, we have successfully pair-housed mice in large cages, separated by a perforated partition, and have replicated identity recognition in this condition12.
For the positive social valence task, it is common for mice to show hesitation to consume novel sucrose pellets. To resolve this issue, it is helpful to increase the food deprivation time or to familiarize mice with the pellets by putting a few pellets in their home cage for 2-3 days prior to the habituation session. In some situations where a majority of mice do not consume any food pellets, it may also be helpful to increase the number of sessions of habituation. Although we noticed that male mice generally ate less during food pellet habituation than female mice, it does not affect their ability to recognize the positively valenced social target.
Finally, the parameters of the identity recognition tasks can be modified to reduce or enhance the difficulty of the task. For instance, to reveal the subtle impact of biological factors on identity recognition, training time and the number of training sessions can be reduced to increase the difficulty of the task.
The behavioral tasks we presented in this protocol are simple, easily set up, and adaptable to male and female mice for studying identity recognition. The flexible use of either appetitive or aversive stimuli in these tasks also allows for the examination of the role of social valence in identity recognition. These tasks will be useful for studying biological mechanisms of identity recognition in healthy mice, or deficits in these mechanisms in diseases-related mouse models that exhibit social impairment.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
We would like to thank Ms. Alice Wong for providing technical support, and Dr. J. Quinn Lee for constructing the shock apparatus. We thank and acknowledge our funding sources: Natural Sciences and Engineering Research Council of Canada (RGPIN-2021-03739), Canadian Institutes of Health Research (PJ8 179866), and Fonds de recherche du Québec – Nature et technologies (326838).
Materials
| Name | Company | Catalog Number | Comments |
| 70% ethanol | |||
| Black-walled cage (24 cm x 36 cm x 19.5 cm) | neutral context, positive social valence experiment | ||
| C57BL/6 mice | Charles River, Jackson Laboratory | Social targets from Jackson Laboratory | |
| Dustless precision pellets | BioServ | F05301 | 20 mg, chocolate flavor |
| Electric tape | |||
| Glass bottles (2) | |||
| hydrogen peroxide-based disinfectant | |||
| Plastic or cardboard | Non-conductive material for enclosure floor | ||
| Rectangular plexiglass enclosures (2, 10 cm x 5 cm x 30 cm) | Identical | ||
| Shock box (triangular, length of each side = 46 cm; height = 29 cm) | |||
| Silent timer | |||
| Single Output Scrambled Animal Shocker | Lafayette Instrument | HSCK100AP | |
| three-chamber box (81 cm x 23 cm x 23 cm), with openings in the middle chamber that connect to the left and the right chambers | social discrimination testing | ||
| Transparent cage (24 cm x 36 cm x 19.5 cm) | Negative social valence | ||
| Transparent cage (26 cm x 47 cm x 21 cm) | Food habituation (positive social valence) | ||
| Webcam | Logitech | C920x | |
| Weighing scale | |||
| white easel pad papers | floor of the neutral context | ||
| White noise generator | 60 dB | ||
| White, plastic dish | |||
| White-walled cage (24 cm x 36 cm x 19.5 cm) with a 1 cm diameter plastic tube as food port | positive social valence context | ||
| Wire cups (circular, four, 8 cm x 8 cm x 10 cm) | |||
References
- Van Der Kooij, M. A., Sandi, C. Social memories in rodents: Methods, mechanisms and modulation by stress. Neurosci Biobehav Rev. 36 (7), 1763-1772 (2012).
- Ferguson, J. N., Young, L. J., Insel, T. R. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 23 (2), 200-224 (2002).
- Tibbetts, E. A., Dale, J. Individual recognition: It is good to be different. Trends Ecol Evol. 22 (10), 529-537 (2007).
- Hitti, F. L., Siegelbaum, S. A. The hippocampal ca2 region is essential for social memory. Nature. 508 (7494), 88-92 (2014).
- Okuyama, T., Kitamura, T., Roy, D. S., Itohara, S., Tonegawa, S. Ventral ca1 neurons store social memory. Science. 353 (6307), 1536-1541 (2016).
- Lai, W. S., Ramiro, L. L., Yu, H. A., Johnston, R. E. Recognition of familiar individuals in golden hamsters: A new method and functional neuroanatomy. J Neurosci. 25 (49), 11239-11247 (2005).
- Cordero, M. I., Sandi, C. Stress amplifies memory for social hierarchy. Front Neurosci. 1 (1), 175-184 (2007).
- Kong, E., Lee, K. H., Do, J., Kim, P., Lee, D. Dynamic and stable hippocampal representations of social identity and reward expectation support associative social memory in male mice. Nat Commun. 14 (1), 2597 (2023).
- Kent, K., Butler, K., Wood, R. I. Ethanol induces conditioned social preference in male mice. Alcohol Clin Exp Res. 38 (4), 1184-1192 (2014).
- Wood, R. I., Rice, R. Ethanol-induced conditioned partner preference in female mice. Behav Brain Res. 243, 273-277 (2013).
- Toth, I., Neumann, I. D., Slattery, D. A. Social fear conditioning: A novel and specific animal model to study social anxiety disorder. Neuropsychopharmacology. 37 (6), 1433-1443 (2012).
- Larosa, A., et al. Social valence dictates sex differences in identity recognition. bioRxiv. , (2024).
- Marwick, K., Hall, J. Social cognition in schizophrenia: A review of face processing. Br Med Bull. 88 (1), 43-58 (2008).
- Sachs, G., Steger-Wuchse, D., Kryspin-Exner, I., Gur, R. C., Katschnig, H. Facial recognition deficits and cognition in schizophrenia. Schizophr Res. 68 (1), 27-35 (2004).
- Whittaker, J. F., Deakin, J. F., Tomenson, B. Face processing in schizophrenia: Defining the deficit. Psychol Med. 31 (3), 499-507 (2001).
- Griffin, J. W., Bauer, R., Scherf, K. S. A quantitative meta-analysis of face recognition deficits in autism: 40 years of research. Psychol Bull. 147 (3), 268-292 (2021).
- Carver, L. J., Dawson, G. Development and neural bases of face recognition in autism. Mol Psychiatry. 7 Suppl 2, S18-S20 (2002).
- Klin, A., et al. A normed study of face recognition in autism and related disorders. J Autism Dev Disord. 29 (6), 499-508 (1999).
- Minio-Paluello, I., Porciello, G., Pascual-Leone, A., Baron-Cohen, S. Face individual identity recognition: A potential endophenotype in autism. Mol Autism. 11 (1), 81 (2020).
- Peca, J., et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 472 (7344), 437-442 (2011).
- Piskorowski, R. A., et al. Age-dependent specific changes in area ca2 of the hippocampus and social memory deficit in a mouse model of the 22q11.2 deletion syndrome. Neuron. 89 (1), 163-176 (2016).
- Xu, Q. W., Larosa, A., Wong, T. P. Roles of ampa receptors in social behaviors. Front Synaptic Neurosci. 16, 1405510 (2024).
- Cai, D. J., et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 534 (7605), 115-118 (2016).
- Mathis, A., et al. Deeplabcut: Markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci. 21 (9), 1281-1289 (2018).
- Zoicas, I., Slattery, D. A., Neumann, I. D. Brain oxytocin in social fear conditioning and its extinction: Involvement of the lateral septum. Neuropsychopharmacology. 39 (13), 3027-3035 (2014).
- Ieraci, A., Mallei, A., Popoli, M. Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural Plast. 2016, 6212983 (2016).
- Kogan, J. H., Frankland, P. W., Silva, A. J. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 10 (1), 47-56 (2000).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved