Se requiere una suscripción a JoVE para ver este contenido. Inicie sesión o comience su prueba gratuita.
Method Article
Construcción de redes metabólicas fuera de equilibrio en vesículas de tamaño nanométrico y micrométrico
En este artículo
Resumen
Presentamos un protocolo para la reconstitución de proteínas de membrana y encapsulación de enzimas y otros componentes solubles en agua en vesículas lipídicas de tamaño submicrométrico y micrométrico.
Resumen
Presentamos un método para incorporar a las vesículas redes complejas de proteínas, que involucran proteínas de membrana integrales, enzimas y sensores basados en fluorescencia, utilizando componentes purificados. Este método es relevante para el diseño y construcción de biorreactores y el estudio de redes complejas de reacciones metabólicas fuera de equilibrio. Comenzamos reconstituyendo (múltiples) proteínas de membrana en grandes vesículas unilaminares (LUVs) de acuerdo con un protocolo previamente desarrollado. A continuación, encapsulamos una mezcla de enzimas purificadas, metabolitos y sensores basados en fluorescencia (proteínas fluorescentes o colorantes) mediante congelación-descongelación-extrusión y eliminamos los componentes no incorporados mediante centrifugación y/o cromatografía de exclusión por tamaño. El rendimiento de las redes metabólicas se mide en tiempo real mediante el seguimiento de la relación ATP/ADP, la concentración de metabolitos, el pH interno u otros parámetros mediante la lectura de fluorescencia. Nuestras vesículas que contienen proteínas de membrana de 100-400 nm de diámetro se pueden convertir en vesículas unilaminares gigantes (GUV), utilizando procedimientos existentes pero optimizados. El enfoque permite la inclusión de componentes solubles (enzimas, metabolitos, sensores) en vesículas de tamaño micrométrico, aumentando así el volumen de los biorreactores en órdenes de magnitud. La red metabólica que contiene los GUV queda atrapada en dispositivos microfluídicos para su análisis mediante microscopía óptica.
Introducción
El campo de la biología sintética ascendente se centra en la construcción de células (mínimas) 1,2 y biorreactores metabólicos con fines biotecnológicos 3,4 o biomédicos 5,6,7,8. La construcción de células sintéticas proporciona una plataforma única que permite a los investigadores estudiar proteínas (de membrana) en condiciones bien definidas que imitan las de los entornos nativos, lo que permite el descubrimiento de propiedades emergentes y funciones bioquímicas ocultas de las proteínas y las redes de reacción9. Como paso intermedio hacia una célula sintética que funcione de forma autónoma, se desarrollan módulos que capturan características esenciales de las células vivas, como la conservación de la energía metabólica, la síntesis de proteínas y lípidos y la homeostasis. Estos módulos no solo mejoran nuestra comprensión de la vida, sino que también tienen aplicaciones potenciales en los campos de la medicina8 y la biotecnología10.
Las proteínas transmembrana están en el corazón de prácticamente cualquier red metabólica, ya que transportan moléculas dentro o fuera de la célula, señalizan y responden a la calidad del medio ambiente y desempeñan numerosas funciones biosintéticas. Así, la ingeniería de módulos metabólicos en células sintéticas requiere en la mayoría de los casos la reconstitución de proteínas de membrana integrales y/o periféricas en una bicapa de membrana compuesta por lípidos específicos y de alta integridad (baja permeabilidad). El manejo de estas proteínas de membrana es un desafío y requiere conocimientos específicos y habilidades experimentales.
Se han desarrollado varios métodos para reconstituir proteínas de membrana dentro de vesículas de fosfolípidos, la mayoría de las veces con el propósito de estudiar la función11,12, la regulación13, las propiedades cinéticas14,15, la dependencia lipídica15,16 y/o la estabilidad17 de una proteína específica. Estos métodos implican la dilución rápida de proteínas solubilizadas con detergente en medios acuosos en presencia de lípidos18, la eliminación de detergentes mediante la incubación de proteínas solubilizadas con detergente con vesículas lipídicas desestabilizadas con detergente y la absorción de los detergentes en perlas de poliestireno19, o la eliminación de detergentes mediante diálisis o cromatografía de exclusión por tamaño20. Los disolventes orgánicos se han utilizado para formar vesículas lipídicas, por ejemplo, a través de la formación de interfases aceite-agua21, pero la mayoría de las proteínas integrales de la membrana se inactivan cuando se exponen a dichos disolventes.
En nuestro laboratorio, reconstituimos principalmente proteínas de membrana por el método de absorción de detergente para formar vesículas unilaminares grandes (LUVs)19. Este método permite la co-reconstitución de múltiples proteínas de membrana y la encapsulación en la luz de la vesícula de enzimas, metabolitos y sondas22,23. Las LUV que contienen proteínas de membrana pueden convertirse en vesículas unilamelares gigantes (GUV) con/sin encapsulación de componentes solubles en agua, utilizando electroformación24 o hinchazón asistida por gel25 y condiciones específicas para preservar la integridad de las proteínas de membrana26.
En este trabajo se presenta un protocolo para la reconstitución en LUVs de una red metabólica fuera de equilibrio que regenera ATP a través de la descomposición de L-arginina en L-ornitina27. La formación de ATP está acoplada a la producción de glicerol-3-fosfato (G3P), un componente importante para la síntesis de fosfolípidos22,28. La vía metabólica consta de dos proteínas de membrana integrales, una arginina/ornitina (ArcD) y un antiportador G3P/Pi (GlpT). Además, se requieren tres enzimas solubles (ArcA, ArcB, ArcC) para el reciclaje de ATP, y GlpK se usa para convertir el glicerol en glicerol 3-fosfato, utilizando el ATP de la descomposición de L-arginina, consulte la Figura 1 para obtener una descripción general esquemática de la vía. Este protocolo representa un buen punto de partida para la futura construcción de redes de reacción aún más complejas, para la síntesis de lípidos o proteínas o la división de células. La composición lipídica de las vesículas apoya la actividad de una amplia variedad de proteínas integrales de membrana y ha sido optimizada para el transporte de diversas moléculas dentro o fuera de las vesículas 27,29,30.
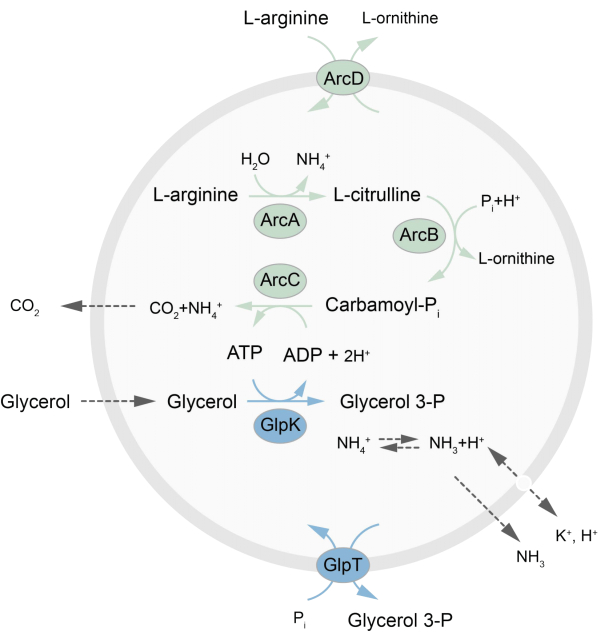
Figura 1: Descripción general de la vía para la producción de ATP y la síntesis y excreción de glicerol 3-fosfato. Haga clic aquí para ver una versión más grande de esta figura.
En resumen, se añaden proteínas de membrana purificadas (solubilizadas en dodecil-β-D-maltósido, DDM) a vesículas lipídicas preformadas que han sido desestabilizadas con Triton X-100, lo que permite la inserción de las proteínas en la membrana. Las moléculas de detergente se eliminan posteriormente (lentamente) mediante la adición de perlas de poliestireno activado, lo que da lugar a la formación de proteoliposomas bien sellados. A continuación, se pueden añadir componentes solubles a las vesículas y encapsularlos mediante ciclos de congelación y descongelación, lo que atrapa las moléculas en el proceso de fusión de la membrana. Las vesículas obtenidas son muy heterogéneas y muchas son multilaminares. Luego se extruyen a través de un filtro de policarbonato con un tamaño de poro de 400, 200 o 100 nm, lo que produce vesículas de tamaño más uniforme; Cuanto menor es el tamaño de los poros, más homogéneas y unilaminares son las vesículas, pero a costa de un menor volumen interno. Las proteínas no incorporadas y las moléculas pequeñas se eliminan de la solución externa mediante cromatografía de exclusión por tamaño. Los proteoLUV se pueden convertir en vesículas de tamaño micrométrico mediante hinchazón asistida por gel, y estos proteoGUV se recogen y atrapan en un chip microfluídico para su caracterización y manipulación microscópica. En la figura 2 se muestra una descripción general esquemática del protocolo completo.
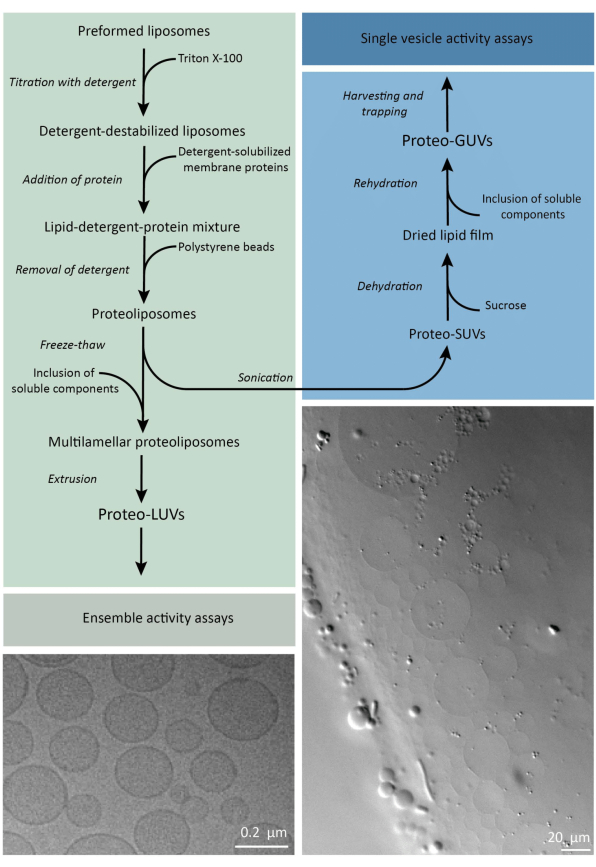
Figura 2: Resumen del protocolo para la reconstitución de proteínas de membrana y la encapsulación de enzimas y componentes solubles en agua en vesículas lipídicas de tamaño submicrométrico (LUVs) y micrométrico (GUVs). Por favor, haga clic aquí para ver una versión más grande de esta figura.
Los protocolos de reconstitución y encapsulación funcionan bien y se conserva la funcionalidad de las proteínas, pero los proteoLUVs y proteoGUVs son heterogéneos en tamaño. Los enfoques microfluídicos31,32 permiten la formación de vesículas de tamaño micrométrico que son más homogéneas en tamaño, pero la reconstitución funcional de las proteínas de membrana generalmente no es posible porque el solvente residual en la bicapa inactiva las proteínas. Los proteoLUVs varían en tamaño de 100 a 400 nm, y a bajas concentraciones de enzimas, la encapsulación puede conducir a vesículas con vías metabólicas incompletas (efectos estocásticos; ver Figura 3). Los LUV son ideales para construir módulos metabólicos específicos, como se muestra aquí para la producción de ATP y bloques de construcción como G3P. Dichos proteoLUV pueden encapsularse potencialmente en GUV y servir como compartimentos similares a orgánulos para las vesículas del huésped.
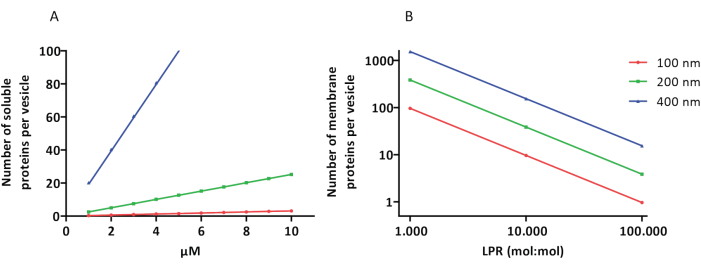
Figura 3: Número de moléculas por vesícula con un diámetro de 100, 200 o 400 nm. (A) Cuando las proteínas encapsuladas (enzimas, sondas) están en el rango de 1-10 μM. (B) La reconstitución se realiza a 1 a 1.000, 1 a 10.000 y 1 a 100.000 proteínas de membrana por lípido (mol/mol). Suponemos que las moléculas se encapsulan en las concentraciones indicadas y se incorporan a la membrana en estas proporciones proteína-lípido. En el caso de algunas enzimas, hemos visto que se unen a las membranas, lo que puede aumentar su concentración aparente en las vesículas. Abreviatura: LPR = Relación Lípido-Proteína Haga clic aquí para ver una versión más grande de esta figura.
Protocolo
1. Preparación general
- Productos químicos
- Disuelva los lípidos (en forma de polvo) a 25 mg/mL en CHCl3 para hacer liposomas preformados.
NOTA: Es preferible preparar caldos lipídicos frescos, pero las soluciones madre también pueden almacenarse a -20 °C durante algunas semanas. Trabajar con lípidos en forma de polvo es más preciso que utilizar lípidos ya solubilizados en CHCl3. El CHCl3 debe manipularse con pipetas de vidrio y/o jeringas y almacenarse en recipientes de vidrio, ya que el CHCl3 disuelve los plásticos. - Disuelva moléculas pequeñas (nucleótidos, aminoácidos, sondas de fluorescencia) para el procedimiento de encapsulación en KPi de 50 mM (tampón A, consulte la tabla 1) y ajuste el pH a 7,00 ± 0,01. Disolver MgCl2 en agua desionizada para evitar la formación de precipitados de fosfato de magnesio.
NOTA: Las soluciones madre pueden almacenarse a -20 °C durante algunas semanas, con la excepción de la TDT, que se prepara fresca el día del experimento. - Disolver los ionóforos (por ejemplo, valinomicina, nigericina) en DMSO o EtOH a una concentración de 100-500 μM. Almacenar a -20 °C durante unas semanas; Evite la evaporación.
NOTA: El DMSO no es volátil y, por lo tanto, se prefiere al EtOH. Utilice viales de vidrio en lugar de plástico, para evitar la adhesión de los ionóforos a la superficie de los viales.
- Disuelva los lípidos (en forma de polvo) a 25 mg/mL en CHCl3 para hacer liposomas preformados.
- Búferes
- Prepare tampones nuevos el día del experimento (Tabla 1). No almacenar durante más de 24 h.
- Purificación de proteínas solubles
- Express ArcA, ArcB, ArcC (usamos una variante específica llamada ArcC1), PercevalHR y GlpK como se describió anteriormente 27,28,33. Asegúrese de agregar un 10% de glicerol v/v al tampón de lisis celular, lo que mejora la estabilidad de las proteínas. Purifique las proteínas solubles como se describeen 27,28,33 y se informa a continuación.
- Descongele 10 ml de lisado celular (~5 g de peso húmedo) en un baño de agua helada. Mientras tanto, aplique 2 mL (1 CV) de resina Ni2+-Sepharose en una columna de flujo por gravedad (20 mL de capacidad) con agua desionizada (12 CV) y tampón B (4 CV) para lavar. Transfiera el lisado descongelado a hielo; Trabajar en hielo, a menos que se indique lo contrario.
- Agregue imidazol a una concentración final de 10 mM al lisado descongelado, luego vierta la solución en la columna de flujo por gravedad. Incubar durante 1 hora a 4 °C con una nutación suave.
- Después de 1 h, desecha el flujo y lava la resina con Buffer C (20 CVs).
- Eluir la proteína con tampón D. Utilice un 60% de CV para el primer paso de elución, seguido de 4-6 pasos de 40% de CV.
- Determinar la concentración de proteínas y añadir Na-EDTA hasta una concentración final de 5 mM.
- Centrifugar la proteína purificada en una centrífuga de sobremesa refrigerada (velocidad máxima, 10 minutos, 4 °C). Purifique mediante cromatografía de exclusión por tamaño utilizando Buffer E. Agrupe las fracciones de elución y concéntrelas a ~ 10 mg / mL con un filtro concentrador con un corte de 30 kDa. Prepare alícuotas de tamaño adecuado (~ 20 μL), congele rápidamente con nitrógeno líquido y almacene a -80 °C para su uso posterior.
NOTA: Es importante concentrar las enzimas a 50-100 μM para minimizar el volumen necesario para la encapsulación.
- Purificación de proteínas de membrana
- Sobreexprese ArcD y GlpT como se describió anteriormente 22,27,33. Asegúrese de agregar un 10% v/v de glicerol al tampón de lisis celular; para la purificación de ArcD, incluya 2 mM de agente reductor (por ejemplo, DTT) en el tampón. Purificar las proteínas marcadas por afinidad mediante cromatografía de Ni2+-Sepharose.
- Descongele una alícuota de vesículas de membrana crudas (10-20 mg de proteína total de membrana) en un baño de agua helada.
NOTA: Una vez descongelado, trabaje siempre sobre hielo a menos que se indique lo contrario. Consulte la Tabla 1 para ver los búferes utilizados en esta sección. - Agregue las vesículas de membrana al tampón F (ArcD) o al tampón G (GlpT) hasta un volumen final de 6 mL. Incubar la muestra durante 1 h a 4 °C con una nutación suave.
- Separar las proteínas de membrana solubilizadas de los restos de membrana mediante ultracentrifugación (337.000 × g, 30 min, 4 °C). Mientras tanto, aplique 0,25 mL (1 CV) de resina Ni2+-Sepharose en una columna de flujo por gravedad (10 mL de capacidad) con agua desionizada (40 CVs) y 20 CVs de Buffer H (ArcD) o Buffer I (GlpT).
- Vierta la proteína solubilizada en la columna de flujo por gravedad y agregue imidazol hasta una concentración final de 10 mM. Incubar durante 1 h a 4 °C con una nutación suave.
- Después de 1 h, desecha el flujo y lava la resina con 20 CV de Buffer J (ArcD) o Buffer K (GlpT).
- Eluir la proteína de membrana en pasos de 60% CV (1st) y 40% CV (2nd-6th) con tampón L (ArcD) o tampón M (GlpT).
- Determine la concentración de proteínas y continúe con la sección 2.2. para la reconstitución de membranas.
NOTA: La purificación por exclusión de tamaño no se realiza necesariamente para las proteínas de membrana, ya que la reconstitución de la membrana produce una purificación similar. Los pasos 1.4 y 2.2 se pueden realizar en 1 día de trabajo. Comience con la purificación de proteínas (paso 1.4) por la mañana y continúe con la reconstitución (paso 2.2) por la tarde. La reconstitución finaliza al día siguiente (consulte la sección 2.2 para obtener más detalles). PUNTO DE PARADA: ArcD y GlpT purificados y solublados en DDM pueden almacenarse a -80 oC para su uso posterior, pero esto no es cierto para todas las proteínas de membrana. Prepare alícuotas de tamaño adecuado (50-200 μL), congele rápidamente con nitrógeno líquido y almacene a -80 °C para su uso posterior. Estas proteínas están activas durante varios meses cuando se almacenan a -80 °C en presencia de glicerol al 10% v/v.
- Descongele una alícuota de vesículas de membrana crudas (10-20 mg de proteína total de membrana) en un baño de agua helada.
- Sobreexprese ArcD y GlpT como se describió anteriormente 22,27,33. Asegúrese de agregar un 10% v/v de glicerol al tampón de lisis celular; para la purificación de ArcD, incluya 2 mM de agente reductor (por ejemplo, DTT) en el tampón. Purificar las proteínas marcadas por afinidad mediante cromatografía de Ni2+-Sepharose.
- Preparación de β-caseína para fines de pasivación
- Resuspender 100 mg de β-caseína en 20 mL de agua desionizada y ajustar con NaOH 1 M hasta que la β-caseína se disuelva por completo. A continuación, añade 1 M de ácido acético para ajustar el pH a 7,0 y rellena el volumen hasta 50 mL con agua desionizada. Filtre la solución a través de un filtro de jeringa de 0,2 μm y haga alícuotas de 500 μL.
NOTA: La β-caseína puede almacenarse a -20 °C durante 6 meses. Se recomienda filtrar la β-caseína nuevamente antes de usarla para evitar que los agregados de β-caseína obstruyan el chip microfluídico.
- Resuspender 100 mg de β-caseína en 20 mL de agua desionizada y ajustar con NaOH 1 M hasta que la β-caseína se disuelva por completo. A continuación, añade 1 M de ácido acético para ajustar el pH a 7,0 y rellena el volumen hasta 50 mL con agua desionizada. Filtre la solución a través de un filtro de jeringa de 0,2 μm y haga alícuotas de 500 μL.
| Búfer | Composición | ||
| Búfer A | 50 mM KPi pH 7.0 | ||
| Búfer B | 50 mM KPi, 100 mM KCl, 10% v/v glicerol, 10 mM imidazol, pH 7,5 | ||
| Tampón C | 50 mM KPi, 100 mM KCl, 10% v/v glicerol, 50 mM imidazol, pH 7,5 | ||
| Búfer D | 50 mM KPi, 100 mM KCl, 10% v/v glicerol, 500 mM imidazol, pH 7,5 | ||
| Búfer E | 50 mM KPi, 100 mM KCl, 10% v/v glicerol, pH 7.0 | ||
| Búfer F | 50 mM KPi, 100 mM KCl, 0,5% p/v DDM, 10% v/v glicerol, 2 mM β-mercaptoetanol, pH 7,5 | ||
| Tampón G | 50 mM de Tris-HCl, 0,5% p/v DDM, 20% v/v glicerol, pH 8 | ||
| Tampón H | 50 mM KPi, 100 mM KCl, 0,02% p/v DDM, 10% v/v glicerol, 2 mM β-mercaptoetanol, 10 mM imidazol, pH 7,5 | ||
| Búfer I | 50 mM de Tris-HCl, 0,04 % p/v de DDM, 20 % v/v de glicerol, 10 mM de imidazol, pH 8,0 | ||
| Búfer J | 50 mM KPi, 200 mM KCl, 0,02% p/v DDM, 10% v/v glicerol, 2 mM β-mercaptoetanol, 50 mM imidazol, pH 7,5 | ||
| Tampón K | 50 mM de Tris-HCl, 0,04% p/v DDM, 20% v/v glicerol, 50 mM de imidazol, pH 8 | ||
| Tampón L | 50 mM KPi, 200 mM KCl, 0,02% p/v DDM, 10% v/v glicerol, 2 mM β-mercaptoetanol, 500 mM imidazol, pH 7,5 | ||
| Búfer M | 50 mM de Tris-HCl, 0,04% p/v DDM, 20% v/v glicerol, 500 mM de imidazol, pH 8 | ||
| Búfer N | 50 mM KPi, 58 mM NaCl, 2 mM TDT, pH 7.0 | ||
| Búfer O | 50 mM KPi, 0,5 mM L-ornitina, 10 mM Na-ADP, 10 mM MgCl2, 2 mM DTT, pH 7,0 | ||
| Búfer P | 50 mM KPi pH 7.0, 2 mM DTT, x mM glucosa (x se varía para que coincida con la osmolaridad del medio externo e interno) | ||
| Búfer Q | 50 mM KPi pH 7.0, 0.5 mM Sacarosa, 2 mM DTT | ||
| Búfer R | 50 mM KPi pH 7.0, 2 mM DTT, 10 mM L-arginina, x mM glucosa | ||
Tabla 1: Búferes utilizados en este protocolo.
2. Proteoliposomas: reconstitución de proteínas de membrana purificadas en vesículas lipídicas preformadas
- Día 1
- Preparación de vesículas lipídicas preformadas
- Elija la composición lipídica (por ejemplo, fosfolípidos sintéticos, lípidos polares de E. coli ) en función de los requisitos de las proteínas de membrana.
NOTA: Una mezcla de DOPE, DOPG y DOPC (25:25:50 mol%) es un buen punto de partida, pero es posible que se requieran esteroles o cardiolipina para algunas proteínas; Para las proteínas de la membrana plasmática de la levadura, incluimos lípidos de palmitoil-oleoilo en lugar de dioleoyl30. Una mezcla de DOPE, DOPG y DOPC (25:25:50 mol%) es suficiente para la reconstitución de ArcD y GlpT. - Mezclar los lípidos deseados (solubilizados en CHCl3) y evaporar el CHCl3 en un evaporador rotativo hasta que se forme una película lipídica. Lave los lípidos añadiendo éter dietílico en el mismo volumen que el CHCl3. Evaporar el éter dietílico y obtener una película lipídica seca.
- Resuspender la película lipídica a 20 mg/mL de lípidos totales en medio acuoso (tampón A). Comience con la mitad del volumen total y agite suavemente; Luego, transfiera cuidadosamente los lípidos a un tubo limpio o botella de tamaño adecuado. Añada tampón A fresco al matraz y repita el procedimiento para disolver los lípidos restantes y transferirlos a un nuevo recipiente. Agregue tampón A adicional para alcanzar una concentración final de 20 mg/mL.
- Sonicar los lípidos resuspendidos utilizando un sonicador de sonda. Utilice los siguientes parámetros para una punta de sonicador con un diámetro de 6 mm: 4 μm de intensidad, 70% de amplitud, 5 s encendido, 45 s apagado, 16 ciclos. Sumerja los lípidos en un baño de agua helada que contenga EtOH para evitar el sobrecalentamiento por sonicación.
- Congelar rápidamente la muestra sonicada (volumen de 40 mL en un tubo de centrífuga de 50 mL) en nitrógeno líquido y descongelar la muestra en un baño de agua a temperatura ambiente. Repita una vez; luego, alícuota los liposomas en los volúmenes deseados (por ejemplo, 1 mL o 20 mg de lípidos totales en un tubo de plástico de 1,5 mL).
PUNTO DE PARADA: El procedimiento se puede detener en este punto. Congele rápidamente cada alícuota una vez más (tercer ciclo) y almacene en nitrógeno líquido hasta por unos meses. Tenga cuidado de perforar las tapas del tubo dos veces con una aguja para evitar la explosión del tubo al hervir rápidamente el nitrógeno líquido.
- Elija la composición lipídica (por ejemplo, fosfolípidos sintéticos, lípidos polares de E. coli ) en función de los requisitos de las proteínas de membrana.
- Preparación de vesículas lipídicas preformadas
- Día 2
- Reconstitución de proteínas de membrana purificadas en liposomas preformados
- Descongelar una alícuota de liposomas (20 mg de lípidos totales) en un baño de agua a temperatura ambiente. Mientras tanto, prepare una extrusora aplicando un filtro de su elección (por ejemplo, policarbonato, diámetro de poro de 400 nm); preequilibrar el extrusor con el tampón A; y cargue los liposomas descongelados ('solución lechosa') en el extrusor y páselos 13 veces a través del filtro. Recoger los liposomas extruidos (ahora grandes vesículas unilamelares; "solución opaca") en un recipiente de vidrio o plástico de tamaño adecuado (por ejemplo, 15 mL). Diluir los liposomas a 4 mg/mL con Tampón A suplementado con 2 mM de DTT.
- Transfiera 1 mL de liposomas de 4 mg/mL a una cubeta transparente de 1 mL. En un espectrofotómetro, mida la densidad óptica inicial a 540 nm. Vuelva a verter la muestra medida y añada 50 μL de Triton X-100 al 10% v/v a los liposomas.
NOTA: Un volumen de titulación de 50 μL de Triton-X100 al 10% es adecuado para 20 mg de lípidos en un volumen de 5 mL; la adición de Triton X-100 diluirá los lípidos en ~5%. Ajuste el volumen de valoración cuando trabaje con diferentes cantidades de liposomas. Para obtener señales de densidad óptica estables, valore los liposomas con Triton X-100 a temperatura ambiente. - Repita el paso 2.2.1.2 y observe cuándo se alcanza una densidad óptica máxima (Rsat). Continúe con la valoración hasta que se alcance una densidad óptica de aproximadamente el 60% R sat (Figura 4). Vierta las vesículas desestabilizadas con detergente de nuevo en el tubo de vidrio/plástico (el volumen final es ahora de aproximadamente 5,2 ml), transfiera la muestra a hielo y deje que se enfríe.
- Agregue la(s) proteína(s) de membrana purificada a los liposomas desestabilizados para alcanzar la proporción deseada de lípidos/proteínas (p/p). Utilice una relación de 400:1 hasta 100:1 p/p; dado que tanto ArcD como GlpT tienen pesos moleculares de ~55 kDa, una relación lípido-proteína de 400:1 p/p corresponde a ~30.000 lípidos por proteína, y ~ 50 moléculas de ArcD y GlpT cada una por vesículas con un diámetro de 400 nm.
NOTA: Aquí utilizamos 400:1 p/p, lo que corresponde a 50 μg de cada proteína por 20 mg de lípidos. - Nutar las muestras a 4 °C durante 15 min para permitir que las proteínas de la membrana se inserten en la membrana liposomal desestabilizada.
- Para eliminar el detergente, agregue 200 mg de perlas de poliestireno secas, preparadas según las instrucciones del fabricante. Nutato a 4 °C durante otros 15 min.
NOTA: Una cantidad de 200 mg de perlas secas es adecuada para 20 mg de lípidos, pero debe ajustarse cuando se utiliza un tamaño de muestra diferente. - Repita el paso 2.2.1.6 2x, para un total de tres adiciones de perlas de poliestireno. A continuación, incubar durante la noche a 4 °C con una nutación suave.
- Reconstitución de proteínas de membrana purificadas en liposomas preformados
- Día 3
- Repita el paso 2.2.1.6; sin embargo, esta vez, nutato a 4 °C durante 1 h.
- Asegure una columna de flujo por gravedad vacía (10 mL de capacidad) sobre un tubo de ultracentrifugación vacío de 6,5 mL sobre hielo. Vierta la muestra en la columna y recoja los proteoliposomas en el tubo de ultracentrifugación (las perlas se retienen en la columna).
- Lave las perlas con 0,5 mL de tampón A suplementado con 2 mM de DTT y recoja el filtrado en el tubo de ultracentrifugación.
- Concentrar los proteoliposomas por ultracentrifugación (337.000 × g, 30 min, 4 oC). Resuspender los proteoliposomas en un volumen total de 200 μL (100 mg de lípidos/mL) en el tampón A suplementado con 2 mM de DTT; el volumen seco de las vesículas después de la centrifugación es de ~40 a 120 μL27. Dividir en alícuotas del tamaño deseado (por ejemplo, tres alícuotas de 6,66 mg de lípidos totales).
NOTA: El tamaño de la alícuota es arbitrario, pero afectará al tamaño del pellet en pasos posteriores (consulte el paso 3.2.3.1). PUNTO DE PARADA: El procedimiento se puede detener aquí. Congele rápidamente cada alícuota y almacene en nitrógeno líquido hasta por unas pocas semanas. Tenga cuidado de perforar las tapas del tubo dos veces con una aguja para evitar la explosión del tubo al hervir rápidamente el nitrógeno líquido.
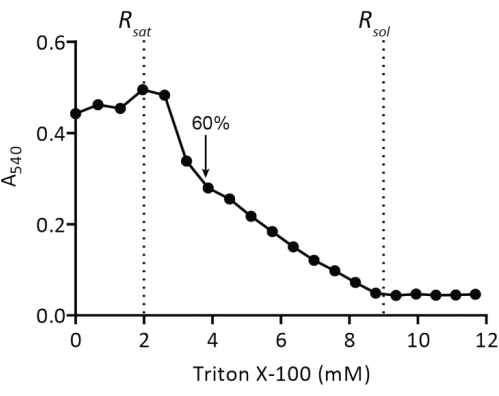
Figura 4: Valoración de liposomas preformados con Triton X-100. Los liposomas a 5 mg de lípidos/mL se extruyen a través de un filtro de policarbonato (400 nm) en 50 mM KPi (pH 7,0) y luego se valoran con Triton X-100 (paso del protocolo 2.2.1.2). La turbidez de las vesículas se mide en A540. La flecha indica la concentración de Triton X-100 en la que las vesículas están lo suficientemente desestabilizadas para la inserción espontánea de proteínas de membrana, como se describe en19. Haga clic aquí para ver una versión más grande de esta figura.
3. Encapsulación de una red metabólica para el reciclaje de ATP y la síntesis de glicerol 3-P en vesículas de tamaño submicrónico
- Día 1
- Mezcla de componentes
- Para una encapsulación estándar, utilice 66,6 μL de proteoliposomas en un volumen final de 200 μL (33,33 mg/mL de lípidos totales). Calcular el volumen de cada componente (enzima, cofactor) necesario para alcanzar la concentración deseada, añadir estos componentes a los proteoliposomas y ajustar el volumen a 200 μL con tampón A (Tabla 2).
NOTA: La concentración de proteínas se puede ajustar en función de la configuración experimental. Sin embargo, se debe tener cuidado de que varias copias (>10) de cada enzima estén presentes en promedio por vesícula, para evitar efectos estocásticos no deseados. Las concentraciones > 1 μM son generalmente seguras; 1 μM en una vesícula con un diámetro de 400 nm corresponde aproximadamente a 20 copias (Figura 3). - Pipetee el tampón A en un tubo vacío de 1,5 ml y añada el DTT, el Na-ADP, el MgCl2, la L-ornitina (y la piranina, si es necesario para las mediciones internas del pH). A continuación, agregue las enzimas y mezcle suavemente. Agregue la solución encima de los proteoliposomas preformados y haga un vórtice breve a baja velocidad.
NOTA: Es importante añadir Na-ADP antes de MgCl2 para evitar la formación no deseada de precipitados de fosfato de magnesio. El vórtice es necesario para la mezcla adecuada de la solución viscosa; sin embargo, minimice la duración y la velocidad para evitar daños mecánicos a las proteínas. PercevalHR y piranina no se pueden coencapsular porque sus espectros se superponen.
- Para una encapsulación estándar, utilice 66,6 μL de proteoliposomas en un volumen final de 200 μL (33,33 mg/mL de lípidos totales). Calcular el volumen de cada componente (enzima, cofactor) necesario para alcanzar la concentración deseada, añadir estos componentes a los proteoliposomas y ajustar el volumen a 200 μL con tampón A (Tabla 2).
- Determinación de la osmolalidad interna
- Prepare una solución de 50 μL como se describe en el paso 3.1.1.1 pero sin proteoliposomas, y mida la osmolalidad con un osmómetro de punto de congelación.
- Prepare una curva de calibración utilizando tampón (50 mM KPi pH 7.0) y concentraciones variables de sal (por ejemplo, NaCl o NaCl) o azúcar. Determine la concentración de osmolitos que coincida con la osmolalidad interna (tampón N).
NOTA: Los componentes permeables a la membrana (por ejemplo, glicerol) no se pueden utilizar para igualar la osmolalidad interna. En el caso de las proteínas solubilizadas en tampón que contiene glicerol (por ejemplo, tampón E), se debe utilizar el mismo tampón sin glicerol. El osmolito elegido para la preparación de un tampón externo isoosmótico debe ser impermeable a la membrana y no interferir con la red metabólica.
- Congelación-descongelación
- Congelar rápidamente (en nitrógeno líquido) los proteoliposomas junto con los componentes solubles y descongelar en un baño de agua y hielo a aproximadamente 10 °C.
- Repita el paso 3.1.3.1 para un total de 5 veces.
PUNTO DE PARADA: El procedimiento se puede detener aquí. Omita el último paso de descongelación y almacene la muestra congelada en nitrógeno líquido durante 1-3 días. Tenga cuidado de perforar las tapas del tubo 2 veces con una aguja para evitar la explosión del tubo al hervir rápidamente el nitrógeno líquido. Se prefiere el almacenamiento en nitrógeno líquido a -80 °C para minimizar la oxidación de lípidos.
- Mezcla de componentes
- Día 2
- Extrusión
NOTA: Todos los pasos de extrusión se realizan a temperatura ambiente, ya que de lo contrario las jeringas herméticas al gas tendrán fugas.- Prepare un extrusor aplicando un filtro de su elección (por ejemplo, policarbonato, diámetro de poro de 400 nm). Lave el extrusor con una solución que contenga el mismo tampón y metabolitos utilizados para la encapsulación de las vesículas (por ejemplo, tampón O más 0,1 mM de piranina).
NOTA: Utilice un extrusor dedicado para la carga de proteoliposomas con piranina, ya que el tinte se adhiere a la superficie del extrusor y puede causar contaminación en muestras posteriores. - Cargue la mezcla de encapsulación en el extrusor y pásela por el filtro 13x. Recoja la solución extruida en un tubo de 1,5 ml.
- Prepare un extrusor aplicando un filtro de su elección (por ejemplo, policarbonato, diámetro de poro de 400 nm). Lave el extrusor con una solución que contenga el mismo tampón y metabolitos utilizados para la encapsulación de las vesículas (por ejemplo, tampón O más 0,1 mM de piranina).
- Cromatografía de exclusión por tamaño (opcional)
NOTA: Este paso se realiza para eliminar moléculas externas, como colorantes como la piranina, mediante cromatografía de exclusión por tamaño. Si los colorantes no están presentes en el sistema, y otros componentes no interfieren a bajas concentraciones (observe que las vesículas también se lavan posteriormente por ultracentrifugación), entonces el paso 3.2.2. se puede omitir.- Rehidrata la resina Sephadex G-75 y viértela en una columna de vidrio (22 cm de largo, 1,5 cm de ancho). Equilibre la resina con un exceso de tampón externo (por ejemplo, tampón N).
- Cargue los proteoliposomas extruidos del paso 3.2.1.2 en la columna de cromatografía de exclusión por tamaño preequilibrada y aplique un flujo de gravedad de tampón externo. Deseche el volumen miccional (aproximadamente 7 mL); luego, recoja diez alícuotas de 1 mL. Visualice las alícuotas que contienen proteoliposomas mediante una breve exposición a una lámpara UV. Agrupar las fracciones que contienen la mayoría de los proteoliposomas (2-4 mL).
- Lavado y resuspensión
- Lavar los proteoliposomas extruidos por ultracentrifugación. Llene un tubo de ultracentrifugación de 6,5 mL con 5,8 mL de tampón N y aplique la muestra extruida encima. Si se realizó una cromatografía de exclusión por tamaño, llene el tubo con las muestras de elución agrupadas (2-4 mL) y agregue tampón N hasta un volumen final de 6 mL.
- Centrífuga a 337.000 × g, 30 min, 4 °C. Deseche el sobrenadante y seque bien el tubo de ultracentrifugación con un pañuelo de papel libre de polvo, prestando atención a no tocar el pellet. Vuelva a suspender el pellet en un pequeño volumen de tampón N (200 μL). Cuando el pellet esté completamente resuspendido, llene el tubo hasta 6 mL con Buffer N.
NOTA: volver a suspender el pellet llevará tiempo y debe hacerse con cuidado. - Repita los pasos 3.2.3.1-3.2.3.2 2 veces, para un total de tres lavados, a menos que se realice una cromatografía de exclusión por tamaño (en cuyo caso basta con un paso de centrifugación). En última instancia, vuelva a suspender el pellet a la concentración deseada (por ejemplo, 5,55 mg/ml de lípido total) añadiendo un volumen adecuado de tampón N.
PUNTO DE PARADA: Los proteoliposomas pueden utilizarse inmediatamente o almacenarse a 4 ºCdurante al menos 48 h. El tamaño de los tubos de ultracentrifugación debe elegirse en función del tamaño de la muestra. Para un pellet de 6,66 mg de lípidos totales, es apropiado un tubo de 6,5 mL. El lavado de 200 μL de proteoliposomas (33,33 mg de lípidos/mL en total; volumen de pellets secos ~40 μL)28 para 3 x 6 mL de tampón diluye los componentes externos en un factor de 100 para cada paso de lavado. Si se utilizan tubos de diferentes tamaños, es conveniente ajustar el número de lavados en consecuencia.
- Extrusión
- Día 3
- Detección de síntesis de ATP por fluorescencia
- Mezclar los componentes de la reacción hasta un volumen final de 120 μL (Tabla 3) en una cubeta de cuarzo negro con una ventana de 3 x 5 mm y un volumen interno mínimo de 100 μL.
NOTA: Se pueden agregar ionóforos para disipar los gradientes iónicos electroquímicos. Una mezcla de valinomicina y nigericina (1 μM cada una) disipa eficazmente cualquier gradiente de protones y potasio. - Precalentar la muestra en un fluorímetro ajustado a 30 °C y adquirir espectros de excitación de PercevalHR (excitación 400-520 nm, ancho de banda 5 nm; emisión 550 nm, ancho de banda 5 nm). Una vez que la señal de la sonda es constante, se inicia la red metabólica mediante la adición de un exceso de L-arginina (5-10 mM) y glicerol (400 μM) cuando el reciclaje de ATP se acopla a la síntesis de glicerol 3-fosfato. Siga la reacción a lo largo del tiempo.
- Mezclar los componentes de la reacción hasta un volumen final de 120 μL (Tabla 3) en una cubeta de cuarzo negro con una ventana de 3 x 5 mm y un volumen interno mínimo de 100 μL.
- Análisis de datos
- Represente la relación F500/F430 en función del tiempo, que es un indicador cualitativo de la relación ATP/ADP. Para una evaluación más cuantitativa de la formación de ATP, construya una curva de calibración en proteoliposomas o utilice un enfoque complementario (por ejemplo, cuantificación de ATP por quimioluminiscencia28).
- Detección de síntesis de ATP por fluorescencia
| Componente | Concentración final |
| Búfer A | 50 mM KPi pH 7.0 |
| TDT | 2 mM |
| Na-ADP | 10 mM |
| MgCl2 | 10 mM |
| L-ornitina | 0,5 mM |
| Sonda fluorescente (PercevalHR o piranina) | 5,8 μM o 0,1 mM, respectivamente |
| ArcA (arginina deiminasa) | 1 μM |
| ArcB (Ornitina carbamoiltransferasa) | 2 μM |
| ArcC1 (carbamato quinasa) | 5 μM |
| GlpK (Glicerol quinasa) | 1,6 μM |
| Proteoliposomas | 33,33 mg/mL de lípidos totales |
Tabla 2: Componentes de encapsulación. Los componentes se enumeran en orden de adición. Las proteínas solubles se encuentran en el tampón E; todos los demás componentes (excepto el MgCl2, en agua desionizada) están en el tampón A.
| Componente | Concentración final |
| Tampón K | 50 mM KPi pH 7.0, 58 mM NaCl, 2 mM DTT, pH 7.0 |
| Proteoliposomas (5,55 mg/mL de lípidos) | 2,7 mg/mL de lípidos |
| Ionóforos (valinomicina, nigericina) | 1 μM cada uno |
Tabla 3: Condiciones experimentales. Los componentes se enumeran en orden de adición. Los proteoliposomas se encuentran en el tampón N, los ionóforos en DMSO o EtOH.
4. Ampliación de una red metabólica para vesículas de tamaño micrométrico
- Día 1
- Preparación de chips microfluídicos
NOTA: Este experimento utiliza un dispositivo microfluídico desarrollado por Robinson et al.34. Otros diseños de chips microfluídicos están disponibles 35,36,37 y pueden ser fácilmente implementados en este protocolo.- Corte una punta de pipeta de 200 μL a un tercio de la parte inferior e inserte la parte inferior de la punta en un dispositivo de captura microfluídica prefabricado.
- Al depósito de entrada del chip, agregue 400 μL de solución de β-caseína (2 mg/mL; ver paso 1.5), teniendo cuidado de evitar la introducción de aire en este paso. Coloque un cubo de placa de 96 pocillos y agregue un pañuelo de laboratorio en una centrífuga de tubo cónico de mesa. Coloque el chip en la parte superior del tejido y centrifuga durante 6 minutos a 900 × g para permitir la pasivación del chip microfluídico. Después de la etapa de centrifugación, el nivel del líquido debe ser igual en el depósito de entrada y en la punta de salida del chip microfluídico; Compruebe si hay fugas en el chip. Incubar la solución de β-caseína en el chip microfluídico durante al menos 30 minutos.
- Eliminar la mayor parte de la solución de pasivación sin introducir aire y añadir 400 μl de tampón de lavado (tampón P). Coloque el chip en el cubo de placa de 96 pocillos y centrifugue a 900 × g durante 6 minutos. Deje el chip en solución de lavado hasta su uso (durante un máximo de 4 horas).
- Preparación de chips microfluídicos
- Preparación en gel para la fabricación de proteo-GUVs
NOTA: La siguiente descripción está tomada y adaptada de25,38.- Disolver el 0,5% (p/p) de una agarosa a baja temperatura de gelificación (LGT) en agua desionizada calentando la solución en el microondas; Asegúrese de que la agarosa se haya disuelto por completo y evite hervir la solución. Mantenga la agarosa a 50 °C hasta su nuevo uso.
NOTA: La agarosa disuelta se puede almacenar a temperatura ambiente durante varias semanas. Para reutilizar la agarosa, simplemente derrita el gel usando un microondas. - Toma dos diapositivas de objetivo y dibuja el contorno del espaciador en las diapositivas. Haga los portaobjetos hidrófilos mediante limpieza con plasma, utilizando plasma con alto contenido de oxígeno durante 1 min.
- Añada la agarosa LGT sobre el portaobjetos hasta que esté completamente cubierto de agarosa (~500 μL); Luego, incline el portaobjetos en un ángulo de 90 ° y drene el exceso de agarosa sobre un pañuelo. Deje los portaobjetos durante 30 minutos a 50 °C.
- Tomar proteoliposomas almacenados en nitrógeno líquido (sección 3) y descongelar en hielo. Diluir las vesículas hasta 5 mg/mL de lípidos utilizando tampón Q.
NOTA: Los proteoliposomas preparados en la sección 3 no contienen proteínas solubles y pueden prepararse con mucha antelación si se almacenan en nitrógeno líquido. Cuando trabaje con proteoGUV, asegúrese de filtrar siempre las soluciones de trabajo para evitar la obstrucción del dispositivo microfluídico. - Sonicar los proteo-liposomas utilizando un sonicador de sonda de mano con una sonda de 1 mm. Sonicato durante 10 ciclos de 0,5 s encendido y 0,5 s apagado al 70% de amplitud. Mantenga las vesículas, en lo sucesivo denominadas proteoSUV, en hielo durante 30 s y repita el proceso de sonicación 5 veces.
- Llene una jeringa de 100 μL (en un dispensador LCP de mano) con la suspensión proteo-SUV. Deposite 0,5 μL de gotas de proteo-SUV en el gel de agarosa previamente preparado; Tenga cuidado de no alterar la capa de agarosa y de mantener una distancia suficiente para evitar que las gotas se fusionen.
NOTA El uso de un dispensador LCP portátil permite la deposición de gotas de manera reproducible, pero también se pueden utilizar sistemas de pipeteo alternativos. El manchado con un capilar de vidrio es menos adecuado porque altera el gel de agarosa seco. - Seque las gotas del SUV en ~ 10 minutos usando un flujo de nitrógeno en lugar de aire comprimido para reducir la posibilidad de oxidar los lípidos.
- Preparar 1 mL de un tampón concentrado O 1,25x (Tabla 4) en el tampón A y pasarlo a través de un filtro de acetato de celulosa de 0,2 μm. Tome 800 μL de la solución concentrada 1,25x y añada las enzimas solubles y las sondas a una concentración como se indica en la Tabla 4. Use agua desionizada filtrada para hacer que el volumen final sea de 1 mL.
- Prepare 100 μL de solución de hinchamiento que contenga todos los componentes de la Tabla 4, a excepción de las proteínas y el glicerol. Mida la osmolalidad de la solución de hinchazón utilizando un osmómetro de punto de congelación calibrado de 3 puntos.
NOTA: Prepare 100 μL de solución de hinchazón sin proteínas ni glicerol para determinar con precisión la osmolalidad de esta solución. El glicerol, que está presente en la solución proteica, afecta a la osmolalidad, pero, en los GUV, el glicerol se difunde rápidamente a través de la membrana y sólo provoca diferencias osmóticas transitorias. - Ensamble la cámara de hinchamiento GUV haciendo un sándwich de dos vasos objetivo que contengan el gel y los SUV secos con un espaciador de teflón de 1,5 o 3,0 mm de grosor en el medio. Luego, agregue la solución de hinchazón en la cámara a través del pequeño orificio en el costado con una jeringa y una aguja.
NOTA: El volumen de la solución de hinchamiento se puede ajustar variando los espaciadores de 1,5 a 3,0 mm.
- Disolver el 0,5% (p/p) de una agarosa a baja temperatura de gelificación (LGT) en agua desionizada calentando la solución en el microondas; Asegúrese de que la agarosa se haya disuelto por completo y evite hervir la solución. Mantenga la agarosa a 50 °C hasta su nuevo uso.
- Hinchazón de vesículas y recolección de GUV
- Permita la hinchazón de las vesículas colocando la cámara a 22 °C durante al menos 30 minutos.
NOTA: La hinchazón de las vesículas puede ser seguida por microscopía óptica (por ejemplo, un microscopio de contraste de fase de mesa o un microscopio de fluorescencia de campo amplio) si las proteínas o lípidos están marcados con fluorescencia. - Extraiga los GUV del gel aplicando una suave agitación física golpeando la cámara sobre una superficie sólida (por ejemplo, una mesa de laboratorio); saque un tercio del volumen y use la burbuja de aire resultante para inducir suavemente el movimiento del líquido restante, separando así los GUV del gel.
- Mientras los GUV se hinchan, prepare el tampón P y haga coincidir la osmolalidad con la solución de hinchazón ajustando la concentración de glucosa. Filtre el tampón a través de un filtro de jeringa de 0,2 μm.
NOTA: En general, la solución de lavado y sustrato debe mantenerse dentro de ± 5 mosmol/kg. Cualquier solución que pase a través del chip debe estar muy limpia, ya que los contaminantes pueden obstruir los canales. Prepare tampones nuevos cada vez o almacene los tampones preparados a -20 °C y filtre antes de usar.
- Permita la hinchazón de las vesículas colocando la cámara a 22 °C durante al menos 30 minutos.
- Atrapamiento de los GUVs para experimentos de microscopía
- Monte el chip microfluídico pasivado en la platina de muestra del microscopio. Compruebe si hay posibles defectos (por ejemplo, fugas, aire atrapado, canales obstruidos).
NOTA: La verificación del chip se puede hacer mucho antes de usarlo para que se pueda preparar un nuevo chip si es necesario. - Conecte el tubo con una aguja doblada a la salida del depósito y conecte el otro extremo a una jeringa de 1 ml. Monte la jeringa en una bomba y ajuste el caudal a un máximo de 10 μL/min.
- Retire el tampón de lavado del depósito (paso 4.1.1.3) y reemplácelo con medio fresco (tampón P). Inicie el flujo de tampón a través del chip mediante la infusión a través de la jeringa a 1-10 μL/min. Lavar con al menos 80 μL de tampón de lavado osmóticamente equilibrado (Tampón P).
- Retire el exceso de tampón de lavado del depósito y añada los (proteo)GUV al depósito. Ajuste el caudal a 0,1-1 μL/min para permitir que los GUV fluyan a través del chip. Supervise el chip a lo largo del tiempo hasta que haya quedado suficiente GUV atrapado en el chip.
NOTA: Las vesículas con cantidades relativamente grandes (>20 mol%) de lípidos cargados como fosfatidilglicerol (PG), fosfatidilserina (PS) o ácido fosfatídico (PA) o lípidos no formadores de bicapas como la fosfatidiletanolamina (PE) se introducen a un caudal más bajo a través del chip para evitar el estallido. Las vesículas compuestas de fosfatidilcolina pura (PC) tienden a ser más estables. - Retire el exceso de solución de GUV y agregue tampón de lavado P, utilizando un flujo constante de 0,1-1 μL/min, para intercambiar el medio externo y lavar los GUV atrapados durante al menos 1 h para eliminar los compuestos no encapsulados y reducir la fluorescencia de fondo cuando se encapsula un fluoróforo. Controle la fluorescencia de fondo a lo largo del tiempo; Si la fluorescencia de fondo no disminuye, es posible que el chip se bloquee.
- Localice las trampas con cantidades suficientes de vesículas y guarde sus posiciones. Aplique los ajustes en el microscopio (por ejemplo, intensidad del láser, ganancia, longitud de onda) e inicie un experimento de serie temporal.
- Añada una solución de sustrato osmóticamente equilibrada (tampón R) al depósito e inicie un caudal de 0,5 μL/min.
- Monte el chip microfluídico pasivado en la platina de muestra del microscopio. Compruebe si hay posibles defectos (por ejemplo, fugas, aire atrapado, canales obstruidos).
| Componentes del búfer L | ||
| Componente | 1,25 x concentración | Concentración de trabajo |
| Búfer A | 62.5 mM KPi pH 7.0 | 50 mM KPi pH 7.0 |
| Sacarosa | 125 mM | 100 mM |
| TDT | 2,5 mM | 2 mM |
| Na-ADP | 12,5 mM | 10 mM |
| MgCl2 | 12,5 mM | 10 mM |
| L-ornitina | 0,625 mM | 0,5 mM |
| Componentes de encapsulación | ||
| Piranina o PercevalHR | 1 mM o 20 μM | |
| ArcA (arginina deiminasa) | 1 μM | |
| ArcB (Ornitina carbamoiltransferasa) | 2 μM | |
| ArcC1 (carbamato quinasa) | 5 μM | |
Tabla 4: Buffer O y componentes de encapsulación. Los componentes se enumeran en orden de adición. Todos los componentes (excepto el MgCl2, en agua desionizada) están en el tampón A. Los componentes de encapsulación se enumeran en orden de adición. Todas las proteínas solubles en agua se encuentran en el tampón E.
Resultados
La reconstitución de las proteínas de membrana solubilizadas en liposomas requiere la desestabilización de las vesículas preformadas. La adición de pequeñas cantidades de Triton X-100 inicialmente resulta en un aumento de la absorbancia a 540 nm (A540) debido a un aumento en la dispersión de la luz por la hinchazón de las vesículas (Figura 4). El valor máximo de A540 es el punto en el que los liposomas están saturados con detergente (Rsat)...
Discusión
Presentamos un protocolo para la síntesis de proteínas (de membrana) que contienen vesículas lipídicas de tamaño submicrométrico (proteoLUVs), y la conversión de proteoLUVs en vesículas unilamelares gigantes (proteoGUVs). El protocolo debería ser aplicable para la reconstitución de otras proteínas de membrana 13,19,30,40 y la encapsulación de redes metabólicas distintas de la descomposición de L-arginina y las vías de síntesis de glicerol 3-fosfato presentadas aquí.
Divulgaciones
Los autores declaran no tener ningún interés financiero contrapuesto.
Agradecimientos
Los autores agradecen a Aditya Iyer por la clonación del gen pBAD-PercevalHR y a Gea Schuurman-Wolters por ayudar con la producción y purificación de proteínas. La investigación fue financiada por el programa de Gravitación del Nuevo Orden Mundial "Construyendo una Célula Sintética" (BaSyC).
Materiales
| Name | Company | Catalog Number | Comments |
| Agarose | Sigma Aldrich | A9414-25g | |
| Amicon cut-off filter | Sigma Aldrich | Milipore centrifugal filter units Amicon Ultra | |
| BioBeads | BioRad | 152-3920 | |
| CHCl3 | Macron Fine Chemicals | MFCD00000826 | |
| D(+)-Glucose | Formedium | - | |
| D(+)-Sucrose | Formedium | - | |
| DDM | Glycon | D97002 -C | |
| Diethyl Ether | Biosolve | 52805 | |
| DMSO | Sigma-Aldrich | 276855-100ml | |
| DOPC | Avanti | 850375P-1g | |
| DOPE | Avanti | 850725P-1g | |
| DOPG | Avanti | 840475P-1g | |
| DTT | Formedium | DTT005 | |
| EtOH | J.T.Baker Avantor | MFCD00003568 | |
| Extruder | Avestin Inc | LF-1 | |
| Fluorimeter | Jasco | Spectrofluorometer FP-8300 | |
| Glycerol | BOOM | 51171608 | |
| Gravity flow column | Bio-Rad | 732-1010 | |
| Hamilton syringe 100 µL | Hamilton | 7656-01 | |
| Hamilton syringe 1000 µL | Hamilton | 81320 | |
| Handheld LCP dispenser | Art Robbins Instruments | 620-411-00 | |
| Handheld Sonicator | Hielscher Ultrasound Technology | UP50H | |
| HCl | BOOM | x76021889.1000 | |
| Imidazole | Roth | X998.4-250g | |
| K2HPO4 | Supelco | 1.05099.1000 | |
| KCl | BOOM | 76028270.1 | |
| KH2PO4 | Supelco | 1.04873.1000 | |
| Kimwipe | Kimtech Science | 7552 | |
| Large Falcon tube centrifuge | Eppendorf | Centrifuge 5810 R | |
| L-Arginine | Sigma-Aldrich | A5006-100G | |
| Light microscope | Leica | DM LS2 | |
| L-Ornithine | Roth | T204.1 | |
| LSM Laser Scanning Confocal Microscope | Zeiss | LSM 710 ConfoCor 3 | |
| MgCl2 | Sigma-Aldrich | M2670-1KG | |
| Microfluidic chip | Homemade | PDMS based | DOI: https://doi.org/10.1039/C8LC01275J |
| Na-ADP | Sigma-Aldrich | A2754-1G | |
| NaCl | Supelco | 1.06404.1000 | |
| Nanodrop Spectrometer | Isogen Life Science | ND-1000 spectrophotometer NanoDrop | |
| NaOH | Supelco | 1.06498.1000 | |
| Needles for GUVs | Henke-Ject | 14-14575 | 27 G x 3/4'' 0.4 x 20 mm |
| Needles for microfluidics | Henke-Ject | 14-15538 | 18 G x 1 1/2'' 1.2 x 40 mm |
| Ni2+ Sepharose | Cytiva | 17526802 | |
| Nigericin | Sigma-Aldrich | N7143-5MG | |
| Nutator | VWR | 83007-210 | |
| Osmolality meter | Gonotec Salmenkipp | Osmomat 3000 basic freezing point osmometer | |
| Plasmacleaner | Plasma Etch | PE-Avenger | |
| Polycarbonate filter | Cytiva Whatman | Nuclepor Track-Etch Membrane Product: 10417104 | 0.4 µm |
| Polycarbonate ultracentrifuge tube | Beckman Coulter | 355647 | |
| Pyranine | Acros Organics | H1529-1G | |
| Quartz cuvette (black) | Hellma Analytics | 108B-10-40 | |
| Sephadex G-75 resin | GE Healthcare | 17-0050-01 | |
| Sonicator | Sonics Sonics & Materials INC | Sonics vibra cell | |
| Syringe filter | Sarstedt | Filtropur S plus 0.2 | 0.2 µm |
| Syringe pump | Harvard Apparatus | A-42467 | |
| Tabletop centrifuge | Eppendorf | centrifuge 5418 | |
| Teflon spacer | Homemade | Teflon based | 45 x 26 x 1.5 or 45 x 26 x 3 or 20 x 20 x 3 mm |
| Tris | PanReac AppliChem | A1086.1000 | |
| Triton X-100 | Sigma Aldrich | T8787-100 ml | |
| Ultracentrifuge | Beckman Coulter | Optima Max-E | |
| UV lamp | Spectroline | ENB-280C/FE | |
| UV/VIS Spectrometer | Jasco | V730 spectrophotometer | |
| Valinomycin | Sigma-Aldrich | V0627-10MG | |
| Widefield fluorescence microscope | Zeiss | AxioObserver | |
| β-Casein | Sigma Aldrich | C5890-500g |
Referencias
- Hirschi, S., Ward, T. R., Meier, W. P., Müller, D. J., Fotiadis, D. Synthetic biology: bottom-up assembly of molecular systems. Chem Rev. 122 (21), 16294-16328 (2022).
- Ivanov, I., et al. Bottom-up synthesis of artificial cells: recent highlights and future challenges. Annu Rev Chem Biomol. Eng. 12 (1), 287-308 (2021).
- Clomburg, J. M., Crumbley, A. M., Gonzalez, R. Industrial biomanufacturing: The future of chemical production. Science. 355 (6320), (2017).
- Shi, T., Han, P., You, C., Zhang, Y. -. H. P. J. An in vitro synthetic biology platform for emerging industrial biomanufacturing: Bottom-up pathway design. Synth Syst Biotechnol. 3 (3), 186-195 (2018).
- Wang, A., et al. Liver-target and glucose-responsive polymersomes toward mimicking endogenous insulin secretion with improved hepatic glucose utilization. Adv Funct Mater. 30 (13), 1910168 (2020).
- Kanter, G., et al. Cell-free production of scFv fusion proteins: an efficient approach for personalized lymphoma vaccines. Blood. 109 (8), 3393-3399 (2007).
- Zeltins, A. Construction and characterization of virus-like particles: a review. Mol Biotechnol. 53 (1), 92-107 (2013).
- Jain, K. K. Synthetic biology and personalized medicine. Med Princ Pract. 22 (3), 209-219 (2013).
- Schwille, P., Frohn, B. P. Hidden protein functions and what they may teach us. Trends Cell Biol. 32 (2), 102-109 (2022).
- Sachsenmeier, P. Industry 5.0-The relevance and implications of bionics and synthetic biology. Engineering. 2 (2), 225-229 (2016).
- Schmidt, D., Jiang, Q. -. X., MacKinnon, R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature. 444 (7120), 775-779 (2006).
- Godoy-Hernandez, A., et al. Rapid and highly stable membrane reconstitution by LAiR enables the study of physiological integral membrane protein functions. ACS Cent Sci. 9 (3), 494-507 (2023).
- Sikkema, H. R., et al. Gating by ionic strength and safety check by cyclic-di-AMP in the ABC transporter OpuA. Sci Adv. 6 (47), 7697 (2020).
- Foucaud, C., Poolman, B. Lactose transport system of Streptococcus thermophilus. Functional reconstitution of the protein and characterization of the kinetic mechanism of transport. J Biol Chem. 267 (31), 22087-22094 (1992).
- Yoneda, J. S., Sebinelli, H. G., Itri, R., Ciancaglini, P. Overview on solubilization and lipid reconstitution of Na,K-ATPase: enzyme kinetic and biophysical characterization. Biophys Rev. 12 (1), 49-64 (2020).
- Simidjiev, I., et al. Self-assembly of large, ordered lamellae from non-bilayer lipids and integral membrane proteins in vitro. Proc Natl Acad Sci. 97 (4), 1473-1476 (2000).
- Harris, N. J., Booth, P. J. Folding and stability of membrane transport proteins in vitro. Biochim Biophys Acta BBA - Biomembr. 1818 (4), 1055-1066 (2012).
- Jackson, M. L., Litman, B. J. Rhodopsin-egg phosphatidylcholine reconstitution by an octyl glucoside dilution procedure. Biochim Biophys Acta BBA - Biomembr. 812 (2), 369-376 (1985).
- Geertsma, E. R., Nik Mahmood, N. A. B., Schuurman-Wolters, G. K., Poolman, B. Membrane reconstitution of ABC transporters and assays of translocator function. Nat Protoc. 3 (2), 256-266 (2008).
- Rigaud, J. -. L., Pitard, B., Levy, D. Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim Biophys Acta BBA - Bioenerg. 1231 (3), 223-246 (1995).
- Szoka, F., Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci. 75 (9), 4194-4198 (1978).
- . Synthetic Organelles for Energy Conservation and Delivery of Building Blocks for Lipid Biosynthesis Available from: https://www.researchsquare.com/article/rs-3385355/v1 (2023)
- Lee, K. Y., et al. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system. Nat Biotechnol. 36 (6), 530-535 (2018).
- Méléard, P., Bagatolli, L. A., Pott, T. Giant unilamellar vesicle electroformation. Methods in Enzymology. , 161-176 (2009).
- Garten, M., Aimon, S., Bassereau, P., Toombes, G. E. S. Reconstitution of a transmembrane protein, the voltage-gated ion channel, KvAP, into giant unilamellar vesicles for microscopy and patch clamp studies. J. Vis. Exp. (95), e52281 (2015).
- Doeven, M. K., et al. lateral mobility and function of membrane proteins incorporated into giant unilamellar vesicles. Biophys J. 88 (2), 1134-1142 (2005).
- Pols, T., et al. A synthetic metabolic network for physicochemical homeostasis. Nat Commun. 10 (1), 4239 (2019).
- Bailoni, E., Poolman, B. ATP recycling fuels sustainable glycerol 3-phosphate formation in synthetic cells fed by dynamic dialysis. ACS Synth Biol. 11 (7), 2348-2360 (2022).
- Van Der Heide, T. On the osmotic signal and osmosensing mechanism of an ABC transport system for glycine betaine. EMBO J. 20 (24), 7022-7032 (2001).
- Van'T Klooster, J. S., et al. Membrane lipid requirements of the lysine transporter Lyp1 from Saccharomyces cerevisiae. J Mol Biol. 432 (14), 4023-4031 (2020).
- Lou, G., Anderluzzi, G., Woods, S., Roberts, C. W., Perrie, Y. A novel microfluidic-based approach to formulate size-tuneable large unilamellar cationic liposomes: Formulation, cellular uptake and biodistribution investigations. Eur J Pharm Biopharm. 143, 51-60 (2019).
- Weiss, M., et al. Sequential bottom-up assembly of mechanically stabilized synthetic cells by microfluidics. Nat Mater. 17 (1), 89-96 (2018).
- Pols, T., Singh, S., Deelman-Driessen, C., Gaastra, B. F., Poolman, B. Enzymology of the pathway for ATP production by arginine breakdown. FEBS J. 288 (1), 293-309 (2021).
- Yandrapalli, N., Robinson, T. Ultra-high capacity microfluidic trapping of giant vesicles for high-throughput membrane studies. Lab Chip. 19 (4), 626-633 (2019).
- Elias, M., et al. Microfluidic characterization of biomimetic membrane mechanics with an on-chip micropipette. Micro Nano Eng. 8, 100064 (2020).
- Robinson, T., Kuhn, P., Eyer, K., Dittrich, P. S. Microfluidic trapping of giant unilamellar vesicles to study transport through a membrane pore. Biomicrofluidics. 7 (4), 044105 (2013).
- Cooper, A., Girish, V., Subramaniam, A. B. Osmotic Pressure Enables High-Yield Assembly of Giant Vesicles in Solutions of Physiological Ionic Strengths. Langmuir. 39 (15), 5579-5590 (2023).
- Tantama, M., Martínez-François, J. R., Mongeon, R., Yellen, G. Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat Commun. 4 (1), 2550 (2013).
- Setyawati, I., et al. In vitro reconstitution of dynamically interacting integral membrane subunits of energy-coupling factor transporters. eLife. 9, e64389 (2020).
- Oropeza-Guzman, E., Ríos-Ramírez, M., Ruiz-Suárez, J. C. Leveraging the coffee ring effect for a defect-free electroformation of giant unilamellar vesicles. Langmuir. 35 (50), 16528-16535 (2019).
- Estes, D. J., Mayer, M. Electroformation of giant liposomes from spin-coated films of lipids. Colloids Surf B Biointerfaces. 42 (2), 115-123 (2005).
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoThis article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados