PH メーターを使用してください。
概要
博士忠祺彼 - アメリカ合衆国農務省のソース: 研究室
酸と塩基は、プロトン (H+) と水酸化物イオン (オハイオ州-) をそれぞれ寄付することができる物質です。彼らは化学物質を記述する 2 つの極端が。酸と塩基を混合することができますアウトをキャンセルしたり、極端な効果を中和します。酸性でも基本的な物質は、中立です。ほとんどのソリューションのためのプロトンの濃度 ([H+]) の値が不便な小さなと比較することは困難より実用的な量、pH、導入されています。pH はもともと陽子のモル濃度の逆数の常用対数として定義された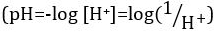 、水素イオン活量の逆数の常用対数に更新されましたが、
、水素イオン活量の逆数の常用対数に更新されましたが、 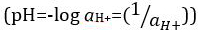 。前の定義は、今、時折 p [H] として表されます。P [H] と pH の違いは非常に小さいです。それはずっとその pH を記載 = p [H] + 0.04。それは測定の両方のタイプの 'pH' という用語を使用するの一般的です。
。前の定義は、今、時折 p [H] として表されます。P [H] と pH の違いは非常に小さいです。それはずっとその pH を記載 = p [H] + 0.04。それは測定の両方のタイプの 'pH' という用語を使用するの一般的です。
PH は 0 から 14 の範囲でスケール通常。強い酸の 1 M 溶液の pH = 0 と強力なベース、pH の 1 M 溶液 = 14。したがって、その範囲外の値が完全に可能、測定された pH 値は範囲 0 に 14 に大抵あるでしょう。純粋な水は pH が中性 = 7。PH は 7 未満は酸性と pH が 7 以上が基本。PH のスケールは対数、pH は無次元量であります。7 以下各全体の pH 値は、10 x より次の整数よりも酸性です。たとえば、4 の pH は 10 x より 5 から 6 の pH よりもより酸性 (10 x 10) x 100 の pH よりも酸性です。7 より基本的な x 10 は、それぞれの上記の pH 値の true (またはアルカリ) を保持する同じ次の下限の全体値より。たとえば、10 の pH は 9 の pH よりもより基本的な x 10 です。
手順
1. pH 校正
- 「パワー」ボタンを押すとメーターの電源を入れます。
- それはおよび/または電極とではない場合は、自動温度補償 (ATC) プローブを取り付けます。
- 測定モードが pH であることを確認します。ない場合は、「モード」ボタンを押して"pH"モードは、液晶ディスプレイに表示されます。
- 必要な場合はヘルプのメーターの近くに下部のクイック リファレンス ガイドを参照してください。
- 常に校正用新鮮、未使用で有効期限内の pH のバッファーを使用します。バッファーは、テスト ソリューションとして同じ温度にする必要があります。
- 蒸留水とし、校正 (すなわち、pH 7.00) に使用されているバッファーの pH 電極をすすいでください。
- PH 電極を中性 pH のバッファー (すなわち、pH 7.00) に浸し。磁気バーを使用してバッファーをかき混ぜる (~ 30 の緩やかなペースで s) 最良の結果のため。
- 「CAL/調べた」を押します (
結果
申請書と概要
スキップ先...
このコレクションのビデオ:

Now Playing
PH メーターを使用してください。
General Chemistry
346.6K 閲覧数

共通の実験室ガラス製品と用途
General Chemistry
657.4K 閲覧数

・濃度
General Chemistry
274.7K 閲覧数

固体と液体の密度を決定します。
General Chemistry
556.5K 閲覧数

水溶液の質量パーセントの組成を決定します。
General Chemistry
383.7K 閲覧数

経験式を決定します。
General Chemistry
183.0K 閲覧数

イオン性化合物の溶解度ルールの決定
General Chemistry
141.5K 閲覧数

滴定の概要
General Chemistry
425.1K 閲覧数

理想気体法律
General Chemistry
78.6K 閲覧数

平衡定数の吸光光度定量
General Chemistry
158.6K 閲覧数

ル Châtelier の原理
General Chemistry
265.7K 閲覧数

未知の化合物を決定するための凝固点降下
General Chemistry
160.7K 閲覧数

率の法律および反作用の順序を決定します。
General Chemistry
196.2K 閲覧数

エンタルピーの示差走査熱量測定の変更を使用してください。
General Chemistry
44.5K 閲覧数

錯体化学
General Chemistry
91.6K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved