A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
وحيدة الخلية التعبير الجيني التنميط عن طريق نظام مراقبة الأصول الميدانية وQPCR مع المعايير الداخلية
In This Article
Summary
We describe a method to sort single mammalian cells and to quantify the expression of up to 96 target genes of interest in each cell. This method includes the use of internal qPCR standards to enable the estimation of absolute transcript counts.
Abstract
Gene expression measurements from bulk populations of cells can obscure the considerable transcriptomic variation of individual cells within those populations. Single-cell gene expression measurements can help assess the role of noise in gene expression, identify correlations in the expression of pairs of genes, and reveal subpopulations of cells that respond differently to a stimulus. Here, we describe a procedure to measure the expression of up to 96 genes in single mammalian cells isolated from a population growing in tissue culture. Cells are sorted into lysis buffer by fluorescence-activated cell sorting (FACS), and the mRNA species of interest are reverse-transcribed and amplified. Gene expression is then measured using a microfluidic real-time PCR machine, which performs up to 96 qPCR assays on up to 96 samples at a time. We also describe the generation and use of PCR amplicon standards to enable the estimation of the absolute number of each transcript. Compared with other methods of measuring gene expression in single cells, this approach allows for the quantification of more distinct transcripts than RNA FISH at a lower cost than RNA-Seq.
Introduction
الخلايا الفردية في عدد السكان يمكن أن تظهر استجابات متباينة على نطاق واسع لحافز موحد الفسيولوجية 1، 2، 3، 4. التباين الوراثي للخلايا في عدد السكان هو آلية واحدة لهذا التنوع من الردود، ولكن هناك أيضا العديد من العوامل غير الوراثية التي يمكن أن تزيد من تنوع الاستجابات، حتى في عدد السكان نسيلي من الخلايا. على سبيل المثال، يمكن للمستويات البروتينات الفردية وغيرها من الجزيئات يشير مهمة تختلف على أساس خلية من خلايا، مما أدى إلى اختلاف في ملامح التعبير الجيني المصب. بالإضافة إلى ذلك، يمكن أن يحدث تنشيط الجينات في رشقات نارية قصيرة المدة من النصوص 5، 6 يمكن أن تقتصر على عدد قليل نسبيا من النصوص في انفجار 7، 8، 9. هذهstochasticity في تفعيل الجينات يمكن أن يساهم إلى حد كبير في التغير في الاستجابات البيولوجية ويمكن أن توفر ميزة انتقائية في الكائنات الحية الدقيقة 10 وفي خلايا الثدييات 1، 2 الاستجابة لحافز الفسيولوجية. بسبب كل من المصادر الوراثية وغير الوراثية من الاختلاف، والوضع التعبير الجيني في أي خلية معينة في استجابة لحافز قد تختلف كثيرا عن متوسط الشخصي التعبير الجيني تم الحصول عليها من قياس استجابة كبيرة. تحديد المدى الذي تظهر الخلايا الفردية التباين في استجابة لحافز يتطلب تقنيات لعزل الخلايا الفردية، وقياس مستويات التعبير عن نسخ من الفائدة، والتحليل الحسابي للبيانات التعبير الناتجة عن ذلك.
هناك عدة طرق لمعايرة التعبير الجيني في الخلايا واحد، وتغطي مجموعة واسعة من التكاليف، وعدد من النصوص سبر، ودقة القياس الكمي. على سبيل المثال، وحيدة الخلية RNA تسلسل يقدم عمق واسعة من تغطية نص والقدرة على تحديد الآلاف من النصوص المتميزة للجينات أكثر أعرب للغاية في الخلايا الفردية. ومع ذلك، فإن التكاليف المرتبطة بهذا العمق التسلسل يمكن أن تكون باهظة، على الرغم من أن تكاليف تستمر في الانخفاض. على العكس من ذلك، واحد جزيء RNA مضان في الموقع التهجين (smRNA FISH) يقدم الكمي الدقيق للنصوص حتى لأدنى مستوى في التعبير عن الجينات بتكلفة معقولة في الجينات في المصالح. ومع ذلك، سوى عدد قليل من الجينات المستهدفة يمكن يعاير في خلية معينة من قبل هذا النهج. الكمية المقايسات PCR القائم، وصفت في هذا البروتوكول، وتوفر حلا وسطا بين هذه التقنيات. هذه المقايسات توظف في الوقت الحقيقي PCR آلة الموائع الدقيقة لقياس ما يصل إلى 96 نسخ من الفائدة في وقت ما يصل الى 96 الخلايا. في حين أن كل الطرق المذكورة أعلاه لديها تكاليف الأجهزة المطلوبة، فإن تكلفة أي فحص QPCR الفردية هي نسبيامنخفض. ويتم تكييف هذا البروتوكول من واحد اقترح من قبل الشركة المصنعة للفي الوقت الحقيقي آلة PCR الموائع الدقيقة (بروتوكول ADP 41، Fluidigm). لتمكين تقدير العدد المطلق للكل نص في النهج القائم على PCR، قمنا بتوسيع بروتوكول للاستفادة من الضوابط الداخلية للamplicons الجين المستهدف مستعدة التي يمكن استخدامها عبر تجارب متعددة.
