需要订阅 JoVE 才能查看此. 登录或开始免费试用。
Method Article
吞噬体偏移和速度与HIV-1感染的活小学的人巨噬细胞测量
摘要
We describe a method to measure the velocity of phagosomes moving towards the cell center in living cells infected with or without the human immunodeficiency virus (HIV) type 1, using spinning disk confocal fluorescence microscopy to identify fluorescent infected cells and bright field microscopy to detect phagosomes.
摘要
Macrophages are phagocytic cells that play a major role at the crossroads between innate and specific immunity. They can be infected by the human immunodeficiency virus (HIV)-1 and because of their resistance to its cytopathic effects they can be considered to be persistent viral reservoirs. In addition, HIV-infected macrophages exhibit defective functions that contribute to the development of opportunistic diseases.
The exact mechanism by which HIV-1 impairs the phagocytic response of macrophages was unknown. We had previously shown that the uptake of various particulate material by macrophages was inhibited when they were infected with HIV-1. This inhibition was only partial and phagosomes did form within HIV-infected macrophages. Therefore, we focused on analyzing the fate of these phagosomes. Phagosome maturation is accompanied by migration of these compartments towards the cell center, where they fuse with lysosomes, generating phagolysosomes, responsible for degradation of the ingested material. We used IgG-opsonized Sheep Red Blood Cells as a target for phagocytosis. To measure the speed of centripetal movement of phagosomes in individual HIV-infected macrophages, we used a combination of bright field and fluorescence confocal microscopy. We established a method to calculate the distance of phagosomes towards the nucleus, and then to calculate the velocity of the phagosomes. HIV-infected cells were identified thanks to a GFP-expressing virus, but the method is applicable to non-infected cells or any type of infection or treatment.
引言
Macrophages play a major role in the innate immune system and in homeostasis. They are professional phagocytes that internalize and eliminate pathogens and debris by a process called phagocytosis 1,2. The phagosome, the closed compartment that forms after the engulfment of particulate material, undergoes a series of fusion and fission events with endocytic compartments, leading to a degradative compartment called the phagolysosome. This compartment has an acidic pH, due to the acquisition of proton-pumping v-ATPases, contains hydrolytic enzymes and is enriched in lysosomal-associated membrane proteins (LAMPs). The maturation of phagosomes is accompanied by their migration on microtubules 3,4 towards the cell center to reach a perinuclear location where lysosomes are accumulated.
Many pathogens have been reported to hijack phagosome maturation, including bacteria with intracellular lifestyles that modify the vacuolar environment where they reside 5. The Human Immunodeficiency Virus (HIV)-1 targets macrophages in addition to T cells. As macrophages are resistant to the cytopathic effects of the virus, unlike T cells, they can be considered as a reservoir for the virus. In addition, macrophages infected with HIV-1 show defective phagocytic functions and contribute to the emergence of opportunistic diseases. In particular, severe invasive non-typhoidal Salmonella disease caused by Salmonella Typhimurium ST313 has been prevalent for the last three decades in sub-Saharan African children or adults infected with HIV 6. It has been estimated that the risk of developing tuberculosis is more than 20 times greater in people living with HIV than among those without HIV infection.
For all these reasons, it is important to better define the molecular mechanisms underlying the phagocytic defects in HIV-infected macrophages. We have shown that the uptake of particulate material, opsonized particles, bacteria or fungi, was inhibited in HIV-infected macrophages 7. Given that this inhibition is partial, we then set out to analyze the fate of the internalized phagosomes in HIV infected human macrophages 8. Because phagosome maturation is tightly connected with migration to the cell center and fusion with lysosomes, a defect in phagosomal maturation can be due to modifications of the trafficking modalities in the infected cell. The method described here uses IgG-opsonized Sheep Red Blood Cells (IgG-SRBCs) as a model to target receptor-mediated phagocytosis and in particular receptors for the Fc portion of immunoglobulins (FcR). These particles are easier to image in bright field (BF) than latex beads because extracellular and intracellular SRBCs show different refraction properties 9. To measure the velocity of phagosomes moving towards the nucleus in HIV-infected macrophages, we used a fluorescent virus 10 and set up a simple manual tracking method that is described here. The method does not require advanced programming and simply uses ImageJ. It is amenable to adherent cells and any type of particle or pathogen that can be visualized with bright field microscopy or with fluorescent imaging.
研究方案
该协议在严格按照国家和国际法规和地方法规来进行。从给他们的同意献血用于研究目的的健康捐献者的血液已经从与该机构签署协议,输血中心获得。特殊的保护必须用人类血液时服用。 HIV-1的实验必须在生物安全3级或2(BSL-3或2)实验室根据当地法规进行。
1.人单核细胞 - 巨噬细胞(hMDMs)通过密度梯度离心与选择附着力的制备
- 开始与新鲜血液来自健康供体(9毫升)中。稀释的新鲜血液,用无菌1×磷酸盐缓冲盐水(PBS)的整个体积不含Ca 2+和Mg 2+,得到70 ml的终体积,并轻轻稀释血液加入到两个50毫升锥形管中(35毫升,每管)上15毫升的顶eutral,高度支化的,高的质量,亲水性多糖在溶液已经在每个管中。
- 离心机无论是在537 XG 20分钟血管在20°C不带刹车。然后收集包含在界面处的混浊细胞环的外周血单核细胞(PBMC),并将其转移到含有15毫升1×PBS中的不含Ca 2+和Mg 2+新50ml管中。
- 离心机在20℃下将细胞在218×g离心5分钟,悬浮在45ml 1×PBS中的沉淀不含Ca 2+和Mg 2+。
- 离心将细胞在20℃218×g离心5分钟,悬浮在10ml 1×PBS中的沉淀不含Ca 2+和Mg 2+,以及通过稀释到1/200在锥虫蓝最终稀释计数细胞。
- 离心将细胞在218×g离心5分钟,在20℃重悬在RPMI(罗斯韦尔园区纪念研究所)粒料1640培养基补充了2mM L-谷氨酰胺和100微克/毫升penicillin -链霉素具有每孔7×10 6个 PBMC中的2ml培养基中在6孔板的各孔中。
- 用5%的CO 2孵育所述板在37℃下2小时。 2小时后单核细胞将已附着于塑料。
- 以允许新鲜分离的单核细胞分化成hMDMs,吸出培养基并用补充用2ml HMDM培养基(RPMI 1640,10%decomplemented胎牛血清(FCS),2mM的L-谷氨酰胺和1%青霉素 - 链霉素)替换它重组人巨噬细胞集落刺激因子(RHM-CSF)在10纳克/毫升的最终浓度。
- 孵育细胞在37℃,5%CO 2的11天( 图1)。
- 第11天除去培养基并用2ml每孔冷HMDM培养基洗2次。接着用1毫升冷1X PBS的清洗每孔2次。
- 要分离完全分化的hMDMs,每孔1次洗1毫升冷PBS 1X与2毫米乙二胺四乙酸(EDTA)中,孵育细胞2毫升冷1×PBS中的每孔2毫摩尔EDTA,在4℃下15-60分钟。
注:替代方法来分离细胞存在( 如胰蛋白酶或胰蛋白酶样的活动)可能得到好的结果11。 - 支队(轻轻吹打向上和向下在很好地完成)后,收集细胞,并把它们放在50毫升管含10毫升冷HMDM中的冰。
- 离心将细胞在20℃218×g离心5分钟,重悬沉淀在10ml冷HMDM培养基中,通过在台盼蓝稀释1/20计数细胞。
- 种子的细胞以每35毫米显微镜级玻璃底培养皿1×10 6 hMDMs并在37℃用5%CO 2孵育平板1天。
HIV-1病毒种群2.生产和定量
注:NLR4.3 HIV-1的Gag iGFP(绿色荧光蛋白)携带R5嗜信封,礼品从M. Schindl器10被用于感染的巨噬细胞,并实时看到感染细胞。
- 通过人胚肾293T细胞(2×10 6个在100mm皿),使用市售的转染试剂中的相应原病毒DNA的6微克的转染产生的病毒的股票。
- 使用指示细胞的HeLa TZM-BL使用后跟细胞及数量的β-半乳糖苷着色病毒种群的连续稀释液(带有β-半乳糖苷基因的HIV-1 LTR控制下)量化病毒种群的感染性蓝小区12。
3.与HIV-1 hMDMs感染
- 添加病毒以感染复数(MOI)的多个0.02-0.03于1ml HMDM介质与hMDMs(镀在1.13细胞)。对照孔添加只需1毫升HMDM培养基中,并在37℃,5%CO 2的2天( 图1)孵育碗碟。
- 在第2天洗细胞与HMDM MEDIUM 3次,并添加每个培养皿新鲜HMDM介质1毫升。孵育细胞在37℃,5%CO 2的6天( 图1)。
4.绵羊红细胞的调理作用
- 对于每盘7×10 6 SRBCs制备,洗SRBCs两次在100μl的1×PBS中的离心分离含有0.1%牛血清白蛋白(BSA)溶液在600 xg离心4分钟。
- 悬浮洗涤SRBCs于500μl1×PBS / BSA 0.1%用兔IgG抗SRBCs以每5微升SRBCs的子凝集的浓度,并在室温与旋转温育30分钟。
注:确定抗SRBCs IgG的子凝集的浓度,(13.1毫克/毫升的库存浓度)从1/50于20μl在96孔板制备的IgG连续稀释至1 / 25,600。加2×10 6 SRBCs在20μl每孔中,并把在黑暗的房间的板几个小时期间。子凝集的浓度的之前的井用凝集抗体(IgG + SRBCs形成网络)的孔的稀释度。 - 旋转后,离心的IgG调理的-SRBCs在600×g离心4分钟,并在600 xg离心用100μl的1×PBS / BSA 0.1%洗用离心4分钟。
- 重悬的IgG调理的-SRBCs在补充有2mM L-谷氨酰胺和1%青霉素 - 链霉素(1毫升/皿)预热无酚红的RPMI培养基中。
5.活细胞显微视频分析吞噬
- 使用共焦成像系统,例如在37℃下装有加热室用CO 2穿过瓶用水加湿纺丝磁盘系统。
- 之前打开所述加热室向实验吞噬测定开始前有显微镜阶段在37℃。打开显微镜和计算机,并加载图像处理软件。
- 优化成像设置诸如扫描速度,尊离子,分辨率等具有每场和图像的一帧60至120分钟之间的每一分钟的至少一个细胞。
注意:在此,将样品60分钟以63X透镜期间成像的每分钟一帧。 - 放置在显微镜舞台成像菜。调整焦距和位置查找只是一个整体的HIV-1在现场感染巨噬细胞。使用基于所使用的成像系统和探头适当的激发/发射设置。包括明视场(BF)通道观察吞噬小体( 图1II)。通过调整透射光和曝光时间的百分比优化不同信道的图像的外观。
注:在这里,NLR4.3 HIV-1的Gag iGFP很兴奋使用491纳米的激光与激光的20%( 图1I),曝光时间为50毫秒。 - 取出成像盘中,在7×10 6 SRBCs / ml至菜( 图1)中添加1毫升SRBC悬浮液。
- 离心500 XG的在RT 2分钟以同步的吞噬能力,记录时间在该离心分离的端部和所述盘返回到阶段。
- 优化的Z堆叠聚焦和捕获的GFP和BF图像(整个细胞的厚度为0.3微米的步距 - 通常是20面)每分钟至少1小时。节省时间推移的视频中所使用的成像系统的本机文件格式。
注:在这里,定时短片被保存在本机格式,* .STK文件。
6.定时短片的分析
- 对于视频编辑,点击下拉菜单中选择"应用程序",并在视频编辑软件标签"审查多维数据"。
- 要打开该文件,点击"选择基本文件",然后在"选择目录"。在"数据集"中,选择采集分析(以.nd格式),点击"查看"。的数据被表示在一个表中的列的时间ðZ-计划行。
- 代表感染的Z-投影( 如图2所示 ,左图),在"波长"框中选择491纳米波长和单击带有"所有平面"的"Z投影"选项卡。
- 分析时间序列,在"波长"框选择高炉波长,并选择在Z轴的最佳平面区分外部SRBCs( 图2,红色箭头),内部SRBCs( 图2,红色圆圈)和细胞核( 图2,蓝色圆圈)。
- 保存视频蒙太奇,单击"选择[X]的"选项卡,然后点击"加载图像(S)"。最后,保存。TIF格式加载的图像和明年打开他们ImageJ的软件。
- 使用上的ImageJ软件插件"手动跟踪"来测量核在高炉通道中观察到的不同吞噬体( 图3)的位置和。
- 道琼斯n载入的ImageJ的网站上的"手动跟踪"插件。上的ImageJ,打开插件和图像序列进行分析( 图3,步骤1和2)。
- 输入诸如"时间间隔"表示的相邻帧之间的时间量,而"x / y的校准",这表示每像素的距离( 图3,步骤3)的设置。
注:在这里,保存图像序列与每一帧和0.205微米X / Y校正之间1分钟的时间间隔,因为63X变焦和6.45点¯x6.45微米2的像素大小相机使用。 - 要开始跟踪,点击"添加轨道"( 图3,第4步),当它是内在的第一次点击一个SRBC中心。在下一帧自动出现。
- 继续点击在所有帧的SRBC中心有在时间不同位置( 图3,步骤6中的红色框)。
- 开始跟踪核通过点击其中心有它在所有帧中的位置。下一页(点击其中心),他们当时的内化(架),在SRBCs功能不同逐个跟踪吞噬体。为方便起见,以查看高炉通道SRBC,跟踪( 图3,步骤5)中使用的"亮度和对比度"的窗口。
- 每个SRBC跟踪之间,点击"添加轨道"( 图3,步骤4)有一个新的轨道。跟踪的数量将在第二列被改变,结果表中的"跟踪N°"( 图3,步骤6)。
- 保存在电子表格中的数据,以继续分析的下一个步骤。
- 使用电子表格软件来计算包含对核和吞噬体的SRBCs的内化后的第5分钟时的速度SRBCs吞噬体的行驶距离( 连接古尔4)。
- 打开电子表格表和一个新的电子表格文件。转移到这个新的文件只有以下参数,时间,x和细胞核和所有SRBCs( 图4A)的y轴坐标。
- 计算SRBCs和细胞核之间的距离,只有它们的坐标,考虑核和SRBC在XY坐标平面( 图4B)。
注:两点之间的距离是连接它们的路径的长度。在飞机上,SRBC和细胞核之间的距离由勾股定理给出。- 如果c(细胞核和SRBC之间的距离)表示斜边和一个的长度和b表示其他两个边的长度,表达毕达哥拉斯定理如毕达哥拉斯方程:

注:同时,在正交的水平距离一为(x 核 -x SRBC)和垂直距离b是(γ 核 -y SRBC)。 - 因此,计算出核和SRBC( 图4A,紫色盒)通过之间的距离:
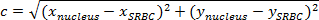
注:乘以所获得的距离值(以像素为单位)由X / Y校准具有在微米的距离。在这里,X / Y校正是0.205微米。 - 在每个时间,减去细胞核与SRBC的初始距离( 图4C)之间的距离。
- 绘制对时间的测量的前5分钟。应用线性趋势线( 图5A)的情节,并确定线性趋势线表示SRBC内化后的第5分钟期间向核吞噬体速度的斜率。
- 整理的数据来计算的平均值和统计误差使用适当的软件( 图5B)的速度值,并绘制它们以适当的形式。
- 如果c(细胞核和SRBC之间的距离)表示斜边和一个的长度和b表示其他两个边的长度,表达毕达哥拉斯定理如毕达哥拉斯方程:
结果
由HIV-1感染和非感染hMDMs的FcR介导的吞噬作用,这里描述了使用的IgG调理的SRBCs作为模型目标( 图1)。此协议的关键步骤是hMDMs编制和感染HIV-1。事实上,差异化的巨噬细胞的产量和质量捐助者之间的差异,以及与在10-40%的范围内效率感染率。此外,IgG的调理-SRBCs的制备也很重要,以避免损坏红细胞,因为这可能导致它们的识别和摄取作为碎片,而不是通过的...
讨论
这种技术有几个关键步骤。首先,hMDMs的制备及其感染HIV-1是至关重要的,因为感染的比例捐助依赖。值得注意的是,我们决定使用未在体外感染前极化巨噬细胞,因为由体内病毒可能遇到的巨噬细胞的状态没有得到很好的迄今表征。我们检查了几种表面标记的表达,表明巨噬细胞既不M1也不M2和已验证的CD4和CCR5的表达。感染率是可变的,从10%至40%,有时低的范围内。这个变量感染...
披露声明
The authors have nothing to disclose.
致谢
We thank Dr Jamil Jubrail for reading the manuscript. This work was supported by grants from CNRS, Inserm, Université Paris Descartes, Agence Nationale de la Recherche (2011 BSV3 025 02), Fondation pour la Recherche Médicale (FRM DEQ20130326518 including a doctoral fellowship for GLB) and Agence Nationale de Recherche sur le SIDA et les hépatites virales (ANRS, including a post-doctoral fellowship for CD) to FN. A. Dumas was supported by doctoral fellowships from Université Paris Descartes and Sidaction.
材料
| Name | Company | Catalog Number | Comments |
| Falcon 100mm TC-Treated Cell Culture Dish | Corning | 353003 | For viral production |
| Glass Bottom Dishes 35 mm uncoated 1.5 | MatTek corporation | P35G-1.5-14-C Case | For acquisition |
| Falcon Tissue Culture Plates 6-well | Thermo Fischer Scientific | Corning. Inc. 353934 | For human monocyte-derived macrophages |
| Ficoll-Plaque PLUS | Dominique Dutscher | 17-1440-03 | a neutral, highly branched, high-mass, hydrophilic polysaccharide in solution for density centrifugation |
| DPBS, no calcium, no magnesium | Thermo Fischer Scientific | 14190-094 | Room temperature |
| Dulbecco's Modified Eagle Medium (DMEM) 1X, liquid (High Glucose) | GIBCO, Molecular probes | 31966-021 | Conserved at 4°C ; for HEK cells culture |
| RPMI 1640 medium GLUTAMAX Supplement | Life technologies | 61870-010 | Conserved at 4°C; for hMDMs culture |
| Fœtal Calf Serum (FCS) | Eurobio | CVFSVF0001 | Conserved at -20°C ; decomplemented |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Fischer Scientific | 15140-122 | Conserved at -20°C ; for hMDMs culture |
| RPMI 1640 medium, no phenol red (10x500 ml) | Life technologies | 11835-105 | Warm in 37°C water bath before use ; for phagocytosis assay |
| FuGENE6 Transfection Reagent | Promega | E2692 | Conserved at 4°C ; for viral production |
| Sheep red blood cells (SRBCs) | Eurobio | DSGMTN00-0Q | Conserved in Alsever buffer at 4°C before use |
| Anti-sheep red blood cells IgG | MP Biomedicals | 55806 | Conserved at 4°C |
| Bovine Serum Albumin heat shock fraction, pH 7, ≥98% | Sigma | A7906 | Conserved at -20°C |
| Inverted microscope DMI600 | Leica | ||
| Confocal Spinning Disk Unit CSU-X1M1 | Yokogawa | ||
| 491 nm 50mW laser | COBOLT CALYPSO | ||
| HCX PL APO CS Objectif | Leica | Objective lens ; Magnification 100x ; Numerical aperture 1.40 ; Immersion oil | |
| CoolSnap HQ2 (FireWire) Camera | Photometrics | Pixel size 6.45 x 6.45 µm ; Definition 1392 x 1040 ; Encoding the image in 14 Bit | |
| Metamorph 7.7.5 software | Molecular Devices | For the control of the microscope | |
| GraphPad Prism software | For the statistics analysis |
参考文献
- Flannagan, R. S., Jaumouille, V., Grinstein, S. The cell biology of phagocytosis. Annu. Rev. Pathol. 7, 61-98 (2012).
- Niedergang, F. . Encyclopedia of Cell Biology. 2, 751-757 (2016).
- Blocker, A., Griffiths, G., Olivo, J. C., Hyman, A. A., Severin, F. F. A role for microtubule dynamics in phagosome movement. J Cell Sci. 111 (Pt 3), 303-312 (1998).
- Blocker, A., et al. Molecular requirements for bi-directional movement of phagosomes along microtubules. J Cell Biol. 137, 113-129 (1997).
- Flannagan, R. S., Cosio, G., Grinstein, S. Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol. 7, 355-366 (2009).
- Feasey, N. A., Dougan, G., Kingsley, R. A., Heyderman, R. S., Gordon, M. A. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 379, 2489-2499 (2012).
- Mazzolini, J., et al. Inhibition of phagocytosis in HIV-1-infected macrophages relies on Nef-dependent alteration of focal delivery of recycling compartments. Blood. 115, 4226-4236 (2010).
- Dumas, A., et al. The HIV-1 protein Vpr impairs phagosome maturation by controlling microtubule-dependent trafficking. J Cell Biol. 211, 359-372 (2015).
- Greenberg, S., el Khoury, J., Kaplan, E., Silverstein, S. C. A fluorescence technique to distinguish attached from ingested erythrocytes and zymosan particles in phagocytosing macrophages. J. Immunol. Methods. 139, 115-122 (1991).
- Koppensteiner, H., Banning, C., Schneider, C., Hohenberg, H., Schindler, M. Macrophage internal HIV-1 is protected from neutralizing antibodies. J Virol. 86, 2826-2836 (2012).
- Gartner, S. The macrophage and HIV: basic concepts and methodologies. Methods Mol Biol. 1087, 207-220 (2014).
- Wei, X., et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 46, 1896-1905 (2002).
- Harrison, R. E., Bucci, C., Vieira, O. V., Schroer, T. A., Grinstein, S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol Cell Biol. 23, 6494-6506 (2003).
- Toyohara, A., Inaba, K. Transport of phagosomes in mouse peritoneal macrophages. J Cell Sci. 94 (Pt 1), 143-153 (1989).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。