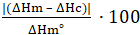Calorimétrie différentielle à balayage
Vue d'ensemble
Source : Danielle N. Beatty et Taylor D. Sparks, Department of Materials Science and Engineering, The University of Utah, Salt Lake City, UT
La calorimétrie différentielle de balayage (DSC) est une mesure importante pour caractériser des propriétés thermiques des matériaux. DSC est utilisé principalement pour calculer la quantité de chaleur stockée dans un matériau pendant qu'il chauffe (capacité de chaleur) ainsi que la chaleur absorbée ou libérée lors de réactions chimiques ou de changements de phase. Cependant, la mesure de cette chaleur peut également conduire au calcul d'autres propriétés importantes telles que la température de transition vitreuse, la cristallinité des polymères, et plus encore.
En raison de la nature longue et en chaîne des polymères, il n'est pas rare que des brins de polymère soient enchevêtrés et désordonnés. En conséquence, la plupart des polymères ne sont que partiellement cristallins, le reste du polymère étant amorphe. Dans cette expérience, nous utiliserons DSC pour déterminer la cristallinité des polymères.
Procédure
- Allumez la machine et laissez-la se réchauffer pendant environ une heure.
- Vérifiez que le réservoir d'azote comprimé et le réservoir d'azote liquide sont à la fois pleins et que la valve qui les relie est ouverte. Le débit de pression d'azote comprimé est réglé à 10 psi par les boutons de réglage sur le régulateur.
- Préparer deux casseroles vides. Poke un petit trou dans le couvercle de chacun et sceller à l'aide de la presse à sertire. Retirez les trois couvercles du four et placez les casseroles sur les deux capteur
Résultats
La figure 3 montre le résultat d'un balayage de l'échantillon de cristallinité pour cent de DSC sur un échantillon de polybutylène téraphtalate (PBT). Le résultat est affiché sous la forme d'une lecture de puissance DSC (en milliwatts par milligramme d'échantillon) versets temps. La lecture de puissance, la trace bleue de la figure 3,indique la puissance supplémentaire nécessaire pour modifier la température de la cas...
Applications et Résumé
La calorimétrie différentielle est une technique utilisée pour déterminer de nombreuses propriétés thermiques des matériaux, telles que la chaleur de la fonte, la chaleur de la cristallisation, la capacité thermique et les changements de phase. Les mesures DSC peuvent également être utilisées pour calculer des propriétés matérielles supplémentaires, y compris la température de transition vitreuse et la cristallinité pour cent de polymère. Le DSC nécessite de très petits échantillons qui doivent se co...
Passer à...
Vidéos de cette collection:

Now Playing
Calorimétrie différentielle à balayage
Materials Engineering
37.4K Vues

Matérialographie optique I : Préparation de l'échantillon
Materials Engineering
15.4K Vues

Matérialographie optique II : Analyse d'image
Materials Engineering
11.0K Vues

Spectroscopie photoélectronique à rayons X
Materials Engineering
21.5K Vues

Diffraction des rayons X
Materials Engineering
88.8K Vues

Faisceaux d'ions focalisés
Materials Engineering
8.8K Vues

Solidification directionnelle et stabilisation de phase
Materials Engineering
6.5K Vues

Diffusivité thermique et méthode du flash laser
Materials Engineering
13.2K Vues

Dépôt électrolytique sur films minces
Materials Engineering
20.0K Vues

Analyse de la dilatation thermique par dilatométrie
Materials Engineering
15.7K Vues

Spectroscopie d'impédance électrochimique
Materials Engineering
23.1K Vues

Matériaux composites à matrice céramique et leurs propriétés de flexion
Materials Engineering
8.1K Vues

Alliages nanocristallins et stabilité de la taille des nano-grains
Materials Engineering
5.1K Vues

Synthèse des hydrogels
Materials Engineering
23.7K Vues