Method Article
无标记技术的单细胞分泌物的时空成像
摘要
细胞间的通信为内和细胞外的控制各种生理活性的关键。本文描述了测量的单细胞的分泌物的时空性质的协议。为了实现这一目标,多学科的方法是使用集成了无标记纳米等离子体激感测与活细胞成像。
摘要
Inter-cellular communication is an integral part of a complex system that helps in maintaining basic cellular activities. As a result, the malfunctioning of such signaling can lead to many disorders. To understand cell-to-cell signaling, it is essential to study the spatial and temporal nature of the secreted molecules from the cell without disturbing the local environment. Various assays have been developed to study protein secretion, however, these methods are typically based on fluorescent probes which disrupt the relevant signaling pathways. To overcome this limitation, a label-free technique is required.
In this paper, we describe the fabrication and application of a label-free localized surface plasmon resonance imaging (LSPRi) technology capable of detecting protein secretions from a single cell. The plasmonic nanostructures are lithographically patterned onto a standard glass coverslip and can be excited using visible light on commercially available light microscopes. Only a small fraction of the coverslip is covered by the nanostructures and hence this technique is well suited for combining common techniques such as fluorescence and bright-field imaging.
A multidisciplinary approach is used in this protocol which incorporates sensor nanofabrication and subsequent biofunctionalization, binding kinetics characterization of ligand and analyte, the integration of the chip and live cells, and the analysis of the measured signal. As a whole, this technology enables a general label-free approach towards mapping cellular secretions and correlating them with the responses of nearby cells.
引言
间的蜂窝通信是对许多生理活动的内部和细胞外部的调节是至关重要的。各种蛋白质和囊泡的可分泌其随后引发复杂的细胞过程如分化,伤口愈合,免疫应答,迁移和增殖。1-5故障的细胞间信号传导途径已牵涉许多疾病,包括癌症,动脉粥样硬化和糖尿病,仅举几例。
最优细胞分泌测定应当能够在空间和时间映射感兴趣的分泌蛋白而不破坏相应信号通路。以这种方式,浓度分布和接收单元的响应之间的因果关系可以推断。不幸的是,许多的最常用的荧光为基础的技术不符合这些标准。荧光融合蛋白可以用于标记分析物瓦特ithin的细胞,而且可以破坏分泌途径,或者如果分泌,结果,其中是难以量化单元外部的扩散辉光。荧光immunosandwich基测定法是最常用的技术,用于检测蜂窝分泌物但通常需要单个细胞的分离。6-11此外,引入感测抗体的通常停止或结束实验和的抗体标签的大小, 150 kDa的对IgG,是妨碍下游信号。
这些路障,因为它是优选无标记技术被用于图像蛋白分泌物和之间现有无标记技术,表面等离子体共振(SPR)和局域型表面等离子体共振(LSPR)传感器是优良的候选12-17这些传感器已被广泛地用于蛋白,外来体和其他生物标志物的分析物结合研究。在18-24 LSPR,电浆nanostr的情况下uctures可以将光刻图案化到玻璃盖玻片,并通过标准的宽视场显微镜的配置利用可见光激发。由于它们的纳米级尺寸,大多数的玻璃基板的可用于诸如亮场和荧光显微镜使这些探针非常适用于与活细胞显微术集成常见的成像技术。25-28我们已经证明了实时测量从使用官能化金电浆纳米结构的225毫秒和10微米,分别时空分辨率杂交瘤细胞抗体的分泌物。基本芯片配置示于图1。28显微镜的输出光路被分离用于成像的CCD照相机和一个光纤光学地耦合分光计用于定量测定纳米结构的给定阵列的分数占用(图2的间)。
该protoc醇在本文提出描述了实验设计为单细胞的分泌物的实时测量,同时监测使用标准明场显微术的细胞的响应。多学科方法包括纳米结构的细胞株的制造,纳米结构为高亲合力的分析物结合的官能化,这两个最小化非特异性结合和使用商业表面等离子体共振表征动力学速率常数(SPR)仪器表面优化,集成到衬底上,并且图像和光谱数据的分析。我们预计这种技术是一种使能技术进行细胞分泌物和接收单元的因果关系的时空映射。
研究方案
1.纳米结构的制备
- 选择直径25mm玻璃盖玻片上为170微米(第1.5)作为用于纳米加工基板的近似厚度。
- 浸入食人鱼溶液盖玻片(3:1的比例的硫酸和过氧化氢的)至少6小时。用超纯水18.2MΩ去离子蒸馏水(DDW)的量丰富的洗净浸泡食人鱼盖玻片。
注意:食人鱼酸发生剧烈反应与有机材料的,必须格外小心。 - 上的盖玻片通过电子束蒸发存款10nm的铬薄膜,以避免纳米结构的图案化和成像期间电荷效应。
- 旋双层的第一层抗蚀剂选自乳酸乙酯甲基丙烯酸甲酯(MMA_EL6)共聚物以2000rpm进行45秒,然后烘烤,在150℃。以3000rpm进行45秒旋的聚甲基丙烯酸甲酯(950PMMA_A2)所述第二层,然后烘烤,在180℃。
- 行话n中的双层抗蚀剂使用电子束光刻(EBL),在25千伏为300μC/ cm 2的区域的剂量。开发中的异丙醇(IPA)/甲基异丁基酮(MIBK):2/1和冲洗在IPA。
- 存款的Ti(5nm)的使用电子束蒸发器在衬底上/金(80纳米)的膜。
- 继金沉积,升空浸泡在丙酮基板4小时的共聚物双层抗拒。
- 使用扫描电子显微镜(SEM)确认纳米结构的形状和大小检查基材,使用的CR-7腐蚀剂通过湿蚀刻除去从基板剩余铬60秒在RT,然后冲洗在DDW。
- 设计的33微米中心对中心阵列间距,以留出空间阵列间的细胞成像。图案在20×20模式为每个阵列的使用电子束写入300nm的间距的纳米结构。每个芯片包含300阵列的80±2.5纳米的高度典型的纳米结构尺寸,70±2.5纳米diame之三。
- 检查使用原子力显微镜(AFM)对尺寸和均匀性的验证阵列的一个子集。
- 附加的支撑环,通常是硅,以使用UV固化环氧树脂盖玻片的背面。
2.芯片清洁和自组装膜中的应用
- 进行清洗和再生芯片,等离子体灰在40瓦,在5%的氢,清洁腔室5分钟在相同条件下经过95%的氩气,持续45秒的300毫乇混合物的功率。
- 通过浸入一种双组分的乙醇硫醇溶液中的芯片组成的3官能的金纳米结构等离子体灰化后,立即:1的比例的SH-(CH 2)8 -EG 3 -OH(SPO)和带胺的成分或羧基官能团,即,SH-(CH 2)11 -EG 3 -NH 2(SPN)或SH-(CH 2)11 -EG 3 -COOH(SPC)。
- 离开芯片的In中的硫醇溶液O / N,以形成自组装单层(SAM)。
- 冲洗用乙醇,干燥,用氮气芯片。
- 如果需要的话,存储该官能化芯片进行长达2周,在4℃。
- 当准备使用反应该SPN或者SPC组分与使用取决于所选择的配位体的化学配位体(见下文)。
注意:该芯片可以再生和再官能数十次。一个给定的芯片可以用于周期,范围从6个月到一年以上。一个给定的阵列上的所测量的光谱通过等离子体灰化反复再生,随后biofunctionalization之后可靠地再现。29
3.表面功能化和动力学特性
注意:使用在商业SPR仪器的官能化芯片与配体和分析物之间的动力学速率常数表征,以及研究的SAM非特异性b上电阻inding。有一个广泛的流量和微流体设计,以便有效的表面功能化。既然我们有市售SPR我们围绕标准化的推荐流量。我们注意到,这些流量是典型的所有SPR仪器等等都没有限制。 SPR仪器不是必须的,因为所有的官能化可以直接在LSPR芯片上进行,但它并减少我们的工作负荷,因为它是一个复用的仪器,而我们的微流体LSPR设置不。
- 功能化商用裸金片与SAM在第2节所述。
- 如果使用SPC基于SAM中,激活羧基用1:133毫1-乙基-3-(3-二甲基氨基丙基)碳二亚胺(EDC)和33 N-羟基的毫琥珀酰亚胺(NHS)的DDW 10 1混合物分钟。
- 缀合的活化的羧基与感兴趣为使用30微升/分钟的流速300秒的抗体/配体。准备配体pH为6的磷酸缓冲液,典型地,但是这可以根据不同的分子变化。
- 所述配体缀合后,流的0.1M乙醇胺在磷酸盐缓冲盐水(PBS),为300秒,在30微升/分的速率失活剂步骤。乙醇胺有助于减少非特异性结合。
- 引入感兴趣的分析物在100微升/ min,使用浓度范围的流速和计算使用动力学分析软件的动力学速率常数。
- 如果非特异性结合是有问题的,增加的SPO到SPC或SPN的比率。
4. LSPR常规设置
- 显微镜设置:
- 使用100瓦的卤素灯克勒照射样品。使用在光路中的长通滤波器(一般593纳米的截止),以消除波长不向谐振移位( 图2)。
- 对于LSPRi数据的收集,使用倒置显微镜与40X石油观鱼ñ目标(1.4 NA)和热电冷却的16位CCD相机。
- 放置一个光束分离器,在显微镜的输出端口,以同时获得图像和光谱。
- 将温度控制的显微镜阶段至37 °C和平衡4小时。
- 合并在显微镜额外孵育组件,以调节CO 2浓度,湿度为5%和95%,分别为。
- 芯片的制备和安装:
- 官能LSPR的芯片作为第2部分所述用来自SPR实验确定最佳的双组分的SAM比率。
- 一个定制的微夹持器内装入芯片如下。放置在铝底片的芯片。夹着该底部片和硅酮密封垫和一个透明的塑料顶部件之间的芯片上。用4个螺钉夹紧组件。
- 对于一个典型的SPC基硫醇应用,降外套300微升1:1混合的133毫的EDC和33毫NHS在DDW TURE激活SPC硫醇组分的羧基。
- 等待10分钟,并手动冲洗表面用10mM PBS中。
- 缀合的活化的羧基与感兴趣的配体(通常是抗体或抗体片段)通过滴涂覆300微升的配体溶液。
- 等待30分钟,用10mM的PBS手动冲洗。
- 降外套300微升的0.1M乙醇胺的PBS在芯片上,以减少非特异性结合。等待10分钟。
- 用含0.005%吐温20的PBS(PBS-T20)的PBS洗乙醇胺。
- 将一个石英片芯片以上,以减少相关的变化半月板的数据波动。
- 保持芯片用PBS-T20缓冲湿而安装在显微镜。
- 放置LSPR芯片组件使其进入加热阶段,样品架,并附上微管。
- 装上微流体管的组装和流动BUFFER(或无血清培养基进行细胞研究),直到达到稳定状态。
- 允许该组件和显微镜下平衡至少2小时。
- 使用操纵杆,使得中央阵列与光纤的光谱对准芯片对齐。光谱数据是使用光谱仪和频谱分析软件。
- 请重点阵列整个实验过程由软件自动对焦,蔡司定聚焦或等效的自动对焦装置。
抗c-myc的分泌物9E10杂交瘤细胞5 LSPR成像
注意:用于此研究的杂交瘤细胞系表达抗c-myc的抗体组成,因此不需要化学触发
- 功能化与c-myc基因肽纳米结构。这具有1.77纳米用于通过克隆9E10杂交瘤细胞分泌的抗c-myc的抗体A K D值 。
- 文化杂交瘤细胞在COMPLETË生长培养基在37℃,5%CO 2条件下10%胎牛血清(FBS)和1%抗生素的T75烧瓶。保持4×10 5个细胞/ ml的细胞密度。
- 为细胞分泌的研究中,从T75烧瓶通过离心沉淀细胞,用RPMI-1640无血清培养基(SFM)洗涤两次以除去分泌的抗体,并调整细胞密度4×10 6个细胞/ ml。
- 他们介绍到LSPR芯片之前收获细胞并测试可行性。
- 引入50微升的细胞溶液的手动到LSPR芯片用微量。几分钟后25到50个细胞附着在LSPR芯片的表面上。
- 与溶液中的新鲜SFM利用微流体灌注系统洗去剩余的细胞。
- 选择在10微米LSPR阵列成像这是接近,但不与细胞重叠。
- 以确保该信号是特定于分泌的抗c-myc的antibodies引入细胞培养基具有和不存在的抗体以及与抗体,但与堵塞的c-myc基因的溶液肽饱和浓度的存在下它们的结合位点。
- 校准传感器在每次运行与抗c-myc的抗体(250纳米)的饱和溶液的末端。这有助于在传感器的响应归一化,并确定基于每个游程的biofunctionalization轮廓分数占用。
- 校正用图像比对软件的X和Y方向上的漂移。
结果
在典型的活细胞分泌研究有数据收集发生的多种模式。 图3示出了LSPRi图像,其中突出的正方形阵列的叠加,和一个透射光照明图像,突出的细胞在左下方。数据通常收集在3小时内,随后通过引入的分析物为后述的归一化计算的饱溶液。荧光图像,也可以通过一个过滤器立方体的自动切换集成到数据收集例程。 在图4染色荧光膜染料若丹明DHPE细胞呈现伪足状延伸(箭头)。如果这样的扩展是与阵列重叠,他们将得到的假阳性蛋白质分泌。有图像的多种模式可以帮助识别这种情况的发生。
图5示出前光谱数据和船尾器引入一个饱和溶液(400纳米)的商购抗c-myc的抗体与c-myc的官能化阵列。没有细胞存在于该实验。光谱显示两个红移和增加强度。两条曲线下的区域之间的差异导致在CCD摄像机上的增加,阵列图像强度LSPRi模式。甲非线性最小二乘数据分析方法已被开发来推断从光谱面结合的配体的分数而定。30,31
在实验结束时,饱和的强度值(即,分数占用≈1)被用来计算使用以下公式中的每个阵列的归一化的反应:
哪里的归一化强度在时间点t,在该实验中,最终的饱和强度的开始初始强度,并且阵列的被测强度在时间点t 分别。
从两个阵列标准化的值示于图6中。一个阵列是在10微米的细胞的受调查的同时,另外,作为对照,从一小区的距离为130微米。在最接近的阵列相对于所述控制阵列的平坦响应的单元的归一化响应的突然增加是表示一个局部突发分泌的抗体的。
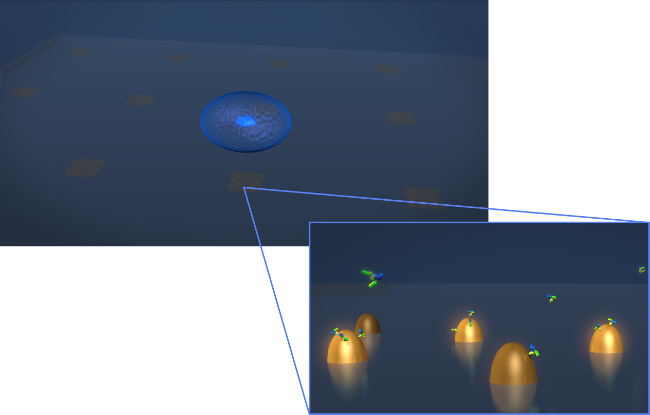
图1.传感器的设计。甲绘图描绘了典型的活细胞分泌实验的几何形状的细胞(蓝色球体)沉积到LSPR芯片,其中包含的biofunctionalized金纳米结构阵列。在放大视图中,感兴趣的细胞分泌,在示出为Y形分子这种情况下的抗体,因为它们结合到表面,测量功能化纳米结构。 请点击此处查看该图的放大版本。
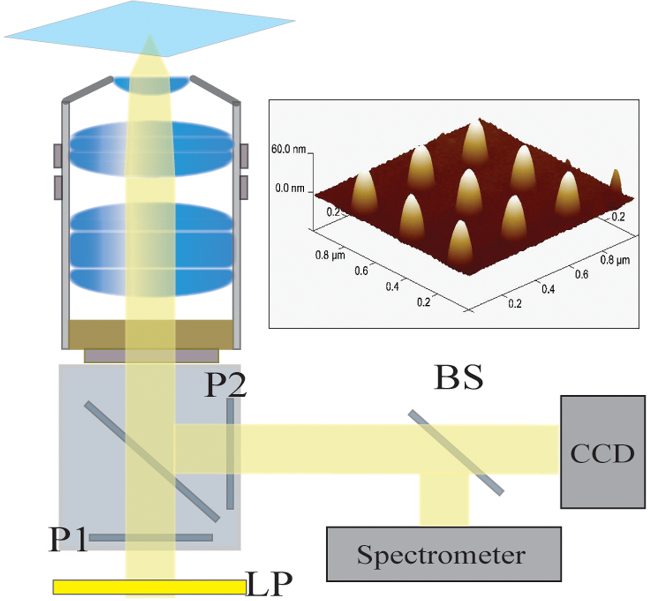
图2.光学设置。来自卤素灯的照明光首先由一个长通滤波器(LP)过滤。的光被线性偏振(P1)和照射经由40X / 1.4 NA物镜的样品。散射光被物镜通过交叉偏振器(P2)的收集和传递。 50/50分束器(BS)被插入到所收集的光路用于同时光谱分析和图像分析。右上:由300纳米的间距隔开9个人纳米结构的原子力显微镜图片请点击这里查看该图的放大版本。
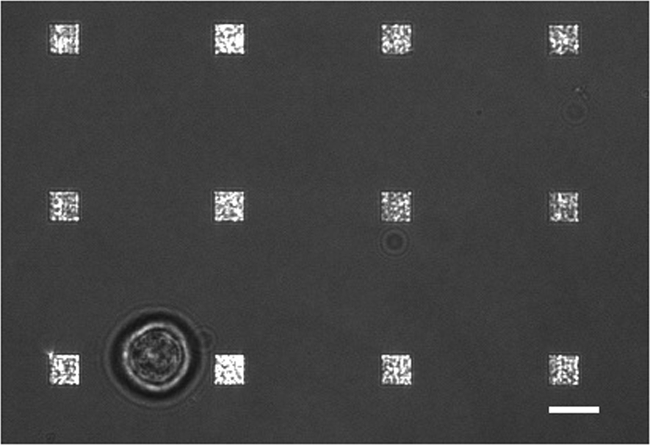
图3.活细胞LSPRi研究。甲合并透射光和LSPRi的图象,显示了12阵列包围的单个杂交瘤细胞(左下)。这是一个对比度增强的图像。比例尺为10微米。 请点击此处查看该图的放大版本。
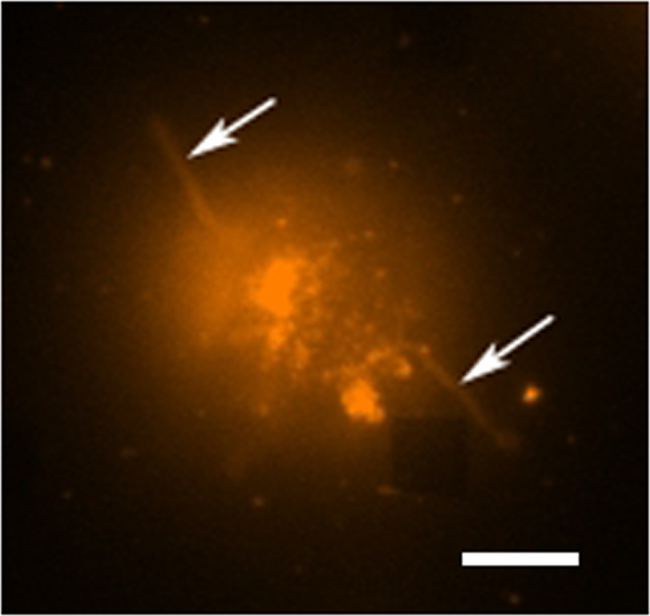
图4.活细胞荧光分析。沾上罗丹明DHPE,这是一种膜染料单个杂交瘤细胞的荧光假彩色图像。在荧光成像模式下的阵列通常不可见的,然而,附近的数组这里可观察到作为从头缺少方在右下角。该细胞可以被看作是从虽然触手状延伸(可能丝状伪足或者伪足)向外从细胞(箭头)延伸的所述阵列分离。比例尺为10微米。 请点击此处查看该图的放大版本。
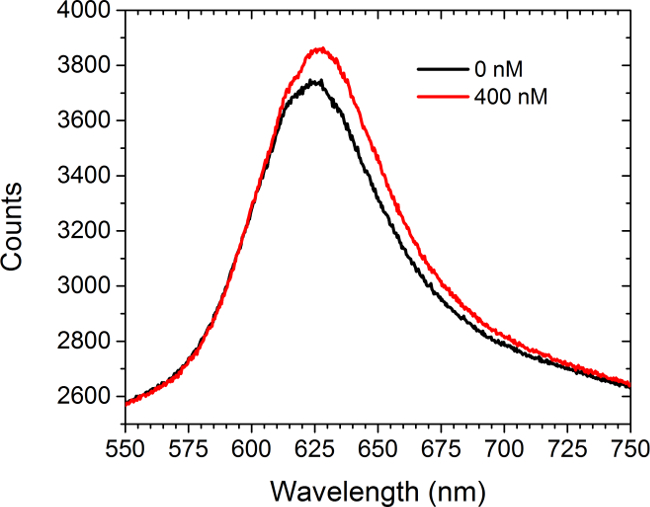
图5.频谱形态。之前和引进抗c-myc基因抗体的400纳米的解决方案后,从c-myc基因功能化的阵列获得的光谱。无细胞存在于这项研究。 请点击此处查看该图的放大版本。

图6.单细胞分泌的影响。位于15μm的单细胞的和一个位于130微米远(控制)的阵列的响应。比例尺为10微米。 请点击此处查看该图的放大版本。
讨论
The LSPR imaging technique described in this work has numerous advantages over more traditional methodologies for detecting cell secretions. First, the time resolution of our technique is on the order of seconds whereas the commercial alternative, an immunosandwhich assay known as EliSpot, has a typical time resolution of 2 to 3 days.7,32 As a result we were able to resolve sudden changes in the rate of protein secretion, such as that shown in Figure 6. Second, having arrays distributed over the chip allows for the secreted signal to be tracked in space and time which enables more rigorous comparisons to diffusion-based models of cell secretion. In addition, arrays like the control array shown in Figure 6 can be used to subtract out global changes in the image that typically arise from instrumental factors such as focus drift. Third, our technique requires no modification of the cells. If desired, the experiment can incorporate commonly used tags such as fluorescent proteins, but if there is concern that such tags may negatively affect cell viability or homeostasis the label-free nature of our approach does not require them. Fourth, using the spectroscopic data we have demonstrated that quantitative information regarding the fractional occupancy of surface bound ligands can be calculated.
There are numerous alternative methods to EBL for fabricating metallic nanoparticles. However, we have found that the EBL provides considerable flexibility for optimizing nanostructure and array dimensions to best suit the optics and the cells under investigation. Also critical is the fact that the chips can be readily regenerated by plasma ashing. In this way, a typical chip can be used dozens of times. Biofunctionalization details must be modified for the specific application. The protocol presented here conjugated the surface with relatively small c-myc peptide ligands. Larger ligands such as whole antibodies typically require more spacing and thus a higher SPO to SPN/SPC ratio. Regardless, a well formed SAM layer is essential for preventing non-specific binding in live-cell experiments. In general, larger molecular weight analytes are more readily detected by LSPR. Thus, in its single-cell manifestation, this technique may not be appropriate for detecting the secretion of small proteins, such as cytokines.
The current setup has been used for studying individual non-adherent cells. There are significant number of secreted signaling proteins and vesicles to which the results reported in this work are directly applicable. For example carcinoembryonic antigen (CEA) which for decades now has been a diagnostic marker for cancer. Colon cancer cells are known to secrete CEA at the rates of thousands of molecules/cell/hr and the molecular weight is 180 kDa which exceeds that of IgG antibodies. CEA is believed to be involved in autocrine and paracrine signaling pathways but the spatio-temporal nature of these secretions have never been measured. Our technique can directly address these signaling questions. An extension of this work will be to measure the spatio-temporal nature of CEA secretion from single cells.33 Future work will also focus on integrating LSPRi with two and three dimensional cell cultures of adherent cells. By incorporating multiplexed arrays capable of detecting a number of secreted proteins in parallel, this technique has the potential to open a new window into cell secretions and how they influence neighboring cells.
披露声明
We thank George Anderson for helpful comments and discussions. This work was supported by the Naval Research Laboratory’s Institute for Nanoscience and the National Research Council Research Associateship Award.
致谢
The authors have nothing to disclose.
材料
| Name | Company | Catalog Number | Comments |
| 25 mm diameter glass coverslips | Bioscience Tools | CSHP-No1.5-25 | 170±5 µm is optimal |
| Poly-methyl methacrylate | Microchem | PMMA 950 A4 | |
| Ethyl lactate methyl metacrylate | Microchem | MMA EL6 | |
| Electron beam evaporator | Temescal | FC-2000 | |
| Electron beam lithography | Raith | Series 150 | |
| Ethanol | Sigma-Aldrich | 459836 | |
| Acetone | Sigma-Aldrich | 320110 | |
| CR-7 chromium etchant | Cyantek | CR-7 | |
| Scanning electron microscope | Zeiss | Ultra 55 | |
| Atomic force microscope | Veeco | Nanoscope III | |
| Plasma ashing system | Technics | Series 85 RIE | |
| SH-(CH2)8-EG3-OH (SPO) | Prochimia | TH 001-m8.n3-0.2 | |
| SH-(CH2)11-EG3-COOH (SPC) | Prochimia | TH 003m11n3-0.1 | |
| SH-(CH2)11-EG3-NH2 (SPN) | Prochimia | TH 002-m11.n3-0.2 | |
| Surface plasmon resonance system | Biorad | XPR36 | |
| Bare gold chip | Biorad | GLC chip | Plasma ashed to remove the monolayer |
| 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide | Thermo | 22980 | |
| N-hydroxysuccinimide (NHS) | Thermo | 24510 | |
| Pentylamine-Biotin | Thermo | 21345 | |
| Ethanolamine | Sigma-Aldrich | E9508 | |
| Neutraavidin | Thermo | 31000 | |
| Phosphate buffered saline | Thermo | 28374 | |
| Tween 20 | Sigma-Aldrich | P2287 | |
| Inverted microscope | Zeiss | Axio Observer | Microscope is equipped with 40X oil immersion objective; CO2 and humidity incubation from Pecon GmbH |
| CCD camera | Hamamatsu | Orca R2 | Thermoelectrically cooled (16 bit) |
| Spectrometer | Ocean Optics | QE65Pro | |
| Spectrasuite | Ocean Optics | version1.4 | |
| c-myc peptide HyNic Tag | Solulink | SP-E003 | |
| monoclonal anti-c-myc antibody | Sigma-Aldrich | M4439 | |
| Hybridoma cell line | ATCC | CRL-1729 | |
| Antibiotic Antimycotic Solution (100×) | Sigma-Aldrich | A5955 | |
| Serum free media RPMI 1640 | Invitrogen | 11835-030 | |
| Fetal bovine serum | ATCC | 30-2020 | |
| Rhodamine DHPE | Life Technologies | L-1392 |
参考文献
- Ludwig, A. -. K., Giebel, B. Exosomes: Small vesicles participating in intercellular communication. The International Journal of Biochemistry & Cell Biology. 44, 11-15 (2012).
- Friedl, P., Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nature Reviews Molecular Cell Biology. 10, 445-457 (2009).
- Letterio, J. J., Roberts, A. B. Regulation of immune responses by TGF-beta. Annual Review of Immunology. 16, 137-161 (1998).
- Werner, S., Grose, R. Regulation of wound healing by growth factors and cytokines. Physiological Reviews. 83, 835-870 (2003).
- Werner, S., Krieg, T., Smola, H. Keratinocyte-fibroblast interactions in wound healing. Journal of Investigative Dermatology. 127, 998-1008 (2007).
- Bailey, R. C., Kwong, G. A., Radu, C. G., Witte, O. N., Heath, J. R. DNA-encoded antibody libraries: A unified platform for multiplexed cell sorting and detection of genes and proteins. Journal of the American Chemical Society. 129, 1959-1967 (2007).
- Gazagne, A., et al. A Fluorospot assay to detect single T lymphocytes simultaneously producing multiple cytokines. Journal of Immunological Methods. 283, 91-98 (2003).
- Han, Q., et al. Polyfunctional responses by human T cells result from sequential release of cytokines. Proceedings of the National Academy of Sciences of the United States of America. 109, 1607-1612 (2012).
- Han, Q., Bradshaw, E. M., Nilsson, B., Hafler, D. A., Love, J. C. Multidimensional analysis of the frequencies and rates of cytokine secretion from single cells by quantitative microengraving. Lab on a Chip. 10, 1391-1400 (2010).
- Ma, C., et al. A clinical microchip for evaluation of single immune cells reveals high functional heterogeneity in phenotypically similar T cells. Nature Medicine. 17, 738-743 (2011).
- Shirasaki, Y., et al. Real-time single-cell imaging of protein secretion. Scientific Reports. 4, (2014).
- Milgram, S., et al. On chip real time monitoring of B-cells hybridoma secretion of immunoglobulin. Biosensors and Bioelectronics. 26, 2728-2732 (2011).
- Abbas, A., Linman, M. J., Cheng, Q. A. New trends in instrumental design for surface plasmon resonance-based biosensors. Biosensors & Bioelectronics. 26, 1815-1824 (2011).
- Ermakova, A., et al. Detection of a Few Metallo-Protein Molecules Using Color Centers in Nanodiamonds. Nano Letters. 13, 3305-3309 (2013).
- Haes, A. J., Van Duyne, R. P. A nanoscale optical blosensor: Sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticles. Journal of the American Chemical Society. 124, 10596-10604 (2002).
- Horowitz, V. R., Aleman, B. J., Christle, D. J., Cleland, A. N., Awschalom, D. D. Electron spin resonance of nitrogen-vacancy centers in optically trapped nanodiamonds. Proceedings of the National Academy of Sciences of the United States of America. 109, 13493-13497 (2012).
- Sepulveda, B., Angelome, P. C., Lechuga, L. M., Liz-Marzan, L. M. LSPR-based nanobiosensors. Nano Today. 4, 244-251 (2009).
- Barbillon, G., et al. Biological and chemical gold nanosensors based on localized surface plasmon resonance. Gold Bulletin. 40, 240-244 (2007).
- Endo, T., et al. Multiple label-free detection of antigen-antibody reaction using localized surface plasmon resonance-based core-shell structured nanoparticle layer nanochip. Analytical Chemistry. 78, 6465-6475 (2006).
- Endo, T., Kerman, K., Nagatani, N., Takamura, Y., Tamiya, E. Label-free detection of peptide nucleic acid-DNA hybridization using localized surface plasmon resonance based optical biosensor. Analytical Chemistry. 77, 6976-6984 (2005).
- Haes, A. J., Hall, W. P., Chang, L., Klein, W. L., Van Duyne, R. P. A localized surface plasmon resonance biosensor: First steps toward an assay for Alzheimer's disease. Nano Letters. 4, 1029-1034 (2004).
- Jonsson, M. P., Jonsson, P., Dahlin, A. B., Hook, F. Supported lipid bilayer formation and lipid-membrane-mediated biorecognition reactions studied with a new nanoplasmonic sensor template. Nano Letters. 7, 3462-3468 (2007).
- Park, J. H., et al. A regeneratable, label-free, localized surface plasmon resonance (LSPR) aptasensor for the detection of ochratoxin A.. Biosensors & Bioelectronics. 59, 321-327 (2014).
- Mayer, K. M., Hao, F., Lee, S., Nordlander, P., Hafner, J. H. A single molecule immunoassay by localized surface plasmon resonance. Nanotechnology. 21, (2010).
- Endo, T., Yamamura, S., Kerman, K., Tamiya, E. Label-free cell-based assay using localized surface plasmon resonance biosensor. Analytica Chimica Acta. 614, 182-189 (2008).
- Huang, Y. X., Cai, D., Chen, P. Micro- and Nanotechnologies for Study of Cell Secretion. Analytical Chemistry. 83, 4393-4406 (2011).
- Oh, B. R., et al. Integrated Nanoplasmonic Sensing for Cellular Functional Immunoanalysis Using Human Blood. ACS Nano. 8, 2667-2676 (2014).
- Raphael, M. P., Christodoulides, J. A., Delehanty, J. B., Long, J. P., Byers, J. M. Quantitative Imaging of Protein Secretions from Single Cells in Real Time. Biophysical Journal. 105, 602-608 (2013).
- Raphael, M. P., et al. A New Methodology for Quantitative LSPR Biosensing and Imaging. Analytical Chemistry. 84, 1367-1373 (2011).
- Raphael, M. P., et al. Quantitative LSPR imaging for biosensing with single nanostructure resolution. Biophysical Journal. 104, 30-36 (2013).
- Raphael, M. P., et al. A new methodology for quantitative LSPR biosensing and imaging. Analytical Chemistry. 84, 1367-1373 (2012).
- Henn, A. D., et al. Modulation of single-cell IgG secretion frequency and rates in human memory B cells by CpG DNA, CD40L, IL-21, and cell division. Journal of Immunology. 183, 3177-3187 (2009).
- Bramswig, K. H., et al. Soluble Carcinoembryonic Antigen Activates Endothelial Cells and Tumor Angiogenesis. Cancer Research. 73, 6584-6596 (2013).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。