Method Article
细胞迁移研究在垂直化学和氧梯度聚二甲基硅氧烷的聚碳酸酯微流体装置
摘要
The control of chemical and oxygen gradients is essential for cell cultures. This paper reports a polydimethylsiloxane-polycarbonate (PDMS-PC) microfluidic device capable of reliably generating combinations of chemical and oxygen gradients for cell migration studies, which can be practically utilized in biological labs without sophisticated instrumentation.
摘要
本文报道具有嵌入式聚碳酸酯(PC)薄膜研究根据化学和氧梯度的组合细胞迁移制成聚二甲基硅氧烷(PDMS)的微流体装置。化学和氧梯度能极大地影响体内细胞迁移;然而,由于技术限制,很少研究已经进行,调查它们的体外作用。在此研究开发的设备需要一系列蛇形的通道,以产生所希望的化学梯度的优势,并利用氧梯度产生在空间上限于化学反应方法。化学和氧梯度的方向是彼此垂直的,以使直接的迁移的结果的解释。为了有效地生成具有最小的化学品消耗的氧梯度,嵌入式PC薄膜被用作气体扩散阻挡层。所开发的微流体装置可以通过注射泵被致动,并在细胞迁移实验放入常规的细胞培养器,以允许设置简化和优化的细胞培养条件。在细胞实验中,我们使用的设备来研究adenocarcinomic人肺泡基底上皮细胞,A549细胞迁移,下的趋化因子的组合(基质细胞衍生因子,SDF-1α)和氧梯度。实验结果表明,该装置能够稳定地产生垂直的趋化因子和氧梯度和与细胞相容。迁移研究结果表明,氧梯度可能在引导细胞迁移下梯度的组合不能从那些在单个梯度预测了至关重要的作用,以及细胞的行为。该设备为研究人员提供研究在细胞培养化学和氧梯度之间的相互作用一个强大而实用的工具,它可以促进更多的体内样microenvi更好的细胞迁移的研究ronments。
引言
的可溶性因子和氧气张力的空间分布可以在体内 1,2,3,4调节一些重要的细胞功能。为了更好地研究其对细胞的影响,体外细胞培养物能够稳定地产生化学和氧梯度平台是高度期望的。各种可溶性因子的生物活性起着关键的作用,影响细胞行为。最近,由于微流体技术的进步,许多能够稳定地产生化学梯度的微流体装置已被开发,研究细胞迁移5。此外,一些研究也揭示了氧分压的体外细胞培养物6,7,8的必要性。然而,氧张力的用于细胞培养的控制主要依赖于直接化学添加用于与加压气瓶的除氧或细胞孵化。直接化学除了改变细胞培养基,影响细胞应答。氧气控制孵化需要特殊培养箱设计,精确的气体流量控制,并以实现低氧条件大量氮气。此外,它是不可行的控制使用此设置,这阻碍了在各种氧张力和渐变的细胞行为研究氧气的空间分布。为了克服这些限制,一些微流体装置已被开发,以产生用于细胞培养应用9氧气梯度。然而,其中大部分是使用加压气体,这会引起蒸发和气泡产生的问题进行操作。因此,它们经常需要复杂的仪器和可能不可靠的长期细胞培养studie秒。
为了克服的挑战,并进一步研究化学和氧梯度之间的相互作用对细胞迁移,我们开发了具有嵌入式聚碳酸酯(PC)薄膜10制成聚二甲基硅氧烷(PDMS)的微流体细胞培养设备。该装置是由通过一个PDMS膜分离的两个微流体通道的层。顶层为氧梯度产生一个PDMS-PC层;底部层是由PDMS制成用于化学梯度的产生和细胞培养的。该设备可以同时产生垂直化学和氧梯度,而不使用气瓶和精密的流量控制系统。在该装置中,PDMS提供了极大的光学透明性,透气性,以及用于细胞培养和成像生物兼容性。嵌入式PC薄膜用作有效的氧张力控制的气体扩散阻挡层。在微流体通道中,我们使用一系列蛇形通道的s到产生化学梯度。设计已经被广泛地利用以产生用于各种用途的微流体装置化学梯度由于其可靠性和容易的实验装置。此外,化学梯度分布可以通过数值模拟事先改变通道的几何形状进行设计。氧梯度产生,我们采取了先前在我们实验室10,11,12开发的空间上的限制化学反应的方法的优点。氧气可以从指定区域无氮气吹扫被清理。用于生物实验室实际使用中,整个实验装置与传统的细胞培养孵化器兼容。通过整合这些方法,我们可以同时产生无散装气瓶和复杂仪器稳定的化学和氧梯度,以便研究细胞迁移。
研究方案
1.微流体装置的制造
注:整个微流体装置是使用软光刻复制品成型方法13制造。
- 在微流体装置模具的PDMS层的制造
- 设计使用市售的插图或绘图软件的微流体通道模式。该文件提交给一个公司的高分辨率的透明度光罩打印14。
- 清洁硅片用丙酮(≥99.5%),异丙醇(IPA;≥99.9%),和缓冲氧化物蚀刻(BOE; 6:1的NH 4 F.HF)。以去离子水冲洗和脱水用氮气枪晶圆。
- 旋涂近似20克负光刻胶的SU-8 2050上以500rpm进行15秒的晶片,然后2,000rpm下持续30秒。
注:旋涂的条件应该产生SU-8 2050的照片抗蚀剂层用光学光刻后的厚度在晶片上约75微米。 - 软烘烤在65℃下加热板的晶片为3分钟,然后在95℃下9分钟。在烘软后,用暴露下紫外线的透明设计口罩掩模对准晶圆;总曝光能量应为约300毫焦/厘米2。后曝光烘烤(PEB),在65℃下将晶片2分钟,然后在95℃下7分钟。
- 该PEB后,浸泡在SU-8显影剂将晶片在强力搅拌或在超声浴中7分钟(37千赫和为180W有效超声波功率)。冲洗,用丙酮和IPA晶片以去除残留的SU-8。
- 放置晶片和100μl的硅烷(1H,1H,2H,2H- perfluorooctyltrichlorosilane)插入,以防止不希望的粘合带隔膜真空泵晶片表面硅烷化连接干燥器一个直径6cm培养皿。打开泵15分钟,将其关闭和密封干燥器以30分钟的真空。
- 就拿硅烷化晶圆出干燥器,并将它们粘在直径为15厘米的培养皿以下软光刻工艺:
- 制造和硅橡胶层组装
- 与PDMS预聚合物的制备
- 混合的PDMS单体(基峰)和固化剂以10:1的比例(体积/体积)。德加干燥器设置为60分钟的混合物。
- 在PC-嵌入式顶层的制造
- 传送2克PDMS预聚合物到与顶层流体通道图案的模具做出的PDMS薄层。在干燥器设置在模具放置到脱气PDMS 60分钟。
- 将模具过夜在60℃的烘箱中的PDMS固化。确保该模具是在水平平面上。
- 冷却模具到室温。倾额外13克PDMS预聚合物到模具和脱气的PDMS在第Ë干燥器设置为60分钟。
- 慢慢嵌入1毫米厚的PC薄膜入新鲜的PDMS层作为气体扩散阻挡;如果需要,驱逐任何气泡。
- 把模具一夜之间在60℃烘箱中,并确保它是在一个水平面上。
- 冷却固化的PDMS至室温。切割用手术刀装置至大约5.5×5厘米2的面积,其可覆盖所有信道的图案,并剥离从模具中的PDMS厚板。
- 冲床使用直径毫米-2-活检穿孔的入口和出口孔。存储制造顶级PDMS-PC层从环境灰尘后装配了。
- 底部PDMS层的制备
- 倾11克与PDMS预聚合物到模具用于底层。在干燥器的模具放置到脱气PDMS 60分钟。保持模具过夜,60℃的烘箱治愈PDMS。请确保它是躺在一个水平面上。
- 冷静下来日ËPDMS至室温。切割设备的大约5.5×5厘米2的面积,其可覆盖所有信道的模式,并把它剥开的模具。存储制造底部PDMS层从环境灰尘后装配了。
- 在PDMS膜的制备
- 旋涂近似4克的PDMS预聚物上以100rpm进行90秒的硅烷空白晶片,然后3,000rpm下4秒。烘烤在60℃下的旋涂晶片烘箱中过夜。
- 该装置的组装
- 放置所制造的PDMS-PC顶层和与旋涂PDMS膜的晶片在O 2等离子体的表面处理机器与键合表面朝上。对待90 PDMS表面W 0 2血浆40秒。
- 在O 2等离子表面处理之后债券顶层到PDMS膜。广场上的粘合层之上的重量(约600克),把它们放在一个60℃烘箱中过夜,以促进粘合。
- 冷却粘合的层,以室温,并粘合到顶层用手术刀膜切成约5.5×5厘米2的面积,其可覆盖所有信道的模式。剥离从硅晶片键合结构和冲头在使用毫米直径的2活检穿孔的化学梯度通道的入口和出口孔。
- 放置膜粘合顶层和所制造的PDMS底层中的O 2的等离子体的表面处理机器,具有粘结表面朝上,在90瓦为40秒,以激活利用等离子体与PDMS表面。
- 的顶层和底层连接在一起的表面处理后,立即粘合。放在整个保税设备顶部的重量(约600克),把它放在一个60℃的烘箱中过夜。
- 取整个制造设备出炉并冷却至室温。
- 与PDMS预聚合物的制备
2.微流控细胞迁移实验
注:在本文中,我们使用了常用的细胞系,adenocarcinomic人肺泡基底上皮细胞(A549)和趋化因子,基质细胞衍生因子(SDF-1α),作为例子。有关其他细胞和趋化因子的研究人员,请调整相应的实验过程。
- 0天:细胞制备
- 培养含有DMEM F12 L-谷氨酰胺替代品,10%(体积/体积)胎牛血清(FBS)和1%(体积/体积)antimicrob - 抗霉菌溶液的A549细胞,在完全生长介质的库存。亚文化与0.25%胰蛋白酶EDTA解离。
- 在140 xg离心离心分离细胞,在室温下3分钟准备用于实验的细胞悬浮液。用于微流体装置的实验中,使用血球计数细胞和种子至少1×10 6个细胞在T75烧瓶用10ml完全生长培养基。
注意:所有的细胞培养过程在培养箱与37℃和5%CO 2的气氛中进行。
- 第1天:设备准备
- 将设备插入O 2等离子机,并在90 W和O 2等离子对待它40秒呈现微流体通道表面亲水性。
- 在表面处理后,立即通过直接插入针头插入冲压成在化学梯度通道入口与PDMS层的直径为2毫米的孔安装两个地下14钝针。确保针头不阻止微流体通道。使用1毫升注射器用钝器地下14针画0.8毫升的DDH 2 O的。注入的DDH 2 O到从出口化学梯度通道,直到水从入口两个针流出。
- 通过稀释纤连蛋白的库存用Dulbecco&#制备将1ml 50微克/毫升纤连蛋白溶液39氏磷酸盐缓冲盐水(DPBS)。
- 注入从用1ml注射器的化学梯度通道的出口处的纤连蛋白溶液用钝地下14针直到溶液从入口两针流出。
- 孵育在常规细胞培养孵化整个设备过夜。
- 第2天:细胞接种和显微成像
- 细胞种植
- 吸从分别因为0培养一天的细胞的培养基,并用5毫升DPBS 2倍洗细胞。吸出DPBS,加2ml胰蛋白酶-EDTA,并孵育在孵化的细胞5分钟以从烧瓶表面脱离的细胞。
- 等到细胞被分离并悬浮于该溶液中,加入8毫升的无血清培养基(DMEM F12 + 1%(体积/体积)antimicrob - 抗真菌剂溶液)的烧瓶中。传送所有的液体的入15ml锥形管中,并在140 xg离心离心它用于在室温下5分钟。
- 吸出上清液离心后,并添加无血清培养基的适量使最终细胞密度1×10 6个细胞/ ml。取出第1天准备的微流体装置计数使用血球或其他自动细胞计数仪细胞。
- 使用1ml注射器的钝地下14针的化学梯度信道的出口处注射的无血清培养基,直到介质从入口两针流出。从化学梯度信道的出口注入200微升细胞悬浮液。
- 观察设备中的细胞培养容器在显微镜下,以确认该细胞已被引入到微流体通道。放置设备插入湿润容器( 例如,一个塑料盒与DDH 2 O的内侧),并保持它在细胞培养孵化5小时,以促进到设备上表面上的细胞的粘附。
- REAG的制备经济需求测试化学梯度产生
- 在无血清培养基中的所需浓度(100毫微克/毫升)制备的化学(SDF-1α)。使用和不使用化学成连接到高纯度的油管两个单独3毫升注射器吸取无血清培养基。设置注射器上与1微升/分钟的流速供以后使用注射器泵。
- 试剂氧气梯度产生的制备
- 使将15ml的1M NaOH溶液和15毫升200毫克/毫升连苯三酚溶液。绘制NaOH和连苯三酚溶液加入到分别连接到高纯度的PTFE管和高纯度的油管,两个单独15毫升注射器。设置注射器上带有的供以后使用5微升/分钟流速注射泵。
- 微流控设备安装
- 5小时培养后,将整个设备并把它放在一个直径约15厘米的培养皿。修正了使用粘性腻子培养皿设备。转移培养皿在培养箱一个活细胞成像的显微镜。
- 管从注射器的化学梯度产生的化学梯度信道的入口连接。的出口连接到管道通向废物储存器。打开与注射器化学梯度产生的注射器泵。
- 连接从含有NaOH和焦棓酚的氧梯度信道的入口的注射器的高纯度的油管。连接油管出口收集垃圾。打开与注射器氧梯度产生的注射器泵。
- 加约15ml的DDH 2 O至培养皿以保持湿润器件。设立时间已过图像采集的活细胞成像显微镜,并拍摄图像,每15分钟。
- 细胞种植
- 第3天:数据收集和分析
- 所捕获的图像文件传送到计算机。使用开放源代码图像的分析图像alysis软件,ImageJ的,有一个开源插件手动跟踪(https://imagej.nih.gov/ij/plugins/track/track.html)和一个免费的工具趋化软件(http://ibidi.com/xtproducts / EN /软件与图像分析/手动图像分析/趋化和迁移工具)15,16。
3.梯度表征
注:化学和氧梯度之前或在细胞实验后来表征。
- 化学梯度的数值模拟
- 估计利用计算流体动力学(CFD)模拟微流体的层流性质。
- 氧梯度实验表征
- 在浓度为5制备的氧敏感荧光染料,三(2,2'-联吡啶)钌(III),氯化六水合物,在水溶液毫克/毫升。
- 绘制氧气敏感的染料溶液成连接到高纯度的油管两个单独3毫升注射器。建立在以1微升/分(等同于用于化学梯度产生的流速)的流速注射泵的注射器。
- 管道从注射器到化学梯度通道的入口连接。的出口连接到管道通向废物储存器。打开与注射器化学梯度产生的注射器泵。
- 使用倒置荧光显微镜与通过一个470±20纳米的光学滤波器的激发光测量荧光强度。通过使用一个很酷的CCD相机515纳米长通发射滤光片收集发射光。
- 连接从含有NaOH和焦棓酚的氧梯度信道的入口的注射器的高纯度的油管。连接油管出口收集垃圾。打开与注射器用于氧气的注射器泵梯度产生。
- 收集在细胞培养容器中流动的氧敏感染料的荧光图像。
- 停氧梯度产生的流量和断开所有油管氧代通道。从气瓶连接高纯度油管向氧含量梯度信道的出口。
- 收集流过空气,纯氮气和纯氧气时插入连接到氧含量梯度通道管中流动的细胞培养室中的氧敏感染料的荧光图像。
- 通过根据参考文献10和11使用Stern-Volmer方程分析荧光图像估计氧梯度。
结果
制成的PDMS-PC混合微流体细胞培养设备。图。图1示出一个相片和微流体装置的示意图。底层包含四个水平蛇形通道,以产生来自六个不同的混合比两个分离的入口引入的试剂溶液。理论上,这六个不同的混合比分别为1:0,4:1,3:2,2:3,1:4和0:1(左:右)从入口引入的两种溶液之间。由六个不同的混合比的解决方案构成的化学梯度所用的细胞培养容器中产生,位于下游侧。顶部和底部层通过PDMS膜分离。在顶层中,对于除氧化学反应试剂被引入到从两个不同入口的微流体通道。将试剂立即在细胞培养容器的顶部流向前彼此对反应混合清除从底部通道中的氧,不直接的化学接触。嵌入式PC膜,用较小的气体扩散系数相比的PDMS,充当扩散屏障,使氧清除更有效。氧气通过在下游区域中的PDMS逐渐扩散回细胞培养容器,以形成沿流动方向的氧梯度。由于除氧化学反应空间上的限制,只有当地的氧张力受到影响。其结果是,该设备可以在常规的细胞培养器可以利用,而不改变它的全球氧气张力。在迁移实验中,细胞被观察的细胞培养容器内接种。生长培养基和化学试剂引入到使用注射泵以精确地控制流率的设备。
化学和氧梯度表征设备内部产生的。由于给T他的层流微流体性质,流动行为可以利用计算流体动力学(CFD)模拟预测。在本文中,我们构建的3D模型,并使用市售的多物理建模软件进行仿真。 图。图2(a)示出了穿过根据荧光强度测量和数值模拟的结果的细胞培养室的宽度实验表征荧光素浓度分布之间的比较。实验和模拟结果之间的协议表明CFD模型能很好地估计所述装置内部产生的化学梯度。 图。图2(b)绘制在细胞培养室中产生的模拟SDF-1α梯度。 图。图3示出由小区实验之前流过的细胞培养容器内的氧敏感荧光染料的氧梯度表征结果。结果表明,氧gradi耳鼻喉科,范围从约1至16%,可使用上述协议来建立。
细胞迁移的结果。作为示范,我们下的趋化因子的4种组合(SDF-1α)和氧梯度进行A549细胞迁移实验:(1)无趋化因子和无氧气梯度作为对照,(2)与趋化因子梯度和没有氧梯度, (3)与氧梯度和不具有趋化因子梯度,和(4)与两个趋化因子和氧梯度。 图。图4示出了整个实验装置的照片。该实验在与整个设置(包括微流体装置,注射泵,和活细胞成像显微镜)置于其内它的常规细胞培养培养箱中的所有执行。细胞迁移的结果示于图5。 图。图5(a)示出了使用活细胞成像ANA实验期间收集的图像仪,而图图5(b)和(c)绘出下通过与插件的ImageJ的软件分析的四种组合的细胞迁移轨迹和平均运动。结果表明,在对照的平均细胞迁移距离接近零,这表明在实验的细胞的随机运动。与此相反,仅与趋化因子梯度,细胞的平均运动是向左,其中所述的SDF-1α浓度较高。结果表明A549细胞,先前已经报道的SDF-1α趋化行为。在只有氧梯度实验中,细胞的平均运动是向上,其中氧张力较低。更有趣的是,在与垂直的趋化因子和氧梯度实验中,细胞的平均运动是向上和没有在水平方向上的任何明显的移动(趋化因子梯度方向)。
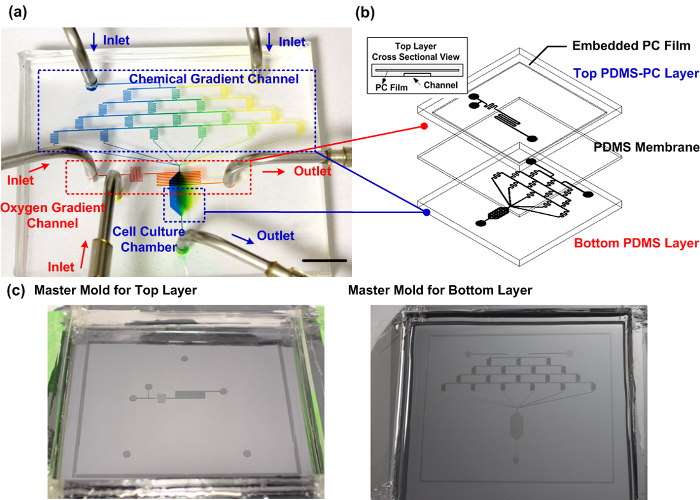
图1:装配式的PDMS-PC微流体细胞培养设备。 (a)能可靠地产生用于细胞迁移研究垂直化学和氧梯度所制造的设备的实验照片。化学梯度通道填充有蓝色和黄色食用色素以证明细胞培养室内部的梯度产生。氧梯度通道填充有红色食用染料。比例尺是1厘米。 (b)该微流体装置的示意图。顶端层使用的PDMS与嵌入式PC层作为用于细胞培养容器内有效的氧梯度控制的气体扩散阻挡制造。 ( 三 )主模具的顶层和底层的制造。 请点击此处查看该图的放大版本。
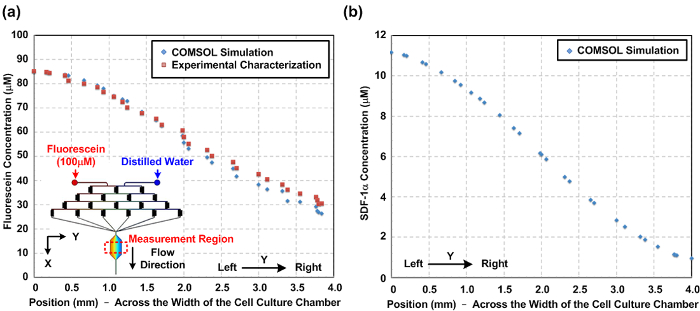
图2: 微流体细胞培养设备内化学梯度。 ( 一 )从数值模拟以及整个细胞培养容器(Y方向)的宽度实验表征细胞培养室内部的荧光素浓度梯度。模拟和实验测量梯度之间的相似性指示该模拟可以很好地预测的化学梯度。该图的插图示出了用于模拟构造的三维(3D)模型。 ( 二 )在整个细胞迁移的研究在细胞培养室的宽度的SDF-1α趋化因子梯度的数值模拟的结果。 请点击这里查看更大的版本这个数字。
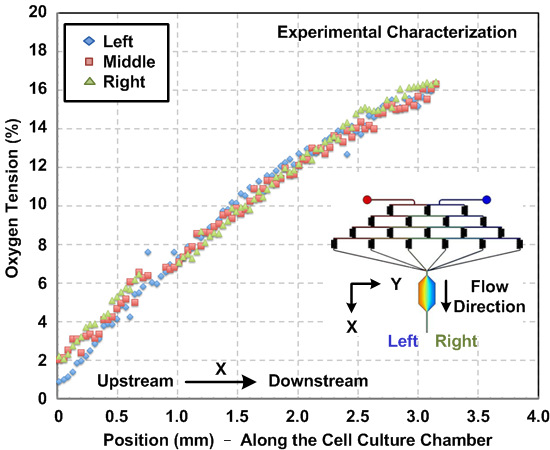
图3: 在微流体细胞培养装置内的氧梯度。实验测得的沿流动方向的细胞培养容器内的氧梯度。梯度用在氧敏感的荧光染料和图像分析估计。梯度,由左到腔室的右侧,为特征值,并且结果显示在整个室的宽度是一致的梯度分布。
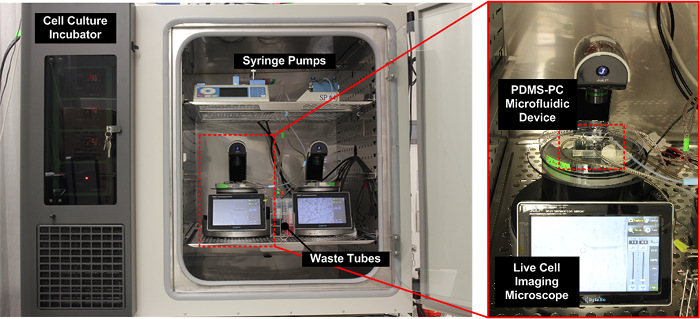
图4: 实验装置的照片。整个设置,包括微流体装置,注射泵和一个活细胞成像显微镜,被放置在通常的细胞培养孵化器内用于最佳在实验过程中的细胞培养条件。 请点击此处查看该图的放大版本。
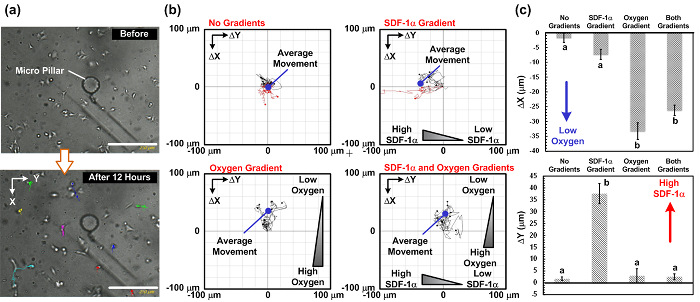
图5: 细胞迁移的研究结果在垂直SDF-1α和氧气梯度。 ( 一 )之前和12小时的细胞迁移研究后的图像捕获。细胞迁移路径可以从使用活细胞成像显微镜拍摄的时间流逝的图像进行分析。 (b)从所述捕获的图像在4种不同的梯度组合的细胞迁移路径和所分析的平均迁移运动:无梯度,仅趋化因子梯度,只有氧梯度,并且两个趋化因子和氧梯度。这些图像被抓获每15分钟。比例尺为250微米。 (c)在该垂直(氧梯度)的平均细胞迁移的距离的地块和水平(趋化因子梯度)根据四种不同梯度组合的方向。数据被表示为平均值±标准差,由三个独立的实验装置得到的,并在每个实验中分析10个细胞。统计显著不同(非配对学生t检验,p <0.01)的结果是由不同字母(a和b)指定。 请点击此处查看该图的放大版本。
讨论
The most critical steps to fabricate the PDMS microfluidic device with an embedded PC thin film are: (1) expelling all the bubbles when inserting the PC film into the PDMS pre-polymer while fabricating the PDMS-PC top layer and (2) making sure all the PDMS curing processes are performed on a well-leveled horizontal plane. For cell migration experiments, the most critical steps are: (1) eliminating the bubbles within the microfluidic device, tubing, and syringe pumps during the experiments; (2) ensuring that the microfluidic device is placed on a well-leveled horizontal plane during live cell imaging for the cell migration observation; and (3) keeping the device moisturized by adding ddH2O to the Petri dish during the experiments and making sure that the water is not dried out.
In order to successfully fabricate the PDMS-PC hybrid microfluidic device without delamination, it is critical to remove all bubbles during the PC film insertion. The PC film can be slowly inserted from an angle (about 15 to 30 degrees away from the PDMS pre-polymer surface) to prevent bubbles generation during the insertion of the PC film into the PDMS pre-polymer. If necessary, the entire PDMS pre-polymer with the embedded PC film can be placed in the desiccator connected to the vacuum pump for 10 min to expel the trapped bubbles. If the PC film floats up after the vacuum process, a pipette tip can be used to press the PC film down onto the cured PDMS layer. Repeat the vacuum and press processes if necessary.
For the cell experiments, a lack of air bubbles is critical for the microfluidic cell culture. Make sure that no air bubbles are introduced into the entire microfluidic setup (including syringe pumps, tubing, and the microfluidic device) when making connections. If air bubbles are created within the microfluidic setup due to the decrease of gas solubility under the elevated temperature of the experiments inside the incubator, all the experimental components (including the syringes and tubing) and reagents (including the growth medium, pyrogallol, and NaOH) can be placed into the incubator beforehand (at least 20 min prior to usage) to minimize the temperature variation. Syringe pumps often generate heat from the operation of the motors within the pumps. It is usually acceptable to operate syringe pumps inside incubators; however, do check the incubator temperature during the experiments. If the temperature elevates during the experiments, additional cooling procedures need to be carried out. Several feasible cooling methods can be employed, such as placing a box of ice into the incubator, reducing the number of syringe pumps placed inside the incubator, or using an incubator with a force cooling system.
The PDMS-PC microfluidic cell culture device developed in this paper is capable of reliably generating perpendicular chemical and oxygen gradients for cell migration studies. The limitation of the developed device is that the generated oxygen gradient profiles depend on the balance between oxygen flux, driven by chemical reaction scavenging, and oxygen diffusion from the ambient atmosphere, through the device, and into the medium. As a result, the oxygen gradient profiles cannot be arbitrarily controlled inside the device. Compared to existing microfluidic cell culture platforms, the device developed in this paper is the first one capable of performing cell culture studies under combinations of chemical and oxygen gradients. The entire device can be fabricated using the conventional soft lithography replica molding process, without tedious alignment and expensive instrumentation. The gradients can be numerically simulated and experimentally characterized to provide more physiological microenvironment-like conditions for in vitro cell studies. By using a spatially confined chemical reaction method with a PC film as a gas diffusion barrier, the oxygen gradient can be generated without using pressurized gas cylinders and sophisticated gas flow control units. In addition, the setup requires only small amounts of chemicals (less than 10 ml per day) to maintain the oxygen gradients. Since the oxygen tension control is confined locally around the microfluidic channel, and does not disturb the ambient oxygen concentration, the entire setup can be placed inside a conventional cell culture incubator without additional temperature, humidity, and CO2 control instrumentation. As a result, the developed device has great potential to be practically used in biological labs.
Due to technical limitations, cellular behaviors under oxygen tensions have seldom been studied in the existing literature. With the help of the device developed in this paper, cell culture under oxygen gradients can be performed in a facile manner that greatly promotes cell studies under oxygen gradients. Furthermore, a similar operation principle can be applied to generate other physiologically relevant gaseous gradients, such as carbon dioxide and nitric oxide, for in vitro cell culture studies17. These capabilities demonstrate that the PDMS-PC microfluidic device shows great potential for various cell culture applications, including drug testing and cell proliferation and migration assays, to advance in vitro cell culture studies.
披露声明
The authors declare that they have no competing financial interests.
致谢
This paper is based on work supported by the National Health Research Institutes (NHRI) in Taiwan under the Innovative Research Grant (IRG) (EX105-10523EI), the Taiwan Ministry of Science and Technology (MOST 103-2221-E-001-001-MY2, 104-2221-E-001-015-MY3, 105-2221-E-001-002-MY2), the Academia Sinica Thematic Project (AS-105-TP-A06), and the Research Program in Nanoscience and Nanotechnology. The authors would like to thank Ms. Rachel A. Lucas for proofreading the manuscript.
材料
| Name | Company | Catalog Number | Comments |
| 1 ml Syringe | Becton-Dickinson, Franklin Lakes, NJ | 302104 | |
| 1.5 ml Microcentrifuge Tube | Smartgene | 6011-000 | |
| 10 ml Syringe | Becton-Dickinson, Franklin Lakes, NJ | 302151 | |
| 15 ml Centrifuge Tube | ThermoFisher Scientific,Waltham, MA | Falcon 352096 | |
| 150 mm Petri dish | Dogger Science | DP-43151 | |
| 1H,1H,2H,2H-Perfluorooctyltrichlorosilane | Alfa Aesar, Ward Hill, MA | 78560-45-9 | |
| 3 ml Syringe | Becton-Dickinson, Franklin Lakes, NJ | 302118 | |
| 4'' Silicon Dummy Wafer | Wollemi Technical, Taoyuan, Taiwan | ||
| Acetone | ECHO Chemical, Miaoli, Taiwan | AH3102-000000-72EC | |
| AG Double Expose Mask Aligner | M&R Nano Technology, Taoyuan, Taiwan | AG500-4D-D-V-S-H | |
| Antibiotic-Antimyotic solution | ThermoFisher Scientific,Waltham, MA | GIBCO 15240-062 | |
| Biopsy punch | Miltex, Plainsboro, NJ | 33-31 | |
| Blunt needle | JensenGlobal, Santa Barbara, CA | Gauge 14 | |
| Bright-Line Hemocytometer | Sigma-Aldrich, St. Louis, MO | Z359629 | for cell counting |
| Buffered Oxide Etch | ECHO Chemical, Miaoli, Taiwan | PH3101-000000-72EC | |
| Cell Culture Incubator | Caron, Marietta, OH | 6016-1 | |
| COMSOL Multiphysics | COMSOL, Burlington, MA | Ver. 4.3b | for numerical simulation of chemical gradients in the device |
| D-PBS | ThermoFisher Scientific,Waltham, MA | GIBCO 14190-144 | |
| Desicattor | A-VAC Industries, Anaheim, CA | 35.10001.01 | |
| DMEM/F12+GlutaMax-1 | ThermoFisher Scientific,Waltham, MA | GIBCO 10565-018 | |
| Fetal Bovine Serum | ThermoFisher Scientific,Waltham, MA | GIBCO 10082 | |
| Fibronectin from Human Plasma | Sigma-Aldrich, St. Louis, MO | F2006 | |
| Inverted Fluorescence Microscope | Leica Microsystems, Wetzlar, Germany | DMIL LED | |
| Isopropyl Alcohol (IPA) | ECHO Chemical, Miaoli, Taiwan | CMOS112-00000-72EC | |
| JuLi Smart Fluorescence Cell Imager | NanoEnTek, Seoul, Korea | DBJ01B | |
| Mechanical Convention Oven | ThermoFisher Scientific,Waltham, MA | Lindberg Blue M MO1450C | |
| NaOH | Showa Chemical Industry, Tokyo, Japan | 1943-0150 | |
| Plasma tretment system | Nordson MARCH, Concord CA | PX-250 | for oxygen plasma surface treatment |
| Polycarbonate (PC) film | Quantum Beam Technologies, Tainan Taiwan | ||
| Polydimehtylsiloxane (PDMS) | Dow Corning, Midland, MI | SYLGARD 184 | |
| Pyrogallol | Alfa Aesar, Ward Hill, MA | A13405 | |
| Removable Adhesive Putty | 3M | 860 | |
| Sorvall Legend Mach 1.6R Tabletop Centrifuge | ThermoFisher Scientific,Waltham, MA | ||
| Spin Coater | ELS Technology, Hsinchu, Taiwan | ELS 306MA | |
| SU-8 2050 | MicroChem, Westborough, MA | SU-8 2050 | |
| SU-8 Developer | MicroChem, Westborough, MA | Y020100 | |
| Surgical blade | Feather, Osaka, Japan | 5005093 | for PDMS cutting |
| Syringe Pump | Chemyx, Houston, TX | Fusion 400 | |
| T75 Cell Culture Flask | ThermoFisher Scientific,Waltham, MA | Nunc 156367 | |
| Trypan Blue Solution, 0.4% | ThermoFisher Scientific,Waltham, MA | 15250061 | |
| Trypsin-EDTA | ThermoFisher Scientific,Waltham, MA | GIBCO 25200 | |
| Tygon PTFE Tubing | Saint-Gobain Performance Plastics, Akron, OH | ||
| Tygon Tubing | Saint-Gobain Performance Plastics, Akron, OH | 621 |
参考文献
- Phillips, J. E., Burns, K. L., Le Doux, J. M., Guldberg, R. E., García, A. J. Engineering graded tissue interfaces. Proc. Natl. Acad. Sci. USA. 105 (34), 12170-12175 (2008).
- Wang, F. The signaling mechanisms underlying polarity and chemotaxis. Cold Spring Harb. Perspect Biol. 1 (4), 1-16 (2009).
- Oh, S. H., Ward, C. L., Atala, A., Yoo, J. J., Harrison, B. S. Oxygen generating scaffolds for enhancing engineering tissue survival. Biomaterials. 30 (5), 757-762 (2009).
- Decaris, M. L., Lee, C. I., Yoder, M. C., Tarantal, A. F., Leach, J. K. Influence of the oxygen microenvironment on the proangiogenic potential of human endothelial colony forming cells. Angiogenesis. 12, 303-311 (2009).
- Chung, B. G., et al. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip. 5 (4), 401-406 (2005).
- Harris, A. L. Hypoxia - a key regulatory factor in tumor growth. Nat. Rev. Cancer. 2 (1), 38-47 (2002).
- Allen, J. W., Bhatia, S. N. Formation of steady-state oxygen gradients in vitro: application to liver zonation. Biotechnol. Bioeng. 82 (3), 253-262 (2003).
- McCord, A. M., Jamal, M., Shankavaram, U. T., Lang, F. F., Camphausen, K., Tofilon, P. J. Physiologic oxygen concentration enhances the stem-like properties of CD133+ human glioblastoma cells in vitro. Mol. Cancer Res. 7 (4), 489-497 (2009).
- Lo, J. F., Sinkala, E., Eddington, D. T. Oxygen gradients for open well cellular cultures via microfluidic substrates. Lab Chip. 10 (18), 2394-2401 (2010).
- Chang, C. W., et al. A polydimethylsiloxane-polycarbonate hybrid microfluidic device capable of generating perpendicular chemical and oxygen gradients for cell culture studies. Lab Chip. 14 (19), 3762-3772 (2014).
- Chen, Y. A., et al. Generation of oxygen gradients in microfluidic devices for cell culture using spatially confined chemical reactions. Lab Chip. 11 (21), 3626-3633 (2011).
- Peng, C. C., Liao, W. H., Chen, Y. H., Wu, C. Y., Tung, Y. C. A microfluidic cell culture array with various oxygen tensions. Lab Chip. 13 (16), 3239-3245 (2013).
- Xia, Y., Whitesides, G. M. Soft Lithography. Ann. Rev. Mate. Sci. 28, 153-184 (1998).
- Friend, J., Yeo, L. Fabrication of microfluidic devices using polydimethylsiloxane. Biomicrofluidics. 4 (2), 026502 (2010).
- Cordelières, F. P. . Manual Tracking. , (2005).
- Asano, Y., Horn, E. . Instructions Chemotaxis and Migration Tool 2.0. , (2013).
- Chen, Y. H., Peng, C. C., Cheng, Y. J., Wu, J. G., Tung, Y. C. Generation of nitric oxide gradients in microfluidic devices for cell culture using spatially controlled chemical reactions. Biomicrofluidics. 7 (6), 064104 (2013).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。