وكمثال على هذا الأسلوب، يتم وصف الكمي للتعبير عن الجينات التي تنظمها القامع البروتين p53 ورم في قدم مكعبة-7 خلايا سرطان الثدي البشرية 11. وتحدى الخلايا مع وكيل الكيميائية التي يدفع الحمض النووي فواصل مزدوج الجديلة. وقد أظهرت دراسات سابقة أن الاستجابة البروتين p53 على الحمض النووي فواصل مزدوج الجديلة يسلك قدرا كبيرا من عدم التجانس في الخلايا الفردية، سواء من حيث مستويات البروتين p53 12 و في تفعيل الجينات المستهدفة متميزة (11). وعلاوة على ذلك، البروتين p53 ينظم التعبير عن أكثر من 100تتميز جيدا الجينات المستهدفة المشاركة في العديد من مسارات المصب، بما في ذلك الاعتقال دورة الخلية، موت الخلايا المبرمج، والشيخوخة 13 و 14. لأن استجابة بوساطة البروتين p53 في كل خلية على حد سواء معقدة ومتغيرة، وتحليل فوائد النظام من توجه فيه ما يقرب من 100 الجينات المستهدفة يمكن سبر في وقت واحد في الخلايا الفردية، مثل تلك التي وصفها أدناه. مع تعديلات طفيفة (مثل طرق بديلة لعزل وحيدة الخلية وتحلل)، وبروتوكول يمكن تكييفه بسهولة لدراسة مجموعة واسعة من أنواع الثدييات الخلية، والنصوص، والاستجابات الخلوية.
مع الإعداد المسبق السليم، ويمكن إجراء جولة من فرز الخلايا وقياس التعبير الجيني وفقا لهذا البروتوكول على مدى ثلاثة أيام. ويقترح توقيت التالية: مقدما، حدد نسخ من الفائدة، وتحديد والتحقق من صحة أزواج التمهيدي أن تضخيم [كدنا من تلك transcrIPTS، وإعداد المعايير ويمزج التمهيدي باستخدام تلك الاشعال. في يوم 1 بعد العلاج بالخلايا، والحصاد وفرز الخلايا، نفذ عكس النسخ ومحددة التضخيم الهدف، ومعالجة العينات مع نوكلياز خارجية لإزالة الاشعال الفردية. في يوم 2، نفذ مراقبة الجودة على خلايا فرز باستخدام QPCR. وأخيرا، في يوم 3، وقياس التعبير الجيني في الخلايا مرتبة باستخدام ميكروفلويديك QPCR. ويلخص الشكل 1 الخطوات المتبعة.
Protocol
1. Advance Preparation
- Select up to 96 genes of interest whose expression will be measured.
NOTE: At least one of these genes should be a "housekeeping gene," such as ACTB or GAPDH, that is known to be expressed at a relatively high and constant level under the conditions used in the experiment. This gene will be used to identify positively sorted wells (step 8.1) and amplified samples (step 10.1).
NOTE: For the example experiment, well-characterized, direct targets of p53 with a variety of known functions11,14 and GAPDH as a housekeeping control were selected. Please see Reference 11 for the complete list of target genes and primer sequences used in this study. - Identify potential primer pairs specific to the genes of interest (e.g., using the scientific literature or a primer design tool such as Primer-BLAST)15. Have the primers synthesized and maintain them at a stock concentration of 100 μM in nuclease-free water.
NOTE: These primers should be 15-25 bases long; produce an amplicon 90-130 base pairs (bp) long; span an exon junction, if one exists, to reduce the chance of amplifying genomic DNA; have melting temperatures of 60 ± 1 °C; and have minimal secondary structure16. These primers will be used to prime reverse transcription, amplify cDNA from the transcripts of interest, and measure gene expression in DNA binding dye-based qPCR. - Validate the primers.
- First, obtain cDNA from the cells of interest. Harvest RNA from the cells using an RNA harvesting kit according to the manufacturer's instructions or standard molecular biology methods17. Reverse-transcribe the RNA into cDNA using a reverse transcription kit with random hexamers according to the manufacturer's instructions or standard methods17.
- For each primer pair, set up qPCR with DNA binding dye master mix, forward and reverse primers, and the cDNA from the previous step, following the master mix manufacturer's recommendations for suggested reaction conditions. Run these reactions on a real-time PCR machine using a thermal cycling protocol recommended by the master mix manufacturer that includes melt curve acquisition.
- After the qPCR, run the amplified DNA samples on a 2% w/v agarose gel and visualize the DNA via a DNA intercalating dye following a standard protocol17.
- Determine the efficiency of the primer pair by constructing a standard curve from serial dilutions of cDNA (made in step 1.3.1) according to a standard protocol16.
NOTE: A good primer pair will yield a melt curve with a single, well-defined peak and a single band in the expected location on the gel16,17 (Figure 2). If the melt curve has multiple peaks or a "shoulder," or if the gel has multiple bands or a smear, the primers are amplifying an off-target sequence. Redesign any primers that have been observed to amplify off-target sequences and repeat the preceding validation steps as necessary. A good primer pair will also have an efficiency of 90-110%, with R2 ≥0.985 for the standard curve16; redesign any primers for which the efficiency or R2 fall outside these ranges.
- Prepare standards from purified DNA amplicons.
- For each validated primer pair:
- Amplify the region of interest from cDNA using a high-fidelity DNA polymerase according to the polymerase manufacturer's recommendations for primer and template DNA concentrations, reaction conditions, and PCR cycling conditions, or using a standard PCR protocol17.
- Run the amplified cDNA on a 2% w/v agarose gel and visualize the band with a DNA intercalating dye17. Excise the band representing the amplicon using a clean razor blade and place the gel piece in a microcentrifuge tube.
- Purify the amplicon from the gel using a standard gel extraction procedure, such as an agarase-based extraction17 or a spin-column gel extraction kit, according to the manufacturer's instructions.
- Measure the concentration of amplicon using a fluorescence-based dsDNA quantification kit according to the manufacturer's instructions. Divide the measured dsDNA concentration by the known molecular weight of the amplicon based on the amplicon sequence to obtain the number of amplicon molecules per µl.
- In a 2.0 ml low-binding microfuge tube, add 2 µl of purified amplicon to a volume of DNA suspension buffer such that the final concentration of amplicon is 5 x 108 molecules/µl.
- When all amplicons have been purified, combine 18 µl of each purified amplicon in a 2.0 ml low-binding microcentrifuge tube. Add DNA suspension buffer to make a total volume of 1,800 µl; thus, the concentration of each amplicon is 5 x 106 molecules/µl.
NOTE: This mixture, which contains cDNA amplicons from all genes of interest in known equimolar amounts, will serve as a standard in single-cell qPCR. - Vortex this "5e6" standard thoroughly, spin for 10 sec at 2,000 x g, and divide into 20 µl aliquots in low-binding PCR tubes. Store the aliquots at -80 °C.
- For each validated primer pair:
- Prepare the specific target amplification (STA) primer mix (10x).
- In a 15 ml conical tube, combine 25 µl of each 100 µM stock primer (up to 192). Add DNA suspension buffer to make a total volume of 5,000 µl.
NOTE: The concentration of each primer in this mixture is 500 nM. Each plate of sorted cells will use 105.6 µl of this primer mix to prime the reverse transcription and specific target amplification. The volume of primer mix given here is enough to make 45 plates to sort. It is advisable to make a large enough volume of primer mix so that it only needs to be made once; scale the volumes as necessary. - Mix thoroughly by vortexing, divide into 110 µl aliquots, and store the aliquots at -20 °C.
- In a 15 ml conical tube, combine 25 µl of each 100 µM stock primer (up to 192). Add DNA suspension buffer to make a total volume of 5,000 µl.
- Prepare assay mixes, one for each primer pair, for use in microfluidic chip-based qPCR.
- In each well of a 96-well PCR plate, combine 3.0 µl of the 100 µM forward primer for a given gene, 3.0 µl of the corresponding 100 µM reverse primer, 24.0 µl of DNA suspension buffer, and 30.0 µl of 2x assay loading reagent.
- Vortex this plate, spin for 10 sec at 500 x g, and store at -20 °C.
- Create two thermal cycling programs: RTSTA (reverse transcription and specific target amplification; Table 1) and EXOI (exonuclease I treatment; Table 2).
2. Treatment
- Plate the mammalian cells on a tissue culture dish such that they will grow to the desired confluence at the time of treatment, depending on the conditions of the experimental study. For the example presented in this study, plate 4 x 105 MCF-7 p53-Venus cells18 per 6 cm dish in RPMI with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 mg/ml streptomycin, and 250 ng/ml amphotericin B. Incubate the cells for two days at 37 °C with 5% CO2 until they reach 50% confluency.
- Treat the cells to establish the condition of interest based on the experimental study. For the example presented in this study, incubate MCF-7 p53-Venus cells with 400 ng/ml neocarzinostatin for 3 hr, 8.5 hr, 14 hr, or 24 hr.
3. Lysis Buffer Preparation for Cell Sorting
NOTE: Making a single plate of lysis buffer takes about 1 hr. It is advisable to make and sort multiple plates, as cell sorting can be inefficient and yield many wells with no detectable cell.
- Clean the bench, pipettes, tube rack, cold PCR plate holders, and gloves with DNA decontaminating solution in preparation for the PCR.
- Dilute the RNAse inhibitor to 2.64 U/µl by adding stock RNAse inhibitor to nuclease-free water in a 1.5 ml tube.
NOTE: This allows RNAse inhibitor to be added to the lysis buffer without pipetting sub-µl volumes. - Make lysis buffer for the cells by combining the components listed in Table 3.
- Transfer 92.4 µl of the lysis buffer for the cells into a separate tube; this will be the lysis buffer for the amplicon standards.
- Using low-binding pipette tips and microcentrifuge tubes, prepare 200 µl and 500 µl of E. coli DNA diluted to 3.1 pg/µl and 6.2 pg/µl, respectively, with DNA suspension buffer.
- Add 184.8 µl of 3.1 pg/µl E. coli DNA to the lysis buffer for the cells.
NOTE: The E. coli carrier DNA serves to broaden the scope of nonspecific primer binding possibilities and thereby reduce the likelihood that primer dimerization will lead to a PCR product. - Prepare standards.
- Label six low-binding microcentrifuge tubes as 5e5, 5e4, … 5e0.
- Add 90 µl of DNA suspension buffer to the "5e5" tube.
- Add 90 µl of 6.2 pg/µl E. coli DNA to each of the other five tubes. Keep all tubes on ice.
NOTE: Incorporating carrier DNA into the standards makes the total mass of DNA in the standard wells comparable to that in the 1-cell wells; this reduces the probability that the concentration of standards will be affected by DNA binding to tubes or pipette tips. - Take an aliquot of the "5e6" standard (5 x 106 molecules/µl of each amplicon, prepared in step 1.4) from storage. Vortex briefly and spin briefly (5 sec at 500 x g) to ensure that the liquid is at the bottom of the tube.
- Using a low-binding pipette tip, add 10 µl of 5e6 standard to the "5e5" tube. Vortex briefly, spin (5 sec at 500 x g), and put the tube back on ice.
- Pipette 10 µl from the "5e5" tube into the "5e4" tube. Vortex briefly, spin (5 sec at 500 x g), and put back on ice.
- Repeat these serial dilutions, with each tube receiving 10 µl from the previous tube, until all tubes have a dilution of standard.
- Prepare a row of 12 PCR tubes with caps. Distribute 67 µl of "cell" lysis buffer to each tube. Cap the tubes and spin down briefly (5 sec at 500 x g) if necessary.
- Using a 12-channel pipette, distribute 9 µl of "cell" lysis buffer from the row of PCR tubes into each well of the first 7 rows of a PCR plate (Figure 3).
- Distribute 7 µl of "standard" lysis buffer to each well of the last row of the plate (Figure 3).
- Using low-binding pipette tips, add 2 µl of standard to each well of the bottom row according to the plate map (Figure 3).
NOTE: 2 µl of 5 x 100 molecules/µl standard amounts to 10 molecules, etc. - Add 2 µl of 3.1 pg/µl E. coli DNA into the 10-cell and 100-cell wells; add 2 µl of 6.2 pg/µl E. coli DNA into the no-cell wells.
NOTE: Each 1-cell, 10-cell, and 100-cell well has 6.2 pg of E. coli DNA, approximating the mass of genomic DNA in a single human cell. Each standard and no-cell well has 12.4 pg of E. coli DNA, approximately two human cells' worth. - Seal the plate using a sterile thermal seal, spin for 5 sec at 500 x g, and store at 4 °C until the cells are ready for sorting.
4. Cell Sorter Setup
- Program the fluorescence-activated cell sorter to sort into a 96-well plate according to the plate map (Figure 3); no cells should be sorted into wells H1-H8 or H11-H12.
- Verify that the cell sorter is well aimed for sorting into a PCR plate.
- Set the angle of the sort stream to the minimum possible; this setting increases the chance that the cells will be sorted into the bottoms of wells rather than hit the sides.
- To aim the machine, seal an empty PCR plate and use the test stream to "sort" droplets of PBS onto the seal of the empty plate, according to the instructions of the machine19; the droplets should land on the surface of the seal at a position above the center of the well. For the best results, verify the aim across the length and width of the plate.
- If the droplets do not land in the correct position, wipe them off the surface of the seal, recalibrate the cell sorter according to the machine manufacturer's instructions, and repeat until the droplets in the sort stream are deposited correctly.
5. Cell Harvest and Sorting
- Trypsinize the cells as appropriate for the cell line. For example, for MCF-7 cells, aspirate the medium from the cells, rinse the cells with 1 ml of 0.05% trypsin, and incubate the cells with 2 ml of 0.05% trypsin for 5 min at 37 °C and 5% CO2.
- Add 8 ml of cell culture medium to the trypsinized cells and transfer all 10 ml of cell suspension to a 15 ml tube. Centrifuge for 4 min at 400 x g.
- Aspirate the supernatant and resuspend the cell pellet in PBS + 2% FBS.
NOTE: A smaller resuspension volume concentrates the cells and facilitates cell sorting; a 500 µl resuspension volume works well for a 6 cm dish of cells at 50% confluency. - Transfer the cell suspension to a 5 ml flow cytometry tube, pipetting through a 40 µm filter to remove clumps of cells.
- Centrifuge the plate of lysis buffer at 400 x g for 30 sec at 4 °C before sorting the cells to ensure that the liquid is at the bottom of the wells.
- Insert the tube with the cell suspension into the cell sorter. Open the seal on the plate of lysis buffer and carefully sort the cells of interest into the plate according to the plate map and following the cell sorter manufacturer's instructions19. To ensure that single cells are sorted at the maximum purity, run the machine in "single cell" mode and use standard flow cytometry gating based on forward and side scatter19.
NOTE: In the example experiment, gate the cells based on p53-Venus fluorescence to isolate the brightest 15% of individual cells11. - Seal the plate with a new, sterile thermal seal and centrifuge at 400 x g for 1 min at 4 °C. Place the plate in the thermal cycler and run the RTSTA program (see step 1.7).
NOTE: This performs reverse transcription on the mRNA from the sorted cells and then amplifies the cDNA of the transcripts of interest.
6. Exonuclease Treatment
- Mix dilute exonuclease I by combining the components listed in Table 4. Prepare a row of 12 PCR tubes with caps. Distribute 30.5 µl of dilute exonuclease I into each tube. Cap the tubes and spin briefly (5 sec at 500 x g) to ensure that the liquid is at the bottom of the tube.
- When the RTSTA is finished, spin the sample plate for 5 sec at 500 x g. Using a 12-channel pipette, distribute 3.6 µl of dilute exonuclease I into each well of the plate.
- Seal the plate with a sterile thermal seal and spin briefly (5 sec at 500 x g). Place the plate in the thermal cycler and run the EXOI program (see step 1.7).
NOTE: This step degrades any primers remaining after specific target amplification so that they will not interfere with future PCR.
7. Sample Dilution
- When the exonuclease treatment is finished, add 32.4 µl of TE buffer to each well of the sample plate; this is an optional stopping point, and samples can be stored at -20 °C for several days.
8. Sort Quality Control Using qPCR
NOTE: Because cell sorting is not perfectly efficient, this step is necessary to identify which wells of the sorted plate actually received a cell. These samples can then be used for further analysis.
- Measure the housekeeping gene expression in each sample by qPCR.
- Prepare 900 µl of DNA binding dye qPCR master mix for 96 reactions according to the polymerase manufacturer's protocol using primers for one of the housekeeping genes selected in step 1.1; the exact concentrations of reaction buffer, polymerase, primers, etc. for this reaction will depend on the specific polymerase being used.
- Distribute 9 µl of master mix to every well of a 96-well PCR plate. Add 1 µl of sample from the sample plate to the corresponding well of the PCR plate.
- Run the plate on a real-time PCR machine using a thermal cycling protocol suggested by the master mix manufacturer or following a standard protocol20.
- Analyze the qPCR data20; an example of qPCR data from the sort quality-control steps listed below is shown in Figure 4.
- Verify that the measurements from the no-cell wells show a low (background) level of gene expression or no expression at all.
- Verify that measurements from 1-cell wells divide clearly into two groups: positive samples with high gene expression and negative samples with background levels of expression or no expression at all, similar to the no-cell wells.
NOTE: The samples with high gene expression represent actual sorted cells; those with background expression should be excluded from further analysis. - Verify that measurements from the 10-cell and 100-cell wells reflect higher gene expression than in the positive 1-cell wells, allowing for the possibility that inefficient sorting could cause those wells to have fewer cells than desired.
- Verify that measurements from the standard wells are evenly distributed in logarithmic space (i.e., that the Ct values are evenly distributed).
- After identifying all positive 1-cell samples, make a "Sample Mixes" plate map to map positive 1-cell samples onto a new 96-well plate. Include eight wells for standards on this plate map. See Figure 5 for an example.
9. Gene Expression Measurement Using Microfluidic qPCR
NOTE: For every step in this section, pipette only to the first stop to minimize the formation of bubbles in the reagents.
- Open a row of 8 PCR tubes. If there are multiple plates of samples, open a second row of 8 PCR tubes and label the tubes 1-8. This row will be for pooling standards from multiple plates.
- In a 1.5 ml tube, mix 360 µl of DNA binding dye qPCR master mix with 36 µl of DNA binding dye sample loading reagent. Vortex briefly and spin for 10 sec at 2,000 x g. Divide this mixture (396 µl) into the unlabeled row of 8 PCR tubes, placing 48 µl in each tube.
- Using an 8-channel pipette, distribute 3.3 µl of the master mix/sample loading reagent mixture into each well of a new 96-well PCR plate. Label this plate, "Sample Mixes."
- For each PCR plate of samples:
- Vortex the plate for 10 sec and spin for 10 sec at 500 x g. Remove the cover and transfer 2.7 µl of each positive 1-cell sample to its corresponding well on the Sample Mixes plate for the 1-cell wells indicated on the plate map.
- If there is only one plate of samples, transfer 2.7 µl of each amplified standard to its corresponding well on the Sample Mixes plate according to the plate map.
- If there are multiple plates of samples, transfer 2.7 µl of each amplified standard to its corresponding position in the labeled row of PCR tubes. Add standards from the current plate of samples to any standards already there. Seal the plate of samples when done using a sealing film.
- If there are multiple plates of samples:
- Vortex the row of PCR tubes containing standards for 10 sec and spin for 10 sec at 2,000 x g. Transfer 2.7 µl from each tube of mixed standards into the corresponding well on the Sample Mixes plate according to the plate map.
- Load the NTC wells with 2.7 µl of nuclease-free water where indicated on the plate map. Seal the Sample Mixes plate and store at 4 °C until needed.
- Peel the sticker off the bottom of a new microfluidic qPCR chip. Using a syringe of control line fluid with the cap still on, push down each accumulator spring to loosen it, and inject each accumulator with 150-200 µl of control line fluid.
- Insert the chip in the loading machine and run the "Prime" script. This takes ~20 min.
- When priming is done, vortex and spin down the plates of assay mixes (prepared in step 1.6) and sample mixes.
- Using an 8-channel pipette, transfer 4.5 µl of each assay mix into the left side of the microfluidic qPCR chip according to the diagram in Figure 6. Pipette very carefully to avoid creating air bubbles at the bottom of the wells. When finished, seal the plate of assay mixes and store at -20 °C for future use.
- Transfer 4.5 µl of each sample mix to the right side of the microfluidic qPCR chip according to Figure 6. Insert the chip into the loading machine and run the "Load Mix" script. This takes ~90 min.
- Turn on the microfluidic real-time PCR machine, start the data collection software, and turn on the lamp to warm it up. This takes ~20 min.
- When the Load Mix script is done, verify that there are no lines across the chip-this would indicate a loading problem. Carefully remove any dust particles from the chip using Scotch tape or lab tape. Insert the chip into the microfluidic real-time PCR machine and run the data collection script. Use the analysis software to inspect the results and export the data for further analysis.
10. Data Analysis
- Remove the measurements for which the qPCR amplification curves show poor quality (i.e., for which the curves do not resemble the standard sigmoidal amplification curve)20.
- Remove samples with more than 30 poor-quality or zero measurements or for which housekeeping gene expression (see step 1.1) is much lower than the majority of other samples (Figure 7).
- For each gene, estimate the correct melt peak location as the median of the melt peak locations from each sample. If a measurement has a melt peak located more than 1 °C from the median, remove that measurement from consideration20.
- For each gene, create a standard curve to estimate the mRNA count in each sample using a spreadsheet or scripting language20.
NOTE: The standards in this experiment consist of 10, 100, 1,000, or 10,000 molecules of dsDNA corresponding to each transcript of interest. If reverse transcription were perfectly efficient, the standards would correspond to 20, 200, 2,000, and 20,000 molecules of mRNA, since mRNA and cDNA are single-stranded. In practice, this is not the case, so we express measurements as an estimate in units of molecules × ERT, where ERT is the efficiency of reverse transcription.- For each standard well, identify m, the number of molecules of ssDNA (twice the number of molecules of dsDNA) before amplification (e.g., 20 molecules, 200 molecules, etc.).
- For each standard well, identify the Ct value from qPCR20.
- With the values for m and Ct, perform a linear regression using the following equation to generate a standard curve with parameters a and b for each gene, and find the R2 value as an indicator of the goodness of fit:
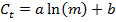
- From the standard curve, compute the PCR efficiency E for each gene:
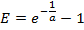
NOTE: A value of E much greater than 1.0 (e.g., E > 1.1) is not physically realistic; an R2 value for the linear regression much less than 1.0 (e.g., R2 < 0.985) means that the standards are not working reliably. These problems generally indicate that the lowest amplicon standard falls below a threshold of reliable quantification, and thus the lowest standard should be excluded from the linear regression. If an adequate linear regression cannot be made using at least three unique standards, discard the expression measurements for the gene. - If the linear regression is adequate, use the resulting fit parameters a and b for each gene to estimate the number of mRNA molecules mest (in units of molecules × ERT) for the corresponding gene in each single-cell sample from the measured Ct value:
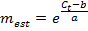
- Designate any measurement mest < 1 as mest = 0. Also, designate any measurement with no amplification in qPCR and thus no Ct value as mest = 0.
NOTE: Measurements with an mest less than the lowest or greater than the highest standard used in the regression are found by extrapolation from the standard curve. These estimates are not necessarily invalid but should be regarded with caution.
- Visualize the single-cell gene expression data.
- Make a beeswarm plot (Dorn, J. and Holy, T., 2012, http://www.mathworks.com/matlabcentral/fileexchange/37105) in which each point represents the gene expression in a particular cell; an example is shown in Figure 8.
- Make a violin plot (Dorn, J., 2012, http://www.mathworks.com/matlabcentral/fileexchange/23661) in which the width of the “violin” represents the frequency of gene expression measurements around a particular level in the population of cells analyzed; an example is shown in Figure 8.
NOTE: More sophisticated analyses can probe the relationships in expression between pairs of genes21,22 and can infer gene expression networks.
النتائج
A general overview of the protocol is shown in Figure 1, including steps for cell treatment, the isolation of single cells by FACS, the generation and pre-amplification of cDNA libraries from single-cell lysates, the confirmation of single-cell cDNA libraries in sorted wells, and the measurement of gene expression by qPCR.
In preparation for single-cell isolation and gene expression analysis, it is necessary to ...
Discussion
We have presented a method for isolating individual mammalian cells from a population of adherent cells grown in culture and for assaying the expression of approximately 96 genes in each cell. Good advance preparation is critical for this method to work well. In particular, designing and testing primer pairs specific to the transcripts of interest (steps 1.2-1.3) are time-consuming but important steps, as the primers determine the quality of the single-cell measurements. Once reliable primer pairs have been obtained, the...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank V. Kapoor in the CCR ETIB Flow Cytometry Core for her aid in performing the cell sorting during the development of this protocol. We also thank M. Raffeld and the CCR LP Molecular Diagnostics Unit and J. Zhu and the NHLBI DNA Sequencing and Genomics Core for their aid in performing the qPCR during the development of this protocol. This research was supported by the Intramural Program of the NIH.
Materials
| Name | Company | Catalog Number | Comments |
| RNeasy Plus Mini Kit | Qiagen | 74134 | |
| High Capacity cDNA Reverse Transcription Kit with RNase Inhibitor | ThermoFisher | 4374966 | |
| Phusion High-Fidelity DNA Polymerase | New England BioLabs | M0530S | |
| QIAquick Gel Extraction Kit | Qiagen | 28704 | |
| Quant-iT High-Sensitivity dsDNA Assay Kit | ThermoFisher | Q33120 | |
| 2.0 ml low adhesion microcentrifuge tubes | USA Scientific | 1420-2600 | |
| DNA Suspension Buffer | Teknova | T0221 | |
| Axygen 0.2 ml Maxymum Recovery Thin Wall PCR Tubes | Corning | PCR-02-L-C | |
| GE 96.96 Dynamic Array DNA Binding Dye Sample & Assay Loading Reagent Kit | Fluidigm | 100-3415 | |
| HyClone RPMI 1640 media | GE Healthcare Life Sciences | SH30027.01 | |
| Fetal Bovine Serum, Certified (US) | ThermoFisher | 16000-044 | |
| Antibiotic-Antimycotic Solution | Corning | 30-004-CI | |
| Neocarzinostatin | Sigma | N9162 | |
| ELIMINase | Decon Labs | 1101 | |
| SUPERase-In | ThermoFisher | AM2696 | |
| CellsDirect One-Step qRT-PCR Kit | ThermoFisher | 11753500 | |
| E. coli DNA | Affymetrix | 14380 10 MG | |
| ThermalSeal Sealing Film, Sterile | Excel Scientific | STR-THER-PLT | |
| BD FACSAria IIu | BD Biosciences | ||
| HyClone Trypsin 0.05% | GE Healthcare Life Sciences | SH30236.01 | |
| PBS, 1x | Corning | 21-040-CV | |
| Falcon 40 µm Cell Strainer | Corning | 352340 | |
| Exonuclease I | New England BioLabs | M0293S | |
| SsoFast EvaGreen Supermix with Low ROX | Bio-Rad | 172-5210 | |
| 96.96 Dynamic Array IFC for Gene Expression (microfluidic qPCR chip) | Fluidigm | BMK-M-96.96 | |
| IFC Controller HX (loading machine) | Fluidigm | ||
| BioMark or BioMark HD (microfluidic qPCR machine) | Fluidigm | ||
| Real-Time PCR Analysis software | Fluidigm | ||
| MATLAB software | MathWorks |
References
- Feinerman, O., Veiga, J., Dorfman, J. R., Germain, R. N., Altan-Bonnet, G. Variability and Robustness in T Cell Activation from Regulated Heterogeneity in Protein Levels. Science. 321 (5892), 1081-1084 (2008).
- Spencer, S. L., Gaudet, S., Albeck, J. G., Burke, J. M., Sorger, P. K. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature. 459 (7245), 428-432 (2009).
- Geva-Zatorsky, N., Rosenfeld, N., et al. Oscillations and variability in the p53 system. Mol. Syst. Biol. 2 (1), (2006).
- Colman-Lerner, A., Gordon, A., et al. Regulated cell-to-cell variation in a cell-fate decision system. Nature. 437 (7059), 699-706 (2005).
- Chong, S., Chen, C., Ge, H., Xie, X. S. Mechanism of Transcriptional Bursting in Bacteria. Cell. 158 (2), 314-326 (2014).
- Raj, A., Peskin, C. S., Tranchina, D., Vargas, D. Y., Tyagi, S. Stochastic mRNA Synthesis in Mammalian Cells. PLoS Biol. 4 (10), e309+ (2006).
- Senecal, A., Munsky, B., et al. Transcription Factors Modulate c-Fos Transcriptional Bursts. Cell Rep. 8 (1), 75-83 (2014).
- Dey, S. S., Foley, J. E., Limsirichai, P., Schaffer, D. V., Arkin, A. P. Orthogonal control of expression mean and variance by epigenetic features at different genomic loci. Mol. Syst. Biol. 11 (5), 806+ (2015).
- Dar, R. D., Razooky, B. S., et al. Transcriptional burst frequency and burst size are equally modulated across the human genome. Proc. Natl. Acad. Sci. U.S.A. 109 (43), 17454-17459 (2012).
- Thattai, M., van Oudenaarden, A. Stochastic gene expression in fluctuating environments. Genetics. 167 (1), 523-530 (2004).
- Porter, J. R., Fisher, B. E., Batchelor, E. p53 Pulses Diversify Target Gene Expression Dynamics in an mRNA Half-Life-Dependent Manner and Delineate Co-regulated Target Gene Subnetworks. Cell Syst. 2 (4), 272-282 (2016).
- Lahav, G., Rosenfeld, N., et al. Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat. Genet. 36 (2), 147-150 (2004).
- Levine, A. J., Oren, M. The first 30 years of p53: growing ever more complex. Nat. Rev. Cancer. 9 (10), 749-758 (2009).
- Riley, T., Sontag, E., Chen, P., Levine, A. Transcriptional control of human p53-regulated genes. Nat. Rev. Mol. Cell Biol. 9 (5), 402-412 (2008).
- Ye, J., Coulouris, G., Zaretskaya, I., Cutcutache, I., Rozen, S., Madden, T. L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinf. 13 (1), 134 (2012).
- . . PCR Technologies: A Technical Guide. , (2014).
- Sambrook, J., Russell, D. W. . Molecular Cloning: A Laboratory Manual. , (2001).
- Batchelor, E., Mock, C. S., Bhan, I., Loewer, A., Lahav, G. Recurrent initiation: a mechanism for triggering p53 pulses in response to DNA damage. Mol. Cell. 30 (3), 277-289 (2008).
- . . Flow Cytometry: Principles and Applications. , (2007).
- . . Real-time PCR. , (2006).
- Song, L., Langfelder, P., Horvath, S. Comparison of co-expression measures: mutual information, correlation, and model based indices. BMC Bioinf. 13 (1), 328 (2012).
- Margolin, A. A., Nemenman, I., et al. ARACNE: An Algorithm for the Reconstruction of Gene Regulatory Networks in a Mammalian Cellular Context. BMC Bioinf. 7 (Suppl 1), (2006).
- Haff, L. A. Improved quantitative PCR using nested primers. PCR Methods Appl. 3 (6), 332-337 (1994).
- Hashimshony, T., Senderovich, N., et al. CEL-Seq2: sensitive highly-multiplexed single-cell RNA-Seq. Genome Biol. 17, 77 (2016).
- Ronander, E., Bengtsson, D. C., Joergensen, L., Jensen, A. T. R., Arnot, D. E. Analysis of Single-cell Gene Transcription by RNA Fluorescent In Situ Hybridization (FISH). J. Vis. Exp. (68), e4073 (2012).
- Raj, A., van den Bogaard, P., Rifkin, S. A., van Oudenaarden, A., Tyagi, S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods. 5 (10), 877-879 (2008).
- Lubeck, E., Cai, L. Single-cell systems biology by super-resolution imaging and combinatorial labeling. Nat. Methods. 9 (7), 743-748 (2012).
- Battich, N., Stoeger, T., Pelkmans, L. Image-based transcriptomics in thousands of single human cells at single-molecule resolution. Nat. Methods. 10 (11), 1127-1133 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved