需要订阅 JoVE 才能查看此. 登录或开始免费试用。
Method Article
单通道分析和钙成像在新鲜分离的肾小球足细胞
摘要
Changes in the intracellular calcium levels in the podocytes are one of the most important means to control the filtration function of glomeruli. Here we explain a high-throughput approach that allows detection of real-time calcium handling and single ion channels activity in the podocytes of the freshly isolated glomeruli.
摘要
Podocytes (renal glomerular epithelial cells) are known to regulate glomerular permeability and maintain glomerular structure; a key role for these cells in the pathogenesis of various renal diseases has been established since podocyte injury leads to proteinuria and foot process effacement. It was previously reported that various endogenous agents may cause a dramatic overload in intracellular Ca2+ concentration in podocytes, presumably leading to albuminuria, and this likely occurs via calcium-conducting ion channels. Therefore, it appeared important to study calcium handling in the podocytes both under normal conditions and in various pathological states. However, available experimental approaches have remained somewhat limited to cultured and transfected cells. Although they represent a good basic model for such studies, they are essentially extracted from the native environment of the glomerulus. Here we describe the methodology of studying podocytes as a part of the freshly isolated whole glomerulus. This preparation retains the functional potential of the podocytes, which are still attached to the capillaries; therefore, podocytes remain in the environment that conserves the major parts of the glomeruli filtration apparatus. The present manuscript elaborates on two experimental approaches that allow 1) real-time detection of calcium concentration changes with the help of ratiometric confocal fluorescence microscopy, and 2) the recording of the single ion channels activity in the podocytes of the freshly isolated glomeruli. These methodologies utilize the advantages of the native environment of the glomerulus that enable researchers to resolve acute changes in the intracellular calcium handling in response to applications of various agents, measure basal concentration of calcium within the cells (for instance, to evaluate disease progression), and assess and manipulate calcium conductance at the level of single ion channels.
引言
肾脏维持自我平衡各种物质和的方式,确定总血压调节血容量。干扰在肾过滤,重吸收或分泌导致或伴随的病理状态,从高血糖或低血压结束末期肾病,最终需要肾移植。肾过滤单元(肾小球)由三层-毛细血管内皮,基底膜和上皮细胞的单细胞层-足细胞,从而起到的狭缝隔膜的完整性和功能1的维持起主要作用。功能障碍的选择性渗透肾小球过滤导致大分子,如蛋白的尿中丢失。各种试剂可以影响足细胞和它们的足突,这确定肾小球滤过屏障的完整性的结构。
足细胞都参与了格莱姆教授的维护eruli过滤功能。它已经确定,不当钙处理由足细胞导致细胞损伤和起着各种形式肾病2,3的发展中起重要作用。因此,一个模型,允许对细胞内钙离子浓度的变化直接测量将有助于对足细胞功能研究的发展。孤立肾小球在许多研究,包括白蛋白反射系数测量变化4,并在全细胞电生理膜片钳测量5,6-积分蜂窝电流的评估以前使用。在本论文中,我们描述的协议,该协议允许研究者测量响应于药理学试剂的应用细胞内钙离子浓度的变化,估算出细胞内钙的基础水平,和评估个体的钙离子通道的活性。 Ratometric钙离子浓度的测量和膜片钳electrophysiology分别用来确定足细胞和信道活动中的变化,在细胞内钙离子浓度。
研究方案
动物使用和福利要坚持NIH指南实验动物以下机构动物护理和使用委员会(IACUC)审查和批准协议的管理和使用。
1.肾冲洗
- 使用8〜12周龄雄性大鼠(建议是只SD菌株,但不同年龄和性别的其他菌株可以适当变化来使用)。
- 根据由IACUC协议所允许的过程麻醉动物;监测麻醉深度,检查动物。将在1.3实施手术的详细描述- 1.8可在Ilatovskaya 等人找到7。
- 适当麻醉后,将动物在一个温度控制的手术台,使腹部中线切口(最多3英寸长),并揭示了腔静脉和主动脉。
- 将周围结扎腹腔及肠系膜上动脉和腹主动脉高于;不结扎。
- 钝解剖低于肾动脉腹主动脉,并把周围的两份结扎,但不结扎,然后夹住上述结扎主动脉和领带底线。
- 导尿用聚乙烯PE50管(连接于注射器泵充满PBS)中的钳下面主动脉和固定与所述第二绑带的导管;除去夹具,打开泵,并结扎主动脉和肠系膜腹腔动脉。迅速使切口肾静脉,以减轻压力。
- 注入与预冷却的PBS主动脉为2或3分钟以6ml / min的速率。
- 停止灌注,消费税和7解封肾脏,并把它们在冰上PBS液。根据经批准的协议IACUC安乐死的动物。
2.分离的大鼠肾小球
- 制备将30ml 5%的BSA的新鲜溶液中的RPMI 1640。
- 用刀片和剪刀,隔离双肾皮质,然后剁碎直到均匀。这个程序已经较早7描述。
- 通过100目的不锈钢筛推在先前步骤剁碎的组织使用刮刀(预先浸泡在5%BSA / RPMI溶液)。收集流过并通过重力允许流动通过穿过一个140目筛。
- 过滤流通从140目筛收集的使用预浸渍200目筛网,弃去滤液,并用10筛的顶部 - 将15ml制备的BSA / RPMI溶液来收集沉淀的肾小球筛。
- 把含有在冰上肾小球BSA / RPMI溶液在一个15毫升管并让肾小球沉淀在试管底部达20分钟。肾小球集中在管的底部将被看得很清楚。除去多余的溶液,留下约2毫升管中。
3.单通道膜片钳萨尔瓦多ectrophysiology
- 准备5×5mm的玻璃盖片与MW 70000涂层他们 - 150000聚ʟ赖氨酸,让干。每护罩玻璃使用约30微升的0.01%的无菌过滤的溶液中的水。
- 预热实验溶液至室温,并填充膜片钳腔和吸管。对于TRPC通道监控,使用沐浴液,在MM:126氯化钠,氯化钙1 2,10 HEPES,2 氯化镁 ,10葡萄糖,pH值7.4;吸管:126氯化钠,氯化钙1.5 2,10 HEPES,10葡萄糖; pH为7.4。
- 添加抑制剂的吸移管溶液来阻断内源性通道,这是不相关的研究(建议的活动有:100μM的尼氟酸或DIDS(方框钙激活Cl -的信道),10mM的TEA(以抑制大电导钙离子-依赖性K +通道),10nM的iberiotoxin(阻止的钙激活K +通道),10μM尼卡地平(方框N型Ca2+直接膜片钳实验前通道))。
- 轻轻地混合含肾小球溶液,然后应用大约50微升到聚ʟ赖氨酸包被的玻璃盖片。让肾小球附加约5分钟。
- 移动的玻璃碎片与肾小球的膜片钳室预先填充的浴溶液;灌注腔室以3毫升/分钟进行1分钟的速率,以保证在除去未附着的肾小球。
- 进行常规的膜片钳实验中一个细胞贴附模式7。用玻璃移液管(7 - 10 M吸液管电阻)形成吸管和通过施加轻柔抽吸(附连到分离的肾小球的表面上的足细胞吸管一个足细胞膜之间的高电阻密封示于图2中 ,上左边)。
- 对于细胞贴附测量,由一个八极贝塞尔滤波器低通的电流,在300赫兹。
- ü本身分离肾小球在膜片钳实验长达4 - 6小时。置于冰上股票肾小球部分。
在足细胞内钙离子浓度成比例4.共聚焦荧光测量
- 将500微升肾小球分数(在2.5描述)的0.5毫升的锥形管,并补充钙染料的Fura红,AM和荧光 - 4,AM。使用2毫米和1mM储备浓度的Fura红,AM和的Fluo-4,AM,分别为(储存在-20℃下,溶解在DMSO中),并使用2.5微升每种染料为500微升肾小球馏分。立即加入染料后用铝箔覆盖的管。
- 地方管在旋转摇动器至少20分钟到1小时,在室温。
注意:药理剂可在此步骤中加入。 - 制备的玻璃盖玻片,用聚ʟ赖氨酸覆盖它们,并允许使用加热板设定为70℃的干燥。
- 一旦钙染料的负载量为COMPLET即,应用100微升含有溶液的聚ʟ赖氨酸包被的盖玻片的肾小球,让他们粘在表面上为约5分钟。安装肾小球附着盖玻片到成像室中,并灌注同浴溶液(含有(以mM计):145氯化钠,4.5氯化钾,2的MgCl 2,10 HEPES,pH值7.35)以3ml / min的速率以除去未连接的肾小球和剩余的染料。
- 设置在共聚焦激光扫描显微镜488nm的激发波长和发射滤光片(25分之525和二十五分之六百五,纳米为的Fluo-4和的Fura红分别)。成像软件设定为所希望的频率和分辨率。
- 发现在明肾小球,然后打开荧光信号的检测。调节激光的强度为每个染料,以避免信号的饱和度。与直接连接到玻璃中的足细胞选择焦平面。这响应于药物最小化由于肾小球的萎缩的效果应用。仔细检查选择的肾小球很好地附着在玻璃;
- 开始成像选择的焦平面(取为512×512的图像与频率设定为4秒来可视化快的Ca 2+瞬时变化。使用60X / NA的1.4或类似物镜,用于高分辨率图像),应用药物的兴趣,并记录的响应。
- 选择期望的焦平面(如靠近玻璃尽可能的表面上,以确保肾小球的表面上足细胞的成像)。荧光检查的Fluo4和FuraRed渠道的力度,并确保肾小球清楚地看到在明。
- 启动成像。应用的任何药物之前,基线荧光记录至少1分钟,以确保该信号是稳定的(没有突然尖峰或衰落的信号)。
- 应用所需的药物用微量的帮助;要小心,并确保药物是能够扩散良好,达到glomer汗国。混合槽液轻轻如果需要的话,监视选定焦平面并检查它没动,因为药物应用失焦。
- 记录为Fluo4和FuraRed信号的荧光强度的变化。确保记录足够长时,通过等待直到信号达到平台期的水平或达到峰值,然后返回到基线。如果需要的话执行解变化或加入任何其它药物。
- 停止录制,并保存在文件中的软件的本机格式。
对于钙测量5图像分析
- 与搭载ND实用插件,允许进口的原生ND2格式图像ImageJ的开源软件进行图像分析。
- 导入图像序列;确保分裂渠道和使用hyperstack灰度模式。
- 按照分析→工具→投资回报率经理路径在ImageJ的软件邻笔投资回报率管理器窗口。选择感兴趣的(足细胞),使用椭圆选择工具和"添加(吨)"中的投资回报率管理器功能几个区域。当最后的投资回报率,选择在后台区域;保存选定的ROI(更多→保存)。
- 突出显示包含选择用于分析所述信道的窗口。使用更多→多功能测量功能的投资回报率经理,标志着在弹出的对话"一排每片"复选框,然后单击确定。结果将显示在可设置在结果窗口中的格式:进入菜单选项结果→设置测量,选择"平均灰度值",并计算每个投资回报率,这将显示在像素强度值结果窗口中的每个单独的投资回报率列。
- 复制所测量的ROI强度值的每个信道(的Fluo-4和的Fura红)到优选的数据分析软件;从每个DAT减去背景强度值一个点。
- 对于每个时间点计算出的Fluo-4的强度来的Fura红信道的比率。剧情分散/的钙瞬变每个ROI线点时间的变化。计算平均/ SE值选定肾小球。
注意:时间列应根据在4.5选定的成像频率来设置。
6.细胞内钙离子浓度计算使用荧光 - 4荧光信号
- 取肾小球的样品,并执行实验方案直到步骤4.7。记录的背景荧光后添加离子霉素(终浓度在浴室应10μM)向浴溶液中,并记录荧光强度的增加。一旦强度达到其最大值和衰减开始,添加的MnCl 2(最终浓度应为5毫米)以猝灭荧光8。
- 分析在6.1中获得的数据。根据获得的荧光 - 4信号ROI强度值复制以在5.1中描述的协议 - 5.5通过优选分析软件。
- 计算出细胞内钙离子浓度(纳米)在使用公式相应的投资回报率足:
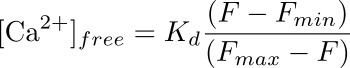
其中K d是预先确定的解离常数的Fluo-4(345纳米),F是强度在您正在计算中的钙浓度为(基线)的时间点,和F 分钟和F 最大值是在强度值点的最大钙负荷(洛诺霉素应用后)和荧光(带有的MnCl 2),骤冷后,分别( 见图3)。
结果
这里我们讨论测量在足细胞中的钙水平急性改变的问题。 图1显示了实验方案设计,以便在新鲜的足细胞进行高分辨率实时荧光共焦成像和单离子通道活性的录音的示意表示孤立的啮齿类动物肾小球。简要地说,将大鼠麻醉后,肾脏应用PBS冲洗以清除血液它们。然后,将肾脏切除和解封装,并且肾小球从肾皮质通过差筛分分离。样品的部分可采取膜片钳分析,其余的可以装载荧光钙染...
讨论
这里所描述的方法允许的钙处理由啮齿类肾小球的足细胞的分析。这种技术允许应用膜片钳单通道电生理和荧光比率共焦成像。然而,这两种方法可以单独使用,在他们自己的。该协议有几个相对简单的步骤,包括:1)肾脏冲洗; 2)肾小球通过差筛分分离; 3)进行膜片钳电生理实验,或用荧光钙标记染料更改胞内钙的比例共焦成像的肾小球的孵化。
为了分离肾小球,基于Gloy?...
披露声明
作者什么都没有透露。
致谢
作者想感谢格伦斯洛克姆(威斯康星医学院)和科琳A.拉文(尼康仪器公司)与显微镜实验优秀的技术援助。格雷戈里·布拉斯是公认的手稿校对至关重要。这项研究是支持的健康补助HL108880和美国糖尿病协会的国家机构给予1-15-BS-172(AS到),以及本J.里普斯研究奖学金由美国肾脏病学会(至DVI)。
材料
| Name | Company | Catalog Number | Comments |
| Fluo4 AM | Life Technologies | F14217 | 500 µl in DMSO |
| FuraRed AM | Life Technologies | F-3020 | |
| Poly-ʟ-lysine | Sigma-Aldrich | P4707 | |
| Pluronic acid | Sigma-Aldrich | F-68 | solution |
| Ionomycin | Sigma-Aldrich | I3909-1ML | |
| Tube rotator | Miltenyi Biotec GmbH | 130-090-753 | Germany |
| Nikon confocal microscope (inverted) | Nikon | Nikon A1R | Laser exitation 488 nm. Emission filters 500-550 nm and 570-620 nm |
| Objective | Nikon | Plan Apo 60x/NA 1.4 Oil | |
| Cover Glass | Thermo Scientific | 6661B52 | |
| High vacuum grease | Dow Corning | Silicone Compound | |
| Software | Nikon | Nikon NIS-Elements | |
| Recording/perfusion chamber | Warner Instruments | RC-26 | |
| Patch clamp amplifier | Molecular Devices | MultiClamp 700B | |
| Data acquisition system | Molecular Devices | Digidata 1440A | Axon Digidata® System |
| Low pass filter | Warner Instruments | LPF-8 | 8 pole Bessel |
| Borosilicate glass capillaries | World Precision Instruments | 1B150F-4 | |
| Micropipette puller | Sutter Instrument Co | P-97 | Flaming/Brown type micropipette puller |
| Microforge | Narishige | MF-830 | Japan |
| Motorized micromanipulator | Sutter Instrument Co | MP-225 | |
| Inverted microscope | Nikon | Eclipse Ti | |
| Microvibration isolation table | TMC | equipped with Faraday cage | |
| Multichannel valve perfusion system | AutoMake Scientific | Valve Bank II | |
| Recording/perfusion chamber | Warner Instruments | RC-26 | |
| Software | Molecular Devices | pClamp 10.2 | |
| Nicardipine | Sigma-Aldrich | N7510 | |
| Iberiotoxin | Sigma | I5904-5UG | |
| Niflumic acid | Sigma-Aldrich | N0630 | |
| DIDS | Sigma-Aldrich | D3514-25MG | |
| TEA chloride | Tocris | T2265 | |
| RPMI 1640 | Life Technologies | 11835030 | without antibiotics |
| BSA | Sigma-Aldrich | A8327 | 30% albumin solution |
| Temperature controlled surgical table | MCW core | for rodents | |
| Steel sieves: | #100 (150 μm), 140 (106 μm) | ||
| Gilson, Inc SIEVE 3 SS FH NO200 | Fisher Sci | 50-871-316 | |
| Gilson, Inc SIEVE 3 SS FH NO270 | Fisher Sci | 50-871-318 | |
| Gilson, Inc SIEVE 3 SS FH NO400 | Fisher Sci | 50-871-320 | |
| mesh 200 | Sigma-Aldrich | s4145 | screen for CD-1 |
| Binocular microscope | Nikon | Eclipse TS100 | |
| Binocular microscope | Nikon | SMZ745 | |
| Syringe pump-based perfusion system | Harvard Apparatus | ||
| Polyethylene tubing | Sigma-Aldrich | PE50 | |
| Isofluorane anesthesia |  VetEquip, Inc. VetEquip, Inc. | 911103 | |
| Other basic reagents | Sigma-Aldrich |
参考文献
- Machuca, E., Benoit, G., Antignac, C. Genetics of nephrotic syndrome: connecting molecular genetics to podocyte physiology. Hum. Mol. Genet. 18, R185-R194 (2009).
- Haraldsson, B., Nystrom, J., Deen, W. M. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol. Rev. 88, 451-487 (2008).
- Patrakka, J., Tryggvason, K. New insights into the role of podocytes in proteinuria. Nat. Rev. Nephrol. 5, 463-468 (2009).
- Savin, V. J., Sharma, R., Lovell, H. B., Welling, D. J. Measurement of albumin reflection coefficient with isolated rat glomeruli. J. Am. Soc. Nephrol. 3, 1260-1269 (1992).
- Gloy, J., et al. Angiotensin II depolarizes podocytes in the intact glomerulus of the Rat. J. Clin. Invest. 99, 2772-2781 (1997).
- Nitschke, R., et al. Angiotensin II increases the intracellular calcium activity in podocytes of the intact glomerulus. Kidney Int. 57, 41-49 (2000).
- Ilatovskaya, D., Staruschenko, A. Single-channel analysis of TRPC channels in the podocytes of freshly isolated glomeruli. Methods Mol Biol. 998, 355-369 (2013).
- Snitsarev, V. A., McNulty, T. J., Taylor, C. W. Endogenous heavy metal ions perturb fura-2 measurements of basal and hormone-evoked Ca2+ signals. Biophys. J. 71, 1048-1056 (1996).
- Fukuda, A., Fujimoto, S., Iwatsubo, S., Kawachi, H., Kitamura, K. Effects of mineralocorticoid and angiotensin II receptor blockers on proteinuria and glomerular podocyte protein expression in a model of minimal change nephrotic syndrome. Nephrology (Carlton). 15, 321-326 (2010).
- Abramowitz, J., Birnbaumer, L. Physiology and pathophysiology of canonical transient receptor potential channels). FASEB J. 23, 297-328 (2009).
- Heeringa, S. F., et al. A novel TRPC6 mutation that causes childhood FSGS. PLoS ONE. 4, e7771 (2009).
- Zhang, X., Song, Z., Guo, Y., Zhou, M. The novel role of TRPC6 in vitamin D ameliorating podocyte injury in STZ-induced diabetic rats. Mol. Cell. Biochem. 399, 155-165 (2015).
- Bohrer, M. P., et al. Mechanisms of the puromycin-induced defects in the transglomerular passage of water and macromolecules. J. Clin. Invest. 60, 152-161 (1977).
- Olson, J. L., Rennke, H. G., Venkatachalam, M. A. Alterations in the charge and size selectivity barrier of the glomerular filter in aminonucleoside nephrosis in rats. Lab. Invest. 44, 271-279 (1981).
- Schiessl, I. M., Castrop, H. Angiotensin II AT2 receptor activation attenuates AT1 receptor-induced increases in the glomerular filtration of albumin: a multiphoton microscopy study. Am J Physiol Renal Physiol. 305, F1189-F1200 (2013).
- Ilatovskaya, D. V., Levchenko, V., Ryan, R. P., Cowley, A. W., Staruschenko, A. NSAIDs acutely inhibit TRPC channels in freshly isolated rat glomeruli. Biochem. Biophys. Res. Commun. 408, 242-247 (2011).
- Peti-Peterdi, J. Calcium wave of tubuloglomerular feedback. Am. J. Physiol. Renal Physiol. 291, F473-F480 (2006).
- Peti-Peterdi, J., Warnock, D. G., Bell, P. D. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J. Am. Soc. Nephrol. 13, 1131-1135 (2002).
- Ilatovskaya, D. V., Palygin, O., Levchenko, V., Staruschenko, A. Pharmacological characterization of the P2 receptors profile in the podocytes of the freshly isolated rat glomeruli. Am. J. Physiol. Cell Physiol. 305, C1050-C1059 (2013).
- Ilatovskaya, D. V., et al. Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli. Kidney Int. 305, C1050-C1059 (2014).
- Schaldecker, T., et al. Inhibition of the TRPC5 ion channel protects the kidney filter. J. Clin. Invest. 123, 5298-5309 (2013).
- Roshanravan, H., Dryer, S. E. ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: role of podocin and reactive oxygen species. Am. J. Physiol. Renal Physiol. 306, F1088-F1097 (2014).
- Anderson, M., Roshanravan, H., Khine, J., Dryer, S. E. Angiotensin II activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species. J. Cell. Physiol. 229, 434-442 (2014).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。