Reazione di accoppiamento catalizzata da palladio
Panoramica
Fonte: Vy M. Dong e Faben Cruz, Dipartimento di Chimica, Università della California, Irvine, CA
Questo esperimento dimostrerà il concetto di accoppiamento incrociato catalizzato dal palladio. Verrà illustrato il set-up di una tipica reazione di accoppiamento incrociato catalizzato pd. Le reazioni di accoppiamento incrociato catalizzato da Pd hanno avuto un profondo effetto sul modo in cui i chimici sintetici creano molecole. Queste reazioni hanno permesso ai chimici di costruire legami in modi nuovi e più efficienti. Tali reazioni hanno trovato applicazioni diffuse nell'industria chimica fine e farmaceutica. Le reazioni di accoppiamento incrociato catalizzato pd aggiungono un altro strumento alla cassetta degli attrezzi del chimico per la costruzione di legami carbonio-carbonio, che sono fondamentali per la chimica organica. La combinazione dell'importanza di creare legami carbonio-carbonio e l'impatto dell'accoppiamento incrociato catalizzato da Pd hanno portato queste reazioni ad essere oggetto del Premio Nobel per la Chimica 2010. Ei-ichi Negishi, uno dei destinatari del Premio Nobel per la chimica 2010, ha spiegato nella sua conferenza Nobel che una delle sue motivazioni per lo sviluppo di questa chimica era quella di sviluppare "metodi simili a Lego semplici ampiamente applicabili per collegare due diversi gruppi organici".
Procedura
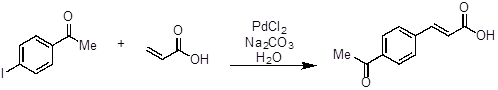
- Aggiungere 4-iodoacetofenone (246 mg, 1 equivalente, 1 mmol), acido acrilico (100 μL, 1,5 equivalenti, 1,5 mmol), carbonatodi sodio(Na 2 CO3, 318 mg, 3 equivalenti, 3 mmol), PdCl2 (2 mg, 0,01 equivalenti, 0,01 mmol) e acqua (5 mL, 0,2 M) a un pallone a fondo rotondo (~ 20 mL) dotato di una barra magnetica.
- Riscaldare la reazione a circa 100 °C e mescolare fino al consumo completo di 4-iodoacetofenone (ci
Risultati
Il prodotto deve essere un solido con il seguito spettro NMR 1H: 1H NMR (400 MHz, DMSO-d6):δ (ppm) 2,60 (s, 3H), 6,67 (d, J = 16,0 Hz, 1H), 7,65 (d, J = 16,0 Hz, 1H). 7,83 (d, J = 8,4 Hz, 2H). 7,97 (d, J = 8,4 Hz, 2H).
Applicazione e Riepilogo
Queste reazioni di accoppiamento incrociato catalizzate dal Pd hanno cambiato il modo in cui le molecole vengono sintetizzate in contesti accademici e industriali. L'impatto di questa tecnologia può essere visto nel modo in cui i chimici costruiscono strutture complesse per prodotti farmaceutici, prodotti chimici agricoli e materiali. Oltre agli accoppiamenti incrociati catalizzato da Pd, la catalisi dei metalli di transizione ha cambiato (e continua a cambiare) il modo in cui i chimici sintetici preparano molecole che .
Vai a...
Video da questa raccolta:

Now Playing
Reazione di accoppiamento catalizzata da palladio
Organic Chemistry II
34.3K Visualizzazioni

Pulizia della vetreria
Organic Chemistry II
123.5K Visualizzazioni

Sostituzione nucleofila
Organic Chemistry II
99.5K Visualizzazioni

Agenti riducenti
Organic Chemistry II
43.0K Visualizzazioni

Reazione di Grignard
Organic Chemistry II
149.0K Visualizzazioni

Titolazione di n-butillitio
Organic Chemistry II
47.7K Visualizzazioni

Dispositivo di Dean Stark
Organic Chemistry II
100.2K Visualizzazioni

Ozonolisi degli alcheni
Organic Chemistry II
67.0K Visualizzazioni

Organocatalisi
Organic Chemistry II
16.6K Visualizzazioni

Sintesi in fase solida
Organic Chemistry II
41.0K Visualizzazioni

Idrogenazione
Organic Chemistry II
49.6K Visualizzazioni

Polimerizzazione
Organic Chemistry II
93.8K Visualizzazioni

Punto di fusione
Organic Chemistry II
149.8K Visualizzazioni

Spettroscopia infrarossa
Organic Chemistry II
214.6K Visualizzazioni

Polarimetro
Organic Chemistry II
99.9K Visualizzazioni