Method Article
从人骨髓分离Mesangiogenic祖细胞(多用途储值卡)
摘要
Here we describe an optimized, highly reproducible protocol to isolate Mesodermal Progenitor Cells (MPCs) from human bone marrow (hBM). MPCs were characterized by flow cytometry and nestin expression. They showed the ability to give rise to exponentially growing MSC-like cell cultures while retaining their angiogenic potential.
摘要
In a research study aimed to isolate human bone marrow (hBM)-derived Mesenchymal Stromal Cells (MSCs) for clinical applications, we identified a novel cell population specifically selected for growth in human serum supplemented medium. These cells are characterized by morphological, phenotypic, and molecular features distinct from MSCs and we named them Mesodermal Progenitor Cells (MPCs). MPCs are round, with a thick highly refringent core region; they show strong, trypsin resistant adherence to plastic. Failure to expand MPCs directly revealed that they are slow in cycling. This is as also suggested by Ki-67 negativity. On the other hand, culturing MPCs in standard medium designed for MSC expansion, gave rise to a population of exponentially growing MSC-like cells. Besides showing mesenchymal differentiation capacity MPCs retained angiogenic potential, confirming their multiple lineage progenitor nature. Here we describe an optimized highly reproducible protocol to isolate and characterize hBM-MPCs by flow cytometry (CD73, CD90, CD31, and CD45), nestin expression, and F-actin organization. Protocols for mesengenic and angiogenic differentiation of MPCs are also provided. Here we also suggest a more appropriate nomenclature for these cells, which has been re-named as "Mesangiogenic Progenitor Cells".
引言
间充质基质细胞(MSCs)是其多谱系分化能力和支持造血分泌生长因子/细胞因子,以及在免疫调节1发挥作用的能力,相关的临床应用价值。在基于MSC的疗法电池生产和应用的定义已经广泛的临床和临床前研究2的对象,尤其要注意对细胞的基础医药产品(自学计划)治疗3的安全性和有效性的具体国际规则。人MSC在含有补充剂和动物来源的试剂媒体广泛培养如胎牛血清(FBS)和牛胰蛋白酶。因此,沿着与细胞操作相关的感染风险,患者还面临朊病毒曝光以及链接到蛋白质,肽或动物来源的其它生物分子的免疫学的风险,可能细胞收获和transplan后持续塔季翁4。
为了规避这个问题,我们培养人的骨髓(HBM)的无动物中衍生的干细胞,用混合人AB型血清(PhABS)替换FBS。在这些条件下,沿着生长的MSC我们鉴定了一种新的细胞群。这些细胞在形态和从干细胞表型的不同而表现出独特的基因表达谱以及特性生长/粘合性能。他们既保留和mesengenic血管生成潜力,因此被命名为中胚层祖细胞 (多用途储值卡)5。随后,我们能够限定选择性的和可重复的培养条件以产生纯度6的高品位的MPC。
我们进一步研究的MPC的形态和生物性质。所述的MPC,证实是巢蛋白阳性,慢于自行车,Ki-67的阴性,并用其特征在于长端粒5染色体。他们表示pluripotency相关转录因子Oct-4和Nanog的而不是MSC主调节Runx2的和Sox9的7。表型,多用途储值卡比的MSCs低表达内皮糖蛋白(CD105),而缺乏的间充质标记CD73,CD90,CD166。的MPC还显示其特征在于PECAM(CD31)的一致的表达粘附分子,整联αL(CD11a),αM(CD11b的),αX(CD11c的)以及整联β2(CD18)的一个独特的图案,专门维持podosome状结构8 。在标准MSC扩容媒体,及时多用途储值卡通过一个中间阶段具有Wnt5 /钙调蛋白细胞信号9的激活分化为干细胞。的MPC还保留血管发生性质,可以通过从在鼠细胞外基质(ECM)蛋白的3D培养物的球状体发芽能力所证明。血管生成潜在沿着mesengenic谱系分化MPC后迅速消失。
这里我们介绍的亲母育酚优化以分离和表征来自HBM血样高度纯化的MPC。为MPC mesengenic和血管发生分化再现的协议也有所说明。
研究方案
注:书面同意后,整形外科髋关节置换过程中获得了HBM样品。紧接着股骨颈截骨和股前扩孔含肝素500 UI 20 ml注射器,用于吸新鲜BM。该协议被认为广泛地适用于任何的BM来源。
1.人骨髓单个核细胞的分离(HBM-跨国公司)
- 稀释5 - - 10毫升新鲜的BM至50毫升,施加Dulbecco磷酸缓冲盐溶液(D-PBS),并通过倒置混合。同样在两个新的50ml锥形管中分发25毫升,将25ml D-PBS添加到每个管中,颠倒混合。
- 允许管子放置在室温下10分钟,对矿物骨片段的分离和脂肪从溶液。
- 小心地用无菌巴斯德吸管和过滤细胞悬液通过70微米的过滤器,而不会干扰矿物骨头碎片颗粒除去浮脂肪。
- 设置四五十毫升管15毫升不连续密度梯度离心介质(1.077克/毫升)。确保该介质是在室温下。
- 轻轻地将20 - 密度梯度介质上(25ml)稀释BM的。小心进行此操作,在管壁上,让细胞悬液滴流以防止混合层。
- 在400×g离心在室温下用制动器30分钟禁用进行密度梯度离心。
- 用无菌巴斯德吸管收集位于两个阶段之间的细胞的发白环,并将其转移到一个新的50ml管中。
- 洗用新鲜培养基的细胞:无酚红,低血糖(1000毫克/升)的Dulbecco改良的Eagle培养基(DMEM),10%(体积/体积)混合的人AB型血清(PhABS),2mM的L-谷氨酰胺和抗生素(DMEM / 10%PhABS)。离心在400×g离心5分钟。
- 吸上清,重新暂停5球团 - 10毫升新鲜的DMEM / 10%PhABS。
- 继续细胞计数。确定白血细胞的1的数目:1稀释在锥虫。应用此血细胞计数仪,和一个相差显微镜下观察。排除从细胞计数小和完美圆形的红细胞和蓝染色的死细胞。
注:强烈建议屏幕PhABS批他们在MPC恢复性能。从美国来源PhABS给予最好的结果而不同来源的大多数血清导致与MSC样细胞的比例较高的MPC培养物。
2.从HBM-跨国公司隔离的多用途储值卡
- 设置疏水的T-75烧瓶用15ml新鲜的DMEM / 10%PhABS而让pH和温度由预孵育在37℃下在5%CO 2平衡30分钟。
- 种子4 -每瓶6×10 7 HBM-跨国公司孵育在37℃下在5%CO 2 48小时。
- 吸液和从烧瓶丢弃培养基和非粘附细胞。加入15mL新鲜的DMEM / 10%PhABS并在37°C孵育在5%CO 2。维持培养6 - 8天,更换培养基每48小时。
可选:为了提高MPC收率从2.3非贴壁细胞可被重新铺板在一个新的培养瓶中,并作为主培养所述维持。 - 吸并丢弃烧瓶中,用新鲜的DMEM洗净,加2ml无动物蛋白酶分离解决方案。孵育在37℃,5 - 15分钟(避免长时间孵育)。
- 在400 xg离心加10毫升的新鲜DMEM / 10%PhABS的,吸出细胞悬浮液离心5分钟
- 吸弃上清,并在1重新悬浮颗粒 - 2毫升新鲜的DMEM / 10%PhABS。如步骤1.10的说明操作的细胞计数。
注:请不要使用胰蛋白酶/ EDTA作为分离试剂。多用途储值卡是胰蛋白酶抗性。培养物的形态学筛选,细胞收获前,强烈建议为了评价纺锤形的MSC样细胞的存在。万一相当数量的MSC样细胞是detected,通过选择性地去除污染的细胞增加电池产品的纯度。这样做,胰蛋白酶消化之前可以MPC采收通过加入2ml胰蛋白酶/ EDTA 0.05%2分钟进行,。洗涤培养物两次用5毫升的DMEM / 10%PhABS的,然后进行蛋白酶作为上述处理。
3.细胞表征
- 流式细胞仪
- 设置10 5新鲜分离的细胞的两份样品在洗涤液:D-PBS补充有0.5%(体积/体积)牛血清白蛋白(BSA)和0.02%(重量/体积)叠氮化钠。离心在400×g离心5分钟。
注意:叠氮化钠是有毒的。 - 重悬在200μl的洗涤液颗粒和添加抗CD90,抗CD45,抗CD73和荧光染料("TEST")偶联的抗CD31抗体;并行设置同型对照("CTRL")。
注:染色抗体的数额应滴定或雅高决定鼎制造商的说明。 - 在4℃孵育样品30分钟。
- 离心在400×g离心5分钟。重悬在500微升的洗涤液的细胞,并获得在多色流至少5×10 4个事件仪7 9 10。
- 分析由点图的结果,并使用"CTRL"记录的事件设置象限。
注:为了定义文化为MPC文化CD73 负 负 CD90 + CD45 + CD31细胞的比例应该是95%以上。对于一些特定的应用, 即 ,基因表达分析,回收切断已被提高到97 - 98%。
- 设置10 5新鲜分离的细胞的两份样品在洗涤液:D-PBS补充有0.5%(体积/体积)牛血清白蛋白(BSA)和0.02%(重量/体积)叠氮化钠。离心在400×g离心5分钟。
- 巢蛋白的检测和F-actin的组织分析
- 文化玻片板新鲜分离的MPC(20,000 /厘米2)。使细胞温育过夜,在37℃在5%CO 2附着。
- 洗涤细胞洗涤溶液和音响中的x 4%(重量/体积)低聚甲醛在室温下15分钟。要删除固定液添加清洗液,孵化2分钟,倒入。
- 重复清洗两次。
- 透细胞的D-PBS补充有0.05%(体积/体积)的Triton X-100在室温下15分钟。
- 停止通过蛋白质自由信号增强剂(在RT 30分钟)或标准阻断溶液反应(D-PBS补充有3%(重量/体积)BSA)中。
- 除去信号增强/封闭溶液。
- 加7微克/毫升抗人巢蛋白第一抗体(重量/体积),并在湿润室中于4℃孵育过夜。同时,使用同型对照抗体,以评估不特定的荧光信号。
- 加入D-PBS洗幻灯片,离开2分钟,倒入。重复两次。
- 加入2微克/毫升的荧光染料缀合的次级抗体(重量/体积),并在4℃下孵育在黑暗1小时。
- 作为洗净上面滑动。
- 添加荧光鬼笔环肽(5 UI /毫升),留在室温30米在黑暗中和洗3次中的D-PBS。
- 取出室的墙壁和在补充了为核检测抗淬灭剂和4',6-二脒基-2-苯基吲哚(DAPI)含水安装介质装入滑动。继续成像7 9 10。
4.多用途储值卡的Mesengenic分化
- 板2×10 4个 / cm 2的中TC-处理的T75培养瓶中新鲜分离的MPC和让细胞在DMEM / 10%PhABS在37℃在5%CO 2附着过夜。
- 与标准的降低血清培养基,约200微升/厘米2,专为间充质干细胞扩增(MSC-RS中)更换DMEM / 10%PhABS。生长细胞从感应7至10天汇合(P1-的MSC),通常。刷新介质每2天。
- 吸并丢弃烧瓶中,用新鲜的MSC-RS中洗净,加2ml无动物蛋白酶分离解决方案。在3776℃持续5 - 15分钟(避免长时间的潜伏期)。
- 在400 XG加入10毫升的新鲜的MSC-RS培养基中,吸出细胞悬浮液离心5分钟
- 吸上清,重新暂停颗粒1 - 2毫升的新鲜MSC-RS介质。继续细胞计数如步骤1.10描述
- 5×10 3个细胞/厘米2 -由播种3继续亚文化它们。细胞生长至汇合(P2-MSCS)。
- 收获细胞蛋白酶消化从步骤4.3中所述步骤4.5和第3节中所述进行鉴定。
注:CD73 + CD90 + CD45 负 负 CD31细胞的比例低于95%,将表明只有部分分化的,需要进一步的培养传代。 - 板P2间充质干以2×10 4 /厘米2在六个孔TC处理板和长到MSC-RS中汇合。
- 马克两口井为"不存在",并刷新MSC-RS网上平台。
- 马克·两所参考译文]"骨",并用200微升/标准成骨介质厘米2,专为MSC分化设计取代网上平台。
- 马克两口井为"Adipo",并用200微升/标准成脂培养基厘米2,专为MSC分化设计取代网上平台。
- 通过改变整个媒体每48小时维持在5%CO 2在37℃下培养。
注:强烈建议使用标准的商用区分媒体测试可重复性。区分条件下2/3周后,钙沉积出现在成骨诱导培养,而细胞内脂滴蓄积在脂肪细胞诱导细胞明显。 - 吸液和丢弃培养基然后用D-PBS洗。
- 通过加入4%1毫升(重量/体积)低聚甲醛在室温下15分钟固定的培养物。
- 要删除固定液添加D-PBS,孵育2分钟后倒。重复洗涤两次。
- 染色一"不存在"连同两个"骨"标志着羟基磷灰石特异性荧光解决方案和一个"无差异"井连同两个"Adipo"标志着200纳米尼罗红液井。孵育在室温下11,12 30分钟。
- 删除染色解决方案,并在D-PBS洗两次。
- 除去的D-PBS中,添加D-PBS补充有50%(体积/体积)甘油和进行成像7 9 10。
5. MPC球体发芽试验
- 为了产生三维球体,打下培养皿盖的内表面上的新鲜分离的MPC悬浮液(1.5×10 4个细胞/滴)的20微升液滴。
注:由于搬运下降可能导致其破裂,这是非常值得推荐的奠定他们过剩。 - 小心使用盖子回顾一下含有D-PBS以防止悬滴蒸发培养皿。在5%CO 2在37℃下过夜,使细胞在3D球体聚集。
- 通过在预冷冻的24孔培养板中加入标准ECM蛋白的300微升等份设定的鼠细胞外基质(ECM)蛋白的厚凝胶,并在37℃下孵育30分钟。
- 小心翻倒培养皿的盖子,轻轻地拿起用无菌巴斯德吸管的球体。
- 躺在球粒上的ECM蛋白凝胶中,添加标准丰富的VEGF-内皮细胞生长培养基的700微升等分试样并在5%CO 2在37℃下孵育。
- 24小时后,在培养7天后,采取在放大4倍电源3D文化的照片。评价从通过测量最后侵入细胞和球体边缘之间的径向距离应用图像分析软件球体发芽。沿至少20个不同的方向上重复的措施。平均距离时50微米或以上视为阳性。
结果
这里所描述的选择性培养条件已经允许的新的粘附和几乎单形细胞群体的分离作为HBM-跨国公司的1.0%(0.5 - 2.0×10 5 6 HBM-跨国公司- 10毫升新鲜骨髓样品)5,6- 。我们发现这些大(40 -直径为60微米),圆形,静止的,Ki-67的阴性细胞的MPC 5。形态学上,它们与由一个平薄周边示出大量的丝状伪足的在更高的放大率(白色箭头在图1中A.1)包围的厚芯区域特征在于独特的煎鸡蛋形。外小区边界的极性伸长经常观察到( 图1中A.1黑色箭头)。这样的形态是标准的MSC文化报道典型的梭形间充质基质细胞的外观明显不同。流式细胞仪检测新鲜分离的MPC的95%以上表达CD31和CD45,而Xesenchymal相关标志物CD90和CD73 13人不到( 图1 A.2)。我们认为这是受限制的组的四个抗原所指示的多用途储值卡。的MPC的进一步区别特征是虚线F-肌动蛋白分布揭示了一些podosome状结构的(红色图1 A.3)和巢蛋白的强烈表达(绿色图1 A.3),其未在细胞中检测到(数据未显示)与同种型对照抗体染色。
在标准的RS培养的MPC媒体设计用于快速分化MSC扩张成果转化为指数增长MSC样细胞( 图1 B.1)。两次传代细胞后,终于切换其从CD73 负 负 CD90 + CD45 + CD31 CD73到CD90 + CD45 + CD31 NEG NEG( 图1 B.2)表型。在这个过程中,的MPC重新ORGAnize F-肌动蛋白成应力纤维,而巢蛋白表达变得仅限于少数稀有细胞( 图1 B.3)。 MPC mesengenic分化成一个明确的MSC样表型发生通过不同的细胞形态显示两个不同的步骤。一周的MSC的RS介质MPC样细胞的残留人口后仍(在图2所述的 P1-的MSC)平的,多边形的多支化的细胞的汇合层内可检测的。进一步的通道才能获得( 图2中 P2-MSCS)纺锤形MSC样细胞几乎单态文化。当转移到选择性培养基中,至少2周,从而证实它们的MSC性质这些指数生长的细胞可以容易地分化为成骨细胞或脂肪细胞。在成骨诱导培养,钙沉积可以通过比色茜素-S染色或特定的荧光染料(绿色如图2 B)进行检测。后成脂诱导细胞显示脂滴的积累由两种油色红或荧光尼罗红染色(红图2 B)透露。
MPC打字是由发芽血管生成实验证实。多用途储值卡显示,他们对24小时后血管内皮生长因子刺激( 图2 C)从三维球体入侵(50微米)的小鼠细胞外基质蛋白胶的能力。 600微米的距离 - 在后300检测1周侵入细胞。相反,入侵容量mesengenic分化( 图2 D)后失去了在P2-干细胞。
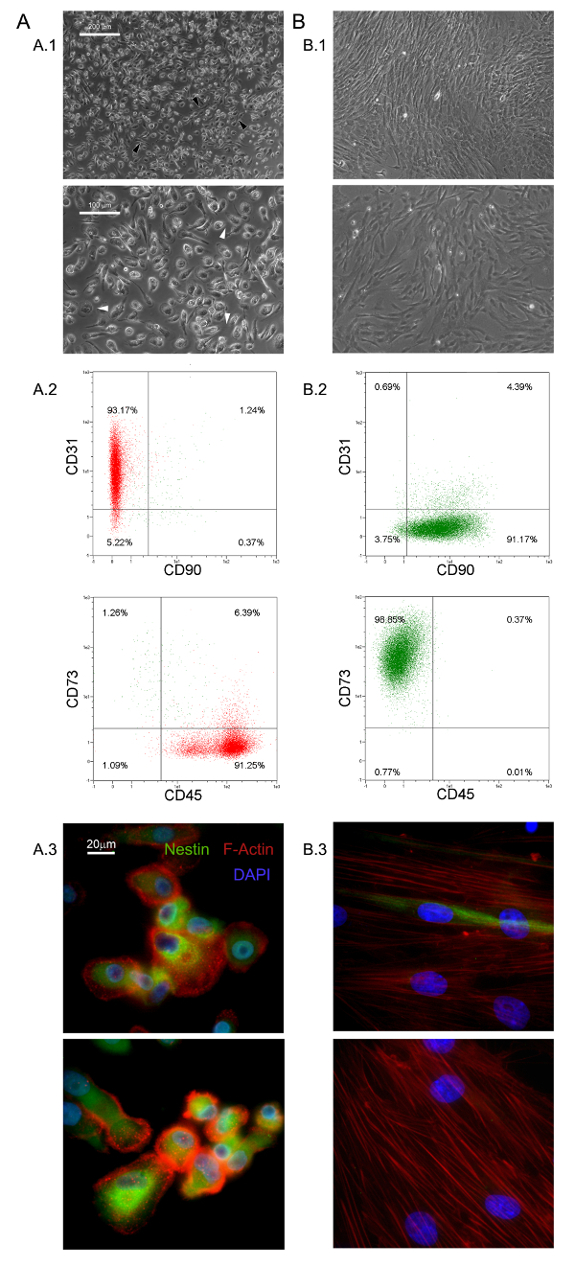
图1. 新鲜分离的MPC具有鲜明的特色:在DMEM / 10%PhABS培养HBM-跨国公司七天引起静态的MPC(A)从干细胞很容易分辨的群体(B) 的形态学方面(A.1,B.1,比例尺= 100微米),表型(A.2,B.2),F-肌动蛋白分布(红A.3中,B.3)和巢蛋白的表达(绿色A.3,B.3,比例尺= 20微米)。 请点击此处查看该图的放大版本。
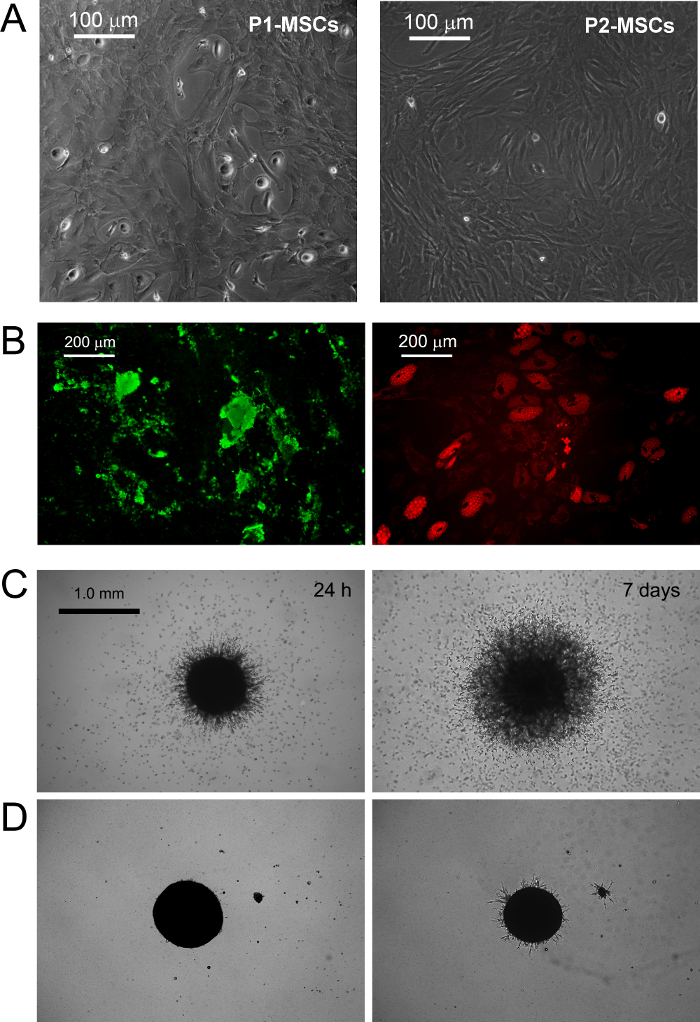
图2 的MPC 分化成中等专为MSC扩张市售RS DMEM / PhABS 体外 。更换标准MSC和显示发芽血管生成触发的MPC的mesengenic诱导。在培养一周后一些残余的MPC仍检测到(P1-的MSC),而在MSC-RS介质进一步通路导致人口汇合的MSC样细胞(P2-的MSC 中,A比例尺= 100微米)。 P2间充质干末端分别分化成下适当的刺激骨细胞或脂肪细胞钙沉积(绿色B)和脂滴的积累(红B,比例尺= 200微米)所揭示。多用途储值卡显示在与P2-干细胞(D,比例尺= 1.0毫米)的区别在小鼠ECM蛋白质凝胶(C)球体一致的萌芽。 请点击此处查看该图的放大版本。
讨论
In the last decades, MSCs have been extensively researched and pre-clinically evaluated for possible application in the treatment of various bone/articular, immunological, neurological, cardiovascular, gastrointestinal and hematological disorders14,15. The easy and inexpensive isolation of multipotent MSCs, from many different tissues, together with their lack of significant immunogenicity16, contribute to make these cells one of the most interesting cell population to be applied in cell based therapies. Nonetheless, the very low frequency in the tissue of origin represents a great limitation to the MSCs application in clinics, forcing the expansion of these cells, in vitro, before the infusion or transplantation.
Expanded MSC cultures have revealed high grades of heterogeneity and variability17-19 making it difficult to reach a consensus about MSC production and characterization protocols. Moreover, recent investigations suggested the presence of multiple in vivo MSC ancestors in a wide range of tissues, which contribute to culture heterogeneity10,20. In fact, it has been proposed that particular culture conditions possibly select or simply promote specific sub-populations of MSCs progenitors present, in various percentages, in "crude" and unprocessed samples like bone marrow (hBM-MNCs) or adipose tissues (stromal vascular fraction)2. Thus, the variability in MSC-initiating cell populations together with the great number of different enrichment/isolation and culture protocols applied, represent a great obstacle to the definition of feasible MSC-based therapies.
A crucial factor affecting heterogeneity of MSC cultures is serum supplementation21. In our hands replacement of FBS with PhABS in primary cultures from hBM-MNCs, combined with high density seeding on hydrophobic plastics, led to the isolation of a novel highly adherent cell population with distinct biological features named MPCs5,6. We observed that the addition of small percentages of PhABS to FBS primary cultures also allowed MPC isolation, suggesting the presence of MPC inducing agents in the human serum6. At the moment, the MPC isolation/characterization protocol is a unique method available to obtain almost pure MPCs. The protocol has been carefully adjusted and it is highly reproducible for quality screening of MPC preparations before further applications.
MPCs could be used as a source for MSC production, thus limiting the variability introduced by use of unfractionated starting material. The precise definition of the multiple steps characterizing MPC mesengenic differentiation reported9 would allow synchronized mesenchymal cell expansion. Nonetheless, this latest condition could be realized exclusively applying highly purified MPC population, as a consequence the characterization of the cell products obtained by the protocol described here, results of crucial importance. This isolating method has been reported allowing MPC recovery with purity generally around 95%. However, donor/patient variability together with the variability related to the different batches of human pooled serum applied, could lead to a significant percentage of MSC-like cells co-isolated together with MPCs, under selective conditions.
It is not clear if these "contaminating" MSC-like cells could arise from the other different in vivo progenitors described in bone marrow22 or from uncontrolled and spontaneous MPC differentiation. In any case, a consistent percentage of MSC-like cells in the MPC products nullify the possibility to applying these cells as homogeneous starting material for the MSC expansion. Thus, here it has been suggested a simple and inexpensive method, based on the MPC resistance to trypsin digestion, increasing the purity of the MPC products. Similar or even better results in purifying MPC cultures could be achieved by fluorescent or magnetic cell sorting performing CD73 and/or CD90 depletion, but significantly prolonging the process time and increasing the costs.
Moreover, MPCs showed expression of pluripotency-associated markers and Nestin, all rapidly lost during mesengenic differentiation7. Sprouting assay revealed MPC ability to invade murine ECM protein gel. Taken together these results indicate that MPCs have to be considered a more immature progenitor, retaining angiogenic potential. Nonetheless, the initial enthusiasm about mesodermal differentiation potential of MPCs is actually waning. In fact, after more than 7 years of studies on MPCs, mesengenic and angiogenic potential have been extensively described5-9, but differentiation toward any other cells of mesodermal origin is still lacking. Thus, here we propose a new, and more rigorous, definition of these cells as "Mesangiogenic Progenitor Cells", maintaining the acronym MPCs.
We also believe that most controversies about MSC angiogenic potential could be related to the heterogeneous composition of expanded cultures consisting of sub-populations of MPCs and MSCs in variable percentages23.
Finally, MPCs could also play a crucial role for the implementation of CBMPs applicable for tissue reconstruction, as these cells could also support the neo-vascularization. In fact, future studies on regeneration should take in consideration that the newly formed tissue growth should be supported by concomitant neo-angiogenesis. The co-existence of mesengenic and angiogenic potential in MPCs could significantly improve the regeneration potential of new therapeutic approaches that involve these interesting cells.
披露声明
The authors have no competing financial interests or other conflicts of interest.
致谢
作者在特别感谢保罗Parchi博士的外科,医学和分子病理学和危重病急救医学,比萨大学,提供骨髓样本和他的人骨祖细胞的专长
材料
| Name | Company | Catalog Number | Comments |
| Matrigel Basement Membrane Matrix | BD Bioscience (San Jose, CA-USA) | 354230 | Murine ECM proteins Stock Concentration: 100% (9 - 12 mg/ml) Final Concentration: 100% |
| Dulbecco's Phosphate-Buffered Saline (D-PBS) | Sigma (St. Louis, MO, USA) | D8537 | |
| 70 μm Filters | Miltenyi Biotec (BergischGladbach, Germany) | 130-095-823 | |
| Ficoll-Paque PREMIUM | GE Healthcare (Uppsala, Sweden) | 17-5442-03 | medium for discontinuos density gradient centrifugation |
| Pooled human AB type serum (PhABS) | LONZA (Walkersville MD-USA) | 14-490E | Final Concentration: 10% |
| Glutamax-I | ThermoFisher (Waltham, MA USA) | 35050-038 | Stabilized L-Glutamine Stock Concentration: 100x Final Concentration: 2 mM |
| Bovine Serum Albumin (BSA) | Sigma (St. Louis, MO, USA) | A8412 | Stock Concentration: 7.5% Final Concentration: 0.5% |
| Sodium Azide | Sigma (St. Louis, MO, USA) | S8032 | Final Concentration: 0.02% |
| Penicillin/Streptomycin (Pen Strep) | Gibco (Grand Island, NY, USA) | 15070-063 | Antibiotics Stock Concentration: 5,000 UI/ml penicillin, 5,000 μg/ml Streptomycin Final Concentration: 50 UI/ml penicillin, 50 μg/ml Streptomycin |
| T-75 culture flask for suspension cultures | Greiner Bio-one (Frickenhausen, Germany) | 658 190 | |
| T-75 culture flask TC treated | Greiner Bio-one (Frickenhausen, Germany) | 658170 | |
| TrypLE Select | ThermoFisher (Waltham, MA USA) | 12563-011 | Animal-free proteases detaching solution Stock Concentration: 1x Final Concentration: 1x |
| Trypsin/EDTA | ThermoFisher (Waltham, MA USA) | 15400-054 | Phenol red free Stock Concentration: 0.5% Final Concentration: 0.25% |
| anti-CD90 APC antibody (CD90) | MiltenyiBiotec (BergischGladbach, Germany) | 130-095-402 | Final Concentration: 1:40 |
| anti-CD45 APC-Vio770 antibody (CD45) | MiltenyiBiotec (BergischGladbach, Germany) | 130-096-609 | Final Concentration: 1:40 |
| anti-CD73 PE antibody (CD73) | MiltenyiBiotec (BergischGladbach, Germany) | 130-095-182 | Final Concentration: 1:40 |
| anti-CD31 PE Vio-770 antibody (CD31) | MiltenyiBiotec (BergischGladbach, Germany) | 130-105-260 | Final Concentration: 1:40 |
| Mouse IgG1 APC antibody | MiltenyiBiotec (BergischGladbach, Germany) | 130-098-846 | Final Concentration: 1:40 |
| Mouse IgG2a APC Vio770 antibody | MiltenyiBiotec (BergischGladbach, Germany) | 130-096-637 | Final Concentration: 1:40 |
| Mouse IgG1 PE antibody | MiltenyiBiotec (BergischGladbach, Germany) | 130-098-845 | Final Concentration: 1:40 |
| Mouse IgG1 PE Vio-770 antibody | MiltenyiBiotec (BergischGladbach, Germany) | 130-098-563 | Final Concentration: 1:40 |
| Low Glucose Dulbecco's Modified Eagle Medium (DMEM) | ThermoFisher (Waltham, MA USA) | 13-1331-82 | Phenol red-free minimal essential medium Stock Concentration: 1,000 mg/L glucose |
| Fetal Bovine Serum (FBS) | ThermoFisher (Waltham, MA USA) | 10500 | Stock Concentration:0.2 mg/ml Final Concentration: 2 μg/ml |
| Prolong Gold antifade reagent with 4’,6-diamidino-2-phenylindole | Invitrogen (Waltham, MA, USA) | P-36931 | Aqueous mounting medium + DAPI Final Concentration: 1x |
| Paraformaldehyde | Sigma (St. Louis, MO, USA) | P6148 | Fixative Final Concentration: 4% |
| LAB-TEK two-well chamber slides | Sigma (St. Louis, MO, USA) | C6682 | |
| Anti-Nestin antibody [clone 10C2] | Abcam (Cambridge, UK) | ab2035 | Stock Concentration: 1 mg/ml Final Concentration: 7 μg/ml |
| Alexa Fluor 555 Phalloidin | ThermoFisher (Waltham, MA USA) | A34055 | Stock Concentration: 200 UI/ml Final Concentration: 5 UI/ml |
| Triton X-100 | Euroclone (Milan, Italy) | EMR237500 | Final Concentration: 0.05% |
| MesenPRO RS Medium (MSC-RS medium) | ThermoFisher (Waltham, MA USA) | 12746-012 | |
| Alexa Fluor 488 anti-mouse SFX kit | ThermoFisher (Waltham, MA USA) | A31619 | Goat anti-mouse secondary antibody + Signal enhancer Stock Concentration: 2 mg/ml Final Concentration: 2 μg/ml |
| Pasteur Pipette | Kartell Labware (Noviglio (MI), ITALY ) | 329 | |
| StemMACS AdipoDiff Media | MiltenyiBiotec (BergischGladbach, Germany) | 130-091-679 | |
| StemMACS OsteoDiff Media | MiltenyiBiotec (BergischGladbach, Germany) | 130-091-678 | |
| Osteoimage Bone mineralization Assay | LONZA (Walkersville MD-USA) | PA-1503 | Hydroxyapatite specific fluorescent staining solution |
| 50 ml Polystyrene conical tube | Greiner bio-one (Kremsmünster Austria) | 227261 | |
| Nile Red | ThermoFisher (Waltham, MA USA) | N1142 | Fluorescent staining solution for lipids Stock Concentration: 100 mM Final Concentration: 200 Nm |
| Glycerin | Sigma (St. Louis, MO, USA) | G2289 | Final Concentration: 50% |
| Polistirene Petri dishes | Sigma (St. Louis, MO, USA) | P5606 | |
| 24-well plates TC-treated | Greiner Bio-one GmbH (Frickenhausen, Germany) | 662160 | |
| Endothelial Growth Medium, EGM-2 BulletKit (EGM-2) | LONZA (Walkersville MD-USA) | CC-3162 | VEGF-rich endothelial cell growth medium |
| Leica Qwin Image Analisys Software | Leica (Wetzlar, Germany) | Image analysis software |
参考文献
- Stoltz, J. F., et al. Stem Cells and Regenerative Medicine: Myth or Reality of the 21th Century. Stem Cells Int. 2015, 734731 (2015).
- Pacini, S. Deterministic and stochastic approaches in the clinical application of mesenchymal stromal cells (MSCs). Front Cell Dev Biol. 2, 50 (2014).
- Galvez, P., Clares, B., Hmadcha, A., Ruiz, A., Soria, B. Development of a cell-based medicinal product: regulatory structures in the European Union. Br Med Bull. 105, 85-105 (2013).
- Herberts, C. A., Kwa, M. S., Hermsen, H. P. Risk factors in the development of stem cell therapy. J Transl Med. 9, 29 (2011).
- Petrini, M., et al. Identification and purification of mesodermal progenitor cells from human adult bone marrow. Stem Cells Dev. 18 (6), 857-866 (2009).
- Trombi, L., et al. Selective culture of mesodermal progenitor cells. Stem Cells Dev. 18 (8), 1227-1234 (2009).
- Pacini, S., et al. Constitutive expression of pluripotency-associated genes in mesodermal progenitor cells (MPCs). PLoS One. 5 (3), 9861 (2010).
- Pacini, S., et al. Specific integrin expression is associated with podosome-like structures on mesodermal progenitor cells. Stem Cells Dev. 22 (12), 1830-1838 (2013).
- Fazzi, R., et al. Mesodermal progenitor cells (MPCs) differentiate into mesenchymal stromal cells (MSCs) by activation of Wnt5/calmodulin signalling pathway. PLoS One. 6 (9), 25600 (2011).
- Tormin, A., et al. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 117 (19), 5067-5077 (2011).
- Greenspan, P., Mayer, E. P., Fowler, S. D. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 100 (3), 965-973 (1985).
- Wang, Y. H., Liu, Y., Maye, P., Rowe, D. W. Examination of mineralized nodule formation in living osteoblastic cultures using fluorescent dyes. Biotechnol Prog. 22 (6), 1697-1701 (2006).
- Horwitz, E. M., et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 7 (5), 393-395 (2005).
- Wang, S., Qu, X., Zhao, R. C. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 5, 19 (2012).
- Si, Y. L., Zhao, Y. L., Hao, H. J., Fu, X. B., Han, W. D. MSCs: Biological characteristics, clinical applications and their outstanding concerns. Ageing Res Rev. 10 (1), 93-103 (2011).
- Le Blanc, K., Tammik, C., Rosendahl, K., Zetterberg, E., Ringden, O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 31 (10), 890-896 (2003).
- Phinney, D. G. Biochemical heterogeneity of mesenchymal stem cell populations: clues to their therapeutic efficacy. Cell Cycle. 6 (23), 2884-2889 (2007).
- Phinney, D. G. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem. 113 (9), 2806-2812 (2012).
- Tolar, J., Le Blanc, K., Keating, A., Blazar, B. R. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 28 (8), 1446-1455 (2010).
- Corselli, M., et al. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 21 (8), 1299-1308 (2012).
- Bieback, K., et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 27 (9), 2331-2341 (2009).
- Watson, L., Elliman, S. J., Coleman, C. M. From isolation to implantation: a concise review of mesenchymal stem cell therapy in bone fracture repair. Stem Cell Res Ther. 5 (2), 51 (2014).
- Pacini, S., Petrini, I. Are MSCs angiogenic cells? New insights on human nestin-positive bone marrow-derived multipotent cells. Front Cell Dev Biol. 2, 20 (2014).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。