A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Single-channel Analysis and Calcium Imaging in the Podocytes of the Freshly Isolated Glomeruli
In This Article
Summary
Changes in the intracellular calcium levels in the podocytes are one of the most important means to control the filtration function of glomeruli. Here we explain a high-throughput approach that allows detection of real-time calcium handling and single ion channels activity in the podocytes of the freshly isolated glomeruli.
Abstract
Podocytes (renal glomerular epithelial cells) are known to regulate glomerular permeability and maintain glomerular structure; a key role for these cells in the pathogenesis of various renal diseases has been established since podocyte injury leads to proteinuria and foot process effacement. It was previously reported that various endogenous agents may cause a dramatic overload in intracellular Ca2+ concentration in podocytes, presumably leading to albuminuria, and this likely occurs via calcium-conducting ion channels. Therefore, it appeared important to study calcium handling in the podocytes both under normal conditions and in various pathological states. However, available experimental approaches have remained somewhat limited to cultured and transfected cells. Although they represent a good basic model for such studies, they are essentially extracted from the native environment of the glomerulus. Here we describe the methodology of studying podocytes as a part of the freshly isolated whole glomerulus. This preparation retains the functional potential of the podocytes, which are still attached to the capillaries; therefore, podocytes remain in the environment that conserves the major parts of the glomeruli filtration apparatus. The present manuscript elaborates on two experimental approaches that allow 1) real-time detection of calcium concentration changes with the help of ratiometric confocal fluorescence microscopy, and 2) the recording of the single ion channels activity in the podocytes of the freshly isolated glomeruli. These methodologies utilize the advantages of the native environment of the glomerulus that enable researchers to resolve acute changes in the intracellular calcium handling in response to applications of various agents, measure basal concentration of calcium within the cells (for instance, to evaluate disease progression), and assess and manipulate calcium conductance at the level of single ion channels.
Introduction
Kidneys maintain homeostatic balance for various substances and regulate blood volume in a way that determines total blood pressure. Disturbances in the renal filtration, reabsorption or secretion lead to or accompany pathological states, ranging from hyper- or hypotension to end stage renal disease that eventually requires kidney transplantation. The renal filtering unit (glomerulus) consists of three layers – the capillary endothelium, basement membrane and a single-cell layer of epithelial cells – podocytes, which play a major role in the maintenance of the slit-diaphragm integrity and function1. Dysfunction in the permselective glomerular filter causes urinary loss of macromolecules, such as proteinuria. Various agents may affect the structure of the podocytes and their foot processes, which determine the integrity of the glomeruli filtration barrier.
The podocytes are involved in the maintenance of the glomeruli filtration function. It has been established that improper calcium handling by the podocyte leads to cell injury and plays an important role in the progression of various forms of nephropathies2,3. Therefore, development of a model which allows for direct measuring of intracellular calcium concentration changes will be instrumental for studies of podocyte function. Isolated glomeruli were previously used in a numerous studies including measurement of albumin reflection coefficient changes4 and assessment of integral cellular currents in the whole-cell electrophysiological patch-clamp measurements5,6. In the present paper we describe the protocol that allows the researcher to measure intracellular calcium concentration changes in response to applications of pharmacological agents, estimate basal levels of calcium within the cells, and assess individual calcium channels activity. Ratometric calcium concentration measurements and patch-clamp electrophysiology were used to determine changes in the intracellular calcium concentration within the podocyte and channel activity, respectively.
Protocol
Animal use and welfare should adhere to the NIH Guide for the Care and Use of Laboratory Animals following protocols reviewed and approved by the Institutional Animal Care and Use Committee (IACUC).
1. Kidney Flush
- Use 8 to 12 week old male rat (suggested is a Sprague Dawley strain, however other strains of different age and gender can be used with appropriate changes).
- Anesthetize the animal according to the procedure allowed by IACUC protocol; monitor depth of anesthesia and inspect the animal. The detailed description of the surgery to be performed in 1.3 - 1.8 can be found in Ilatovskaya et al.7
- After proper anesthesia, place the animal on a temperature-controlled surgical table, make a midline incision of the abdomen (up to 3 inches in length), and uncover the vena cava and the aorta.
- Insert ligature around the celiac and superior mesenteric arteries and the abdominal aorta above those; do not ligate.
- Blunt dissect the abdominal aorta below the renal arteries, and place two ligatures around it, but do not ligate, then clamp the aorta above the ligatures and tie the lower thread.
- Catheterize the aorta with a polyethylene PE50 tubing (attached to a syringe pump filled with PBS) below the clamp and fix the catheter with the second ligature; remove the clamp, turn on the pump, and ligate the aorta and mesenteric with celiac arteries. Quickly make incision in renal vein to relieve the pressure.
- Infuse the aorta with the pre-chilled PBS for 2 or 3 min at a rate of 6 ml/min.
- Stop perfusion, excise and decapsulate7 the kidneys, and put them on ice in the PBS solution. Euthanize the animal according to protocol approved by IACUC.
2. Isolation of the Rat Glomeruli
- Prepare 30 ml of fresh solution of 5% BSA in RPMI 1640.
- Using a razor blade and scissors, isolate the cortex of both kidneys, and then mince until homogenous. This procedure has been described earlier7.
- Push the tissue minced during the previous step through a 100 mesh stainless steel sieve (pre-soaked in 5% BSA/RPMI solution) using a spatula. Collect the flow-through and by gravity force allow the flow-through to pass through a 140 mesh sieve.
- Filter the flow-through collected from the 140 mesh sieve using a pre-soaked 200 mesh sieve, discard the filtrate, and wash the top of the sieve with 10 - 15 ml of the prepared BSA/RPMI solution to collect the glomeruli that sediment on the sieve.
- Put the BSA/RPMI solution containing glomeruli on ice in a 15 ml tube and let the glomeruli sediment at the bottom of the tube for up to 20 min. The glomeruli concentrate on the bottom of the tube will be clearly seen. Remove the excess solution, leaving approximately 2 ml in the tube.
3. Single-channel Patch-clamp Electrophysiology
- Prepare 5 x 5 mm cover glass chips by coating them with MW 70,000 - 150,000 poly-ʟ-lysine, and let dry. Use approximately 30 µl of 0.01% sterile filtered solution in water per cover glass.
- Warm up the experimental solutions to RT and fill the patch-clamp chamber and pipette. For TRPC channels monitoring, use a bath solution, in mM: 126 NaCl, 1 CaCl2, 10 HEPES, 2 MgCl2, 10 glucose, pH 7.4; pipette: 126 NaCl, 1.5 CaCl2, 10 HEPES, 10 glucose; pH 7.4.
- Add inhibitors to the pipette solution to block activity of endogenous channels, which are not relevant for the studies (recommended are: 100 µM niflumic acid or DIDS (to block Ca2+-activated Cl- channels), 10 mM TEA (to inhibit the large-conductance Ca2+-dependent K+ channel), 10 nM iberiotoxin (to block the Ca2+-activated K+ channels), 10 µM nicardipine (to block N-type Ca2+ channels)) directly before the patch-clamp experiment.
- Gently mix the glomeruli-containing solution, and then apply approximately 50 µl of it to the poly-ʟ-lysine coated cover glass chips. Let the glomeruli attach for approximately 5 min.
- Move the glass chips with glomeruli to the patch-clamp chamber pre-filled with the bath solution; perfuse the chamber at a rate of 3 ml/min for 1 min to ensure the removal of the unattached glomeruli.
- Conduct a conventional patch-clamp experiment in a cell-attached mode7. With a glass pipette (7 - 10 MΩ pipette resistance) form a high-resistance seal between a pipette and a podocyte membrane by applying gentle suction (a pipette attached to a podocyte on the surface of the isolated glomerulus is shown in Figure 2, on the left).
- For the cell-attached measurements, low-pass the currents at 300 Hz by an eight-pole Bessel filter.
- Use isolated glomeruli in patch-clamp experiments for up to 4 - 6 hr. Keep the stock glomeruli fraction on ice.
4. Ratiometric Confocal Fluorescence Measurements of Intracellular Calcium Concentration in the Podocytes
- Place 500 µl of the glomeruli fraction (described at 2.5) in 0.5 ml conical tube and add calcium dyes Fura Red, AM and Fluo-4, AM. Use 2 mM and 1 mM stock concentrations of Fura Red, AM and Fluo-4, AM, respectively (store at -20 °C, dissolved in DMSO) and use 2.5 µl of each dye for a 500 µl of glomeruli fraction. Immediately after addition of the dyes cover the tube with aluminum foil.
- Place tubes on a rotating shaker for at least 20 min up to 1 hr at RT.
Note: Pharmacological agents can be added during this step. - Prepare glass coverslips, cover them with poly-ʟ-lysine and allow drying using heated plate set to 70 °C.
- Once the loading of calcium dyes is complete, apply 100 µl of the glomeruli containing solution to the poly-ʟ-lysine coated coverslips and let them stick to the surface for about 5 min. Mount the glomeruli-attached coverslips into an imaging chamber, and perfuse with the bath solution (containing (in mM): 145 NaCl, 2 CaCl2, 4.5 KCl, 2 MgCl2, 10 HEPES, pH 7.35) at a rate of 3 ml/min to remove the unattached glomeruli and the remaining dyes.
- Set the confocal laser scanning microscope to a 488 nm excitation wavelength and emission filters (525/25 and 650/25 nm for Fluo-4 and Fura Red, respectively). Set the imaging software to a desired frequency and resolution.
- Find the glomeruli in brightfield and then turn on the detection of the fluorescence signal. Adjust the intensity of the laser for each dye to avoid saturation of the signal. Choose the focal plane with the podocytes which are directly attached to the glass. This minimizes the effect caused by contraction of a glomerulus in response to drug application. Double-check that the glomerulus of choice is well attached to the glass;
- Start imaging the focal plane of choice (take 512 x 512 images with the frequency set at 4 sec to visualize fast Ca2+ transient changes. Use a 60X/NA 1.4 or similar objective lens for high resolution image), apply drugs of interest, and record the response.
- Select a desired focal plane (as close to the surface of the glass as possible, to ensure imaging of podocytes on the surface of the glomerulus). Check the intensity of fluorescence on the Fluo4 and FuraRed channels, and make sure that the glomerulus is clearly seen in brightfield.
- Start imaging. Before application of any drugs, record at least 1 min of baseline fluorescence to make sure that the signal is stable (there are no sudden spikes or fading of the signal).
- Apply desired drugs with the help of a micropipette; be careful and ensure the drug was able to diffuse well and reach the glomerulus. Mix the bath solution gently if needed, monitor the selected focal plane and check that it did not move out of focus because of the drug application.
- Record the changes in fluorescence intensity for the Fluo4 and FuraRed signals. Make sure the recording is long enough, by waiting until the signal reaches the plateau level or reaches a peak and then returns to baseline. Perform solution change or addition of any other drugs if needed.
- Stop the recording, and save the file in the native format of the software.
5. Image Analysis for the Calcium Measurements
- Perform image analysis with the ImageJ open software equipped with the ND Utility plugin that allows importing images in the native ND2 format.
- Import the image sequence; make sure to split the channels and use a hyperstack grayscale mode.
- Follow the Analyze → Tools → ROI manager path in the ImageJ software to open a ROI manager window. Select several regions of interest (podocytes) using an oval selection tool and the “Add (t)” function in the ROI Manager. As the last ROI, select the area in the background; save the selected ROIs (More → Save).
- Highlight the window containing the channel selected for analyses. Use the More → Multi Measure function in the ROI Manager, mark the “one row per slice” box in the dialogue that pops out, and then click OK. The results will be shown in the format that can be set up in the Results window: enter into the menu option Results → Set Measurements, select “Mean gray value” and calculate the pixel intensity values for each ROI, which will be displayed in the Results window in separate columns for each ROI.
- Copy the measured ROI intensity values for each channel (Fluo-4 and Fura Red) into preferred data analysis software; subtract background intensity values from each data point.
- For each time point calculate the ratio of intensities of the Fluo-4 to Fura Red channels. Plot scatter/line point-time changes of Ca2+ transient for each ROI. Calculate Mean/SE values for selected glomeruli.
Note: Time column should be set according to imaging frequency selected at 4.5.
6. Intracellular Calcium Concentration Calculations Using Fluo-4 Fluorescence Signal
- Take a sample of the glomeruli and perform the experimental protocol up to step 4.7. After recording of the background fluorescence add Ionomycin (final concentration in the bath chamber should be 10 µM) to the bath solution, and record fluorescence intensity increase. Once the intensity reaches its maximum and the decay starts, add MnCl2 (final concentration should be 5 mM) to quench the fluorescence8 .
- Analyze the data obtained in 6.1. Copy the Fluo-4 signal ROI intensity values obtained according to the protocol described at 5.1 - 5.5 by preferred analysis software.
- Calculate intracellular calcium concentration (in nM) in the podocyte corresponding to the ROI using a formula:
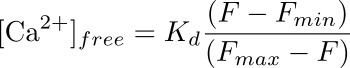
where Kd is a pre-determined dissociation constant for Fluo-4 (345 nM), F is the intensity at the timepoint that you are calculating the calcium concentration for (baseline), and Fmin and Fmax are the intensity values at the point of maximum calcium load (after ionomycin application) and after quenching of the fluorescence (with MnCl2), respectively (see Figure 3).
Results
Here we addressed the problem of measuring acute changes in the calcium levels in the podocytes. Figure 1 shows a schematic representation of the experimental protocol designed in order to perform high resolution live fluorescence confocal imaging and single ion channel activity recordings in the podocytes of the freshly isolated rodent glomeruli. Briefly, after the rat is anaesthetized, the kidneys should be flushed with PBS to clear them of blood. Then, the kidneys are excised and decapsulated, and glo...
Discussion
The approach described here allows for the analysis of calcium handling by the podocytes of the rodent glomeruli. This technique allows application of patch-clamp single channel electrophysiology and fluorescence ratiometric confocal imaging. However, both approaches can be used separately, on their own. The proposed protocol has several relatively simple steps, including 1) kidney flush; 2) isolation of the glomeruli by differential sieving; 3) performing patch-clamp electrophysiological experiments, or incubation of th...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Glen Slocum (Medical College of Wisconsin) and Colleen A. Lavin (Nikon Instruments, Inc.) for excellent technical assistance with microscopy experiments. Gregory Blass is acknowledged for critical proofreading of the manuscript. This research was supported by the National Institutes of Health grant HL108880 and American Diabetes Association grant 1-15-BS-172 (to AS), and the Ben J. Lipps Research Fellowship from the American Society of Nephrology (to DVI).
Materials
| Name | Company | Catalog Number | Comments |
| Fluo4 AM | Life Technologies | F14217 | 500 µl in DMSO |
| FuraRed AM | Life Technologies | F-3020 | |
| Poly-ʟ-lysine | Sigma-Aldrich | P4707 | |
| Pluronic acid | Sigma-Aldrich | F-68 | solution |
| Ionomycin | Sigma-Aldrich | I3909-1ML | |
| Tube rotator | Miltenyi Biotec GmbH | 130-090-753 | Germany |
| Nikon confocal microscope (inverted) | Nikon | Nikon A1R | Laser exitation 488 nm. Emission filters 500-550 nm and 570-620 nm |
| Objective | Nikon | Plan Apo 60x/NA 1.4 Oil | |
| Cover Glass | Thermo Scientific | 6661B52 | |
| High vacuum grease | Dow Corning | Silicone Compound | |
| Software | Nikon | Nikon NIS-Elements | |
| Recording/perfusion chamber | Warner Instruments | RC-26 | |
| Patch clamp amplifier | Molecular Devices | MultiClamp 700B | |
| Data acquisition system | Molecular Devices | Digidata 1440A | Axon Digidata® System |
| Low pass filter | Warner Instruments | LPF-8 | 8 pole Bessel |
| Borosilicate glass capillaries | World Precision Instruments | 1B150F-4 | |
| Micropipette puller | Sutter Instrument Co | P-97 | Flaming/Brown type micropipette puller |
| Microforge | Narishige | MF-830 | Japan |
| Motorized micromanipulator | Sutter Instrument Co | MP-225 | |
| Inverted microscope | Nikon | Eclipse Ti | |
| Microvibration isolation table | TMC | equipped with Faraday cage | |
| Multichannel valve perfusion system | AutoMake Scientific | Valve Bank II | |
| Recording/perfusion chamber | Warner Instruments | RC-26 | |
| Software | Molecular Devices | pClamp 10.2 | |
| Nicardipine | Sigma-Aldrich | N7510 | |
| Iberiotoxin | Sigma | I5904-5UG | |
| Niflumic acid | Sigma-Aldrich | N0630 | |
| DIDS | Sigma-Aldrich | D3514-25MG | |
| TEA chloride | Tocris | T2265 | |
| RPMI 1640 | Life Technologies | 11835030 | without antibiotics |
| BSA | Sigma-Aldrich | A8327 | 30% albumin solution |
| Temperature controlled surgical table | MCW core | for rodents | |
| Steel sieves: | #100 (150 μm), 140 (106 μm) | ||
| Gilson, Inc SIEVE 3 SS FH NO200 | Fisher Sci | 50-871-316 | |
| Gilson, Inc SIEVE 3 SS FH NO270 | Fisher Sci | 50-871-318 | |
| Gilson, Inc SIEVE 3 SS FH NO400 | Fisher Sci | 50-871-320 | |
| mesh 200 | Sigma-Aldrich | s4145 | screen for CD-1 |
| Binocular microscope | Nikon | Eclipse TS100 | |
| Binocular microscope | Nikon | SMZ745 | |
| Syringe pump-based perfusion system | Harvard Apparatus | ||
| Polyethylene tubing | Sigma-Aldrich | PE50 | |
| Isofluorane anesthesia |  VetEquip, Inc. VetEquip, Inc. | 911103 | |
| Other basic reagents | Sigma-Aldrich |
References
- Machuca, E., Benoit, G., Antignac, C. Genetics of nephrotic syndrome: connecting molecular genetics to podocyte physiology. Hum. Mol. Genet. 18, R185-R194 (2009).
- Haraldsson, B., Nystrom, J., Deen, W. M. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol. Rev. 88, 451-487 (2008).
- Patrakka, J., Tryggvason, K. New insights into the role of podocytes in proteinuria. Nat. Rev. Nephrol. 5, 463-468 (2009).
- Savin, V. J., Sharma, R., Lovell, H. B., Welling, D. J. Measurement of albumin reflection coefficient with isolated rat glomeruli. J. Am. Soc. Nephrol. 3, 1260-1269 (1992).
- Gloy, J., et al. Angiotensin II depolarizes podocytes in the intact glomerulus of the Rat. J. Clin. Invest. 99, 2772-2781 (1997).
- Nitschke, R., et al. Angiotensin II increases the intracellular calcium activity in podocytes of the intact glomerulus. Kidney Int. 57, 41-49 (2000).
- Ilatovskaya, D., Staruschenko, A. Single-channel analysis of TRPC channels in the podocytes of freshly isolated glomeruli. Methods Mol Biol. 998, 355-369 (2013).
- Snitsarev, V. A., McNulty, T. J., Taylor, C. W. Endogenous heavy metal ions perturb fura-2 measurements of basal and hormone-evoked Ca2+ signals. Biophys. J. 71, 1048-1056 (1996).
- Fukuda, A., Fujimoto, S., Iwatsubo, S., Kawachi, H., Kitamura, K. Effects of mineralocorticoid and angiotensin II receptor blockers on proteinuria and glomerular podocyte protein expression in a model of minimal change nephrotic syndrome. Nephrology (Carlton). 15, 321-326 (2010).
- Abramowitz, J., Birnbaumer, L. Physiology and pathophysiology of canonical transient receptor potential channels). FASEB J. 23, 297-328 (2009).
- Heeringa, S. F., et al. A novel TRPC6 mutation that causes childhood FSGS. PLoS ONE. 4, e7771 (2009).
- Zhang, X., Song, Z., Guo, Y., Zhou, M. The novel role of TRPC6 in vitamin D ameliorating podocyte injury in STZ-induced diabetic rats. Mol. Cell. Biochem. 399, 155-165 (2015).
- Bohrer, M. P., et al. Mechanisms of the puromycin-induced defects in the transglomerular passage of water and macromolecules. J. Clin. Invest. 60, 152-161 (1977).
- Olson, J. L., Rennke, H. G., Venkatachalam, M. A. Alterations in the charge and size selectivity barrier of the glomerular filter in aminonucleoside nephrosis in rats. Lab. Invest. 44, 271-279 (1981).
- Schiessl, I. M., Castrop, H. Angiotensin II AT2 receptor activation attenuates AT1 receptor-induced increases in the glomerular filtration of albumin: a multiphoton microscopy study. Am J Physiol Renal Physiol. 305, F1189-F1200 (2013).
- Ilatovskaya, D. V., Levchenko, V., Ryan, R. P., Cowley, A. W., Staruschenko, A. NSAIDs acutely inhibit TRPC channels in freshly isolated rat glomeruli. Biochem. Biophys. Res. Commun. 408, 242-247 (2011).
- Peti-Peterdi, J. Calcium wave of tubuloglomerular feedback. Am. J. Physiol. Renal Physiol. 291, F473-F480 (2006).
- Peti-Peterdi, J., Warnock, D. G., Bell, P. D. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J. Am. Soc. Nephrol. 13, 1131-1135 (2002).
- Ilatovskaya, D. V., Palygin, O., Levchenko, V., Staruschenko, A. Pharmacological characterization of the P2 receptors profile in the podocytes of the freshly isolated rat glomeruli. Am. J. Physiol. Cell Physiol. 305, C1050-C1059 (2013).
- Ilatovskaya, D. V., et al. Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli. Kidney Int. 305, C1050-C1059 (2014).
- Schaldecker, T., et al. Inhibition of the TRPC5 ion channel protects the kidney filter. J. Clin. Invest. 123, 5298-5309 (2013).
- Roshanravan, H., Dryer, S. E. ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: role of podocin and reactive oxygen species. Am. J. Physiol. Renal Physiol. 306, F1088-F1097 (2014).
- Anderson, M., Roshanravan, H., Khine, J., Dryer, S. E. Angiotensin II activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species. J. Cell. Physiol. 229, 434-442 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved