Method Article
Human Neutrophil Extracellular Trap release의 실시간 고처리량 현미경 정량화 및 길항제의 약리학 평가
요약
이 정의된 프로토콜은 in vitro에서 인간 호중구 세포외 트랩(NET) 방출을 시각화하고 정량화하기 위한 실시간 고처리량 현미경 접근 방식을 설명합니다. 재현 가능한 방법을 통해 뚜렷한 NETosis 유도 인자를 자극 시 NET 방출의 특성과 동역학을 조사할 수 있으며 NETosis 길항제의 약리학을 평가할 수 있습니다.
초록
호중구는 NETosis라고 하는 과정에서 호중구 세포외 트랩(NET)을 방출하는 것을 포함하여 여러 전략을 사용하여 선천성 면역 방어에 중요한 역할을 합니다. 그러나 지난 20년 동안 조직에 NET이 축적되는 것이 여러 염증성 및 자가면역 질환의 병태생리학에 기여한다는 것이 분명해졌습니다. 따라서 NETosis 길항제 개발에 대한 관심이 높아졌습니다. NETosis를 탐지하고 분석하기 위한 가변적이고 표준화되지 않은 방법이 동시에 개발되었으며, 각각 고유한 장점과 한계가 있습니다. 여기에서는 인간 NET 방출의 정량화를 위한 실시간 현미경 방법을 설명하여 고처리량 방식으로 NETosis와 NET 억제를 연구할 수 있습니다. 표면적 기반 반자동 분석은 NET을 인식하고 이를 non-netting 활성화 호중구와 구별합니다. 우리는 비생리학적 NETosis 유도제인 칼슘 이오노포어와 phorbol-12-myristate-13-acetate (PMA)가 서로 다른 특성과 역학을 가진 NET의 방출을 유발한다는 것을 입증합니다. 또한, 우리는 이 접근 방식을 통해 면역 복합체, N-Formylmethionine-leucyl-phenylalanine(fMLF), 요산나트륨 결정 및 피로인산칼슘 결정을 포함한 질병 관련 자극에 대한 반응으로 NET 방출을 연구할 수 있음을 보여줍니다. NETosis 길항제 연구를 위한 이 방법의 유용성을 예시하기 위해 NET 방출의 first-in-class 단클론 항체 억제제인 CIT-013을 사용했습니다. CIT-013은 시트룰린 화 히스톤 H2A 및 H4를 표적으로 하며 4.6nM의 IC50 으로 NET 방출을 효율적으로 억제합니다. 테스트된 다른 항히스톤 항체는 이러한 NETosis-억제 능력이 부족했습니다. 전체적으로 우리는 이 프로토콜이 NET의 특이적이고 신뢰할 수 있으며 재현 가능한 고처리량 정량화를 가능하게 하여 NETosis 길항제의 NET 방출 특성, 역학 및 약리학에 대한 연구를 향상시킨다는 것을 입증합니다.
서문
호중구는 혈액에 풍부하게 존재하며 감염이나 염증이 발생하면 조직으로 이동합니다. 그들은 미생물로부터 숙주를 보호하기 위해 광범위한 무기를 사용하여 선천성 면역 방어에 중요한 역할을 합니다. 호중구는 식세포작용(phagocytosis), 탈과립화(degranulation), 반응성 산소종(ROS) 생성, NETosis1이라는 과정에 의한 호중구 세포외 트랩(NET)이라고 하는 응축된 염색질의 방출을 통해 병원체를 죽입니다. NET은 무엇보다도 과립 단백질과 칼프로텍틴(calprotectin)으로 장식된 염색질의 세포외 구조이며, 2,3 , 광범위한 분자4로 자극을 받으면 방출됩니다. NETosis는 크게 두 가지 주요 경로로 분류할 수 있습니다: NADPH 산화효소 의존성또는 독립 5,6,7. 또한, 펩티딜 아르기닌 데이미나제 4(PAD4)에 의한 N-말단 히스톤 꼬리의 아르기닌 시트룰린화는 NETosis와 밀접한 관련이 있으며 염색질 축합을 촉진하여 궁극적으로 응축된 염색질을 세포 외 환경으로 배출합니다.
NET 방출은 병원균 제거에 관여하지만, 수많은 연구에서 비정상적이고 장기간의 NET 방출이 급성 폐 손상8, 류마티스 관절염(RA)9, 혈관염10 및 화농 한선염11을 포함한 다양한 염증성 질환의 발병과 관련이 있음을 보여주었습니다. NET은 전염증성이고, 자가항원의 원천이며, 주변 조직에 세포독성을 일으키고, 면역혈전증을 유발하고, 파골세포 분화 및 뼈 미란을 촉진하기 때문에 질병에서 NET의 해로운 역할은 다면적입니다 9,12,13. 소분자 PAD4 억제제에 의한 NETosis 경로의 약리학적 억제는 NETosis를 표적으로 하는 치료제가 NET 축적이 발병의 중요한 동인인 질병의 치료법으로서 잠재력을 가지고 있음을 보여줍니다14. PAD4 효소를 표적으로 하는 대신, 시트룰린 화 히스톤 H2A 및 H415에 특이적으로 결합하는 인간화 항시트룰린 히스톤 단클론 항체인 CIT-013을 억제하는 동급 최초의 NETosis를 사용했습니다. CIT-013은 NET 방출을 억제하고 대식세포 매개 NET 식세포작용을 강화함으로써 독특한 이중 작용 기전을 가지고 있습니다16. CIT-013 및 전구체 분자는 NET 관련 염증의 여러 마우스 모델에서 치료 효능을 보여주었습니다17.
NET 방출을 연구하기 위해 수년에 걸쳐 다양한 방법이 개발되었는데, 예를 들면 1) 면역형광 플레이트 리더와 함께 원형질막 불투과성 DNA 추적자를 사용한 DNA 검출, 2) 상층액에서 NET 특이적 단백질과 복합체를 이루는 DNA 및 DNA의 ELISA(enzyme-linked immunosorbent assay) 기반 검출, 3) 면역조직화학을 통한 NET 관련 분자와 세포외 DNA의 공동 국소화, 4) 그물 호중구를 검출하기 위한 유세포 분석 접근법. 이러한 모든 방법에는 고유한 장점과 한계가 있습니다. 우리는 원형질막 불투과성 DNA 염료16,18을 사용하여 인간 NET 방출의 현미경 정량화를 위한 실시간 고처리량 접근 방식을 개발했습니다. 설명된 방법을 사용하면 쉽고 신뢰할 수 있으며 재현 가능한 방식으로 NETosis 역학 및 특성을 조사할 수 있으며 CIT-013과 같은 NETosis 길항제의 약리학을 평가할 수 있습니다.
프로토콜
모든 헌혈자는 헬싱키 선언에 따라 정보에 입각한 동의를 했으며, 연구는 인간 연구를 위한 시트릴 윤리 지침에 따라 수행되었습니다.
참고: 인간의 혈액 및 분리된 호중구를 사용한 모든 활동은 층류 캐비닛에서 멸균 상태에서 수행해야 합니다. 원심분리를 위한 브레이크 및 가속 설정이 프로토콜에 언급되어 있지 않은 경우 최대로 간주될 수 있습니다.
1. 혈액으로부터의 호중구 분리
- 건강한 지원자의 말초 혈액을 리튬-헤파린 튜브에 채취하여 새로운 50mL 튜브로 옮깁니다.
- 1x DPBS로 리튬-헤파린 튜브를 헹구고 혈액을 옮긴 것과 동일한 50mL 튜브로 옮깁니다. 최종 혈액 대 DPBS 비율이 1:1인지 확인합니다. 피펫팅을 통해 혈액과 DPBS를 혼합하여 균일한 용액을 얻을 수 있습니다.
- 13mL의 밀도 구배 용액을 다른 새 50mL 튜브에 추가하고 밀도 구배 용액 위에 1:1 희석된 혈액을 최대 50mL까지 천천히 추가합니다(25mL 피펫 사용).
- 400 x g 및 실온(RT)에서 40분 동안 최소한의 가속과 브레이크로 원심분리기.
참고: 레이어가 형성됩니다. 위에서 아래로 층은 다음과 같습니다 : 1. 플라즈마; 2. 말초 혈액 단핵 세포(PBMC); 3. 밀도 구배 용액; 4. 적혈구/호중구. - 먼저 10mL 피펫으로 혈장을 버린 다음 적혈구/호중구층을 방해하지 않고 플라스틱 파스퇴르 피펫으로 PBMC와 밀도 구배 용액 층을 최대한 폐기합니다.
- 적혈구/호중구층을 흔들어 부드럽게 재현탁하고 1x DPBS 15mL에 25mL 피펫으로 재현탁합니다.
- 6% Dextran/0.9% NaCl 용액 25mL를 넣고 튜브를 10회 뒤집어 혼합한 다음 상온에서 25분 동안 튜브를 똑바로 세웁니다.
참고: 레이어가 형성됩니다. 위에서 아래로 층은 다음과 같습니다 : 1. 호중구; 2 개의 적혈구. - 10mL 피펫과 원심분리를 사용하여 호중구층을 새 50mL 튜브로 500 x g 및 RT에서 10분 동안 이동합니다.
알림: 호중구 펠릿에는 여전히 약간의 적혈구가 포함되어 있고 튜브에 단단히 부착되어 있지 않으므로 주의하십시오. - 상등액을 디캔팅하여 폐기하고 10mL의 ACK(Ammonium-Chloride-Potassium) 용해 완충액(155mMNH4Cl, 10mMKHCO3 및 0.1mM Na2EDTA; pH = 7.2)에 10mL 피펫으로 세포 펠릿을 재현탁한 다음 즉시 40mL의 ACK 용해 완충액을 추가합니다.
- 용액이 반투명해질 때까지 튜브를 지속적으로 뒤집으면서 RT에서 배양하고(1-5분 소요, 각 기증자마다 다를 수 있음) 350 x g 및 RT에서 10분 동안 원심분리합니다.
- 상등액을 제거하고 호중구를 현탁액으로 만들지 않고 호중구 펠렛 위에 10%(v/v) 열 비활성화 소 태아 혈청(FBS), 50 U/mL 페니실린 및 50 μg/mL 스트렙토마이신(이하 배양 배지 10%)이 보충된 L-글루타민이 포함된 배양 배지 5mL를 천천히 적가합니다. 이렇게 하면 호중구 펠릿에서 대부분의 적혈구를 효율적으로 제거할 수 있습니다.
- 호중구 펠릿 위에 존재하는 대부분의 적혈구가 배양 배지에 10% 재현탁될 때까지 50mL 튜브를 부드럽게 소용돌이칩니다. 그런 다음 튜브를 디캔팅하여 상층액을 제거합니다.
- 호중구 펠릿을 10mL의 배양 배지 10%에 재현탁하고, 완전히 재현탁되면 최대 50mL의 배양 배지 10%를 추가합니다. 350 x g 및 RT에서 10분 동안 원심분리기.
- 상등액을 제거하고 세포 펠렛을 10mL의 배양 배지 10%에 재현탁합니다.
2. 유세포 분석으로 순도를 확인하기 위한 호중구 염색
- 세포 농도를 측정하려면 호중구 현탁액을 0.4% 트리판 블루 용액(비율 1:1)으로 희석하고 명시야 세포 계수기로 호중구를 계수합니다.
참고: 다른 계산 방법도 사용할 수 있습니다. - 배양 배지 10%에 있는 1 x 105 호중구를 V-bottom 96-well plate의 well로 옮기고 400 x g 및 RT에서 3분 동안 원심분리합니다.
- 상등액을 버리고 Fc 수용체 블록(50배 희석)을 포함하는 1%(w/v) 소 혈청 알부민(BSA) 및 0.1%(v/v) NaN3 (이하 형광 활성화 세포 분류(FACS) 버퍼라고 함)가 보충된 50μL의 1x DPBS에 세포를 재현탁합니다.
- RT에서 15분 동안 배양하고 50x 희석된 APC-Cy7 결합 마우스 항-human CD45 항체, 600x 희석된 PerCP-Cy5.5-conjugated 마우스 항-human CD16 항체, 133x 희석된 FITC-conjugated mouse anti-human CD66b 항체 및 500x 희석된 고정성 생존도 염료 eFluor 506을 포함하는 50μL의 FACS buffer를 추가합니다.
참고: CD45는 백혈구에서 발현됩니다. CD66b는 과립구에서만 독점적으로 발현됩니다. CD16은 호중구에서 많이 발현되고, 호산구에서는 낮게 발현되며, 호염기구에서는 발현되지 않습니다. - 어둠 속에서 RT에서 30분 동안 배양하고 400 x g 및 RT에서 3분 동안 원심분리합니다.
- 상층액을 버리고 175μL의 FACS 완충액에 호중구를 재현탁한 후 400 x g 및 RT에서 3분 동안 원심분리합니다.이 단계를 한 번 반복합니다.
- 상층액을 버리고 호중구 펠릿을 175μL의 FACS 완충액에 재현탁합니다. 유세포 분석 시스템 및 관련 소프트웨어를 사용하여 시료를 분석합니다.
3. 유세포 분석 소프트웨어를 사용한 호중구 순도 분석
참고: 유세포 분석 데이터 분석은 Table of Materials에 표시된 대로 유세포 분석 소프트웨어를 사용하여 수행되었습니다.
- 다음 단계별 절차에 따라 게이팅을 수행합니다.
- FSC-A 대 시간 플롯에서 시간 게이트를 설정하여 적절한 세포 흐름이 있는 영역을 선택합니다.
- SSC-A 대 FSC-A 플롯에서 셀 게이트를 설정하여 셀을 선택하고 파편을 제외합니다.
- FSC-A 대 AmCyan-A 플롯에서 사용 가능한 세포 게이트를 설정하여 사용 가능한 세포를 선택합니다.
- 플롯 FSC-A 대 APC-Cy7에서 백혈구 게이트를 설정하여 CD45+ 세포를 선택합니다.
- CD66b+CD16+(호중구), CD66b-CD16+(호산구) 및 CD66b-CD16-(호염구, 단핵구 및 림프구)를 구별하기 위해 플롯 FITC 대 PerCP-Cy5.5 에서 호중구 게이트를 설정합니다.
- 단일 호중구를 선택하기 위해 플롯 FSC-H 대 FSC-A 에서 단일 세포 게이트를 설정합니다.
- 단일 호중구를 세포의 빈도(cell gate)로 제시하여 호중구 순도를 결정합니다.
참고: 계속 진행하려면 호중구 순도가 85%를 초과해야 합니다.
4. 라이브 이미징 현미경 검사
참고: 이 분석은 여러 개의 96-well 이미징 플레이트와 다양한 NETosis 자극 및 길항제에 최적화되어 있습니다. 아래 프로토콜은 포함된 테이블을 사용하여 지정할 수 있는 접근 방식의 일반적인 보기를 설명합니다.
- 0.01% 폴리-L-라이신을 멸균 H2O에 1:10의 비율로 희석하여 0.001% 폴리-L-라이신 용액을 준비합니다.
- 각 웰에 0.001% 폴리-L-라이신 용액을 추가하고 37°C에서 최소 1시간 동안 배양합니다.
참고: 웰당 부피는 96웰 이미징 플레이트에 따라 다릅니다(표 1). - 200μL의 DPBS로 웰을 3회 세척하여 과도한 폴리-L-라이신을 제거합니다. 층류 캐비닛에서 모든 단계를 수행합니다. 플레이트의 뚜껑을 제거하고 마를 때까지(약 1시간) 층류 캐비닛의 플레이트를 열어 웰을 자연 건조합니다.
- 실험에 필요한 호중구의 수를 계산하고 잉여분을 15mL 튜브로 옮깁니다. 350 x g 및 RT에서 10분 동안 원심분리기.
참고: 각 웰에 있는 호중구의 양은 96웰 이미징 플레이트의 유형에 따라 다릅니다(표 1). 세포 밀도는 적절한 분석에 필요한 개별 세포를 잘 분리할 수 있도록 최적화되어 있습니다. 호중구 밀도가 너무 높으면 세포와 NET이 인접한 세포 및 NET과 겹쳐 분석 품질에 영향을 미칩니다. - 상층액을 버리고 2%(v/v) FBS, 50 U/mL 페니실린 및 50 μg/mL 스트렙토마이신, 10 mM HEPES 및 1 mM CaCl2 (이하 NET 분석 버퍼라고 함)가 보충된 페놀 레드가 없는 배양 배지에 호중구를 재현탁합니다.
- 다음 작업 솔루션을 준비합니다.
- NET 분석 버퍼에서 80nM DNA 염료를 준비합니다.
- NET 분석 버퍼에서 NETosis 자극을 준비합니다.
- NET 분석 버퍼에서 NETosis 길항제를 준비합니다.
알림: 위에서 설명한 작업 용액의 농도는 우물에 필요한 최종 농도의 4배입니다. NETosis 자극과 NETosis 길항제의 권장 농도는 다양하다(표 2 및 표 3).
- NET 분석 버퍼의 4x 농축 DNA 염료를 각 웰에 추가합니다.
- NET 분석 완충액의 4배 농축된 NETosis 자극을 해당 웰에 추가하고, 자극이 없는 웰 또는 코팅된 면역 복합체(cIC, 표 2의 프로토콜)를 포함하는 웰에만 NET 분석 완충액을 추가합니다.
- NET 분석 버퍼의 4x 농축 NETosis 길항제를 해당 웰에 추가합니다. NET 분석 버퍼는 길항작용제가 없는 웰에만 추가합니다.
- 각 웰에 호중구 현탁액을 추가합니다.
참고: NET 분석 완충액에서 4배 농축된 DNA 염료의 부피, NET 분석 완충액에서 4배 농축된 NETosis 자극, NET 분석 완충액에서 4배 농축된 NETosis 길항제, 호중구의 수 및 웰당 호중구 현탁액의 부피는 96웰 이미징 플레이트에 따라 다릅니다(표 1). 피펫팅할 때 기포가 형성되지 않도록 하십시오. - 100 x g 및 RT에서 2분 동안 플레이트를 원심분리하고 37°C 및 5% CO2의 인큐베이터에 배치된 라이브 셀 현미경 분석 시스템에 96웰 이미징 플레이트를 삽입합니다.
참고: 배양 첫 1분 동안 96웰 이미징 플레이트의 바닥에 응결이 발생할 수 있습니다. 이것은 티슈로 제거해야 합니다.
5. 획득을 위한 라이브 셀 현미경 분석 시스템 소프트웨어 설정
참고: 위상차 및 면역형광 이미지는 분석 소프트웨어로 제어되는 라이브 셀 현미경 분석 시스템에 의해 획득되었습니다.
- 라이브 셀 현미경 분석 시스템 소프트웨어를 열고 장치에 연결을 클릭합니다. 사용자 이름과 비밀번호를 입력합니다. Schedule을 클릭하여 획득하고 더하기 버튼(Launch add vessel)을 클릭합니다. 그런 다음 Scan on Schedule(일정에 따라 스캔)을 선택하고 Next(다음)를 클릭합니다.
- 처음부터 새 선박을 생성하려면 Create Vessel 섹션에서 New를 선택하고 Next를 클릭합니다. Scan Type(스캔 유형) 섹션에서 Standard(표준)를 선택하고 Next(다음)를 클릭합니다.
- 다음 스캔 설정을 선택하고 다음을 클릭합니다: 셀별: 없음; 이미지 채널: 위상차 및 녹색 (획득 시간 100ms); 목표: 20x.
- Vessel Selection(용기 선택) 섹션에서 스캔할 적절한 선박 유형을 선택하고 Next(다음)를 클릭합니다.
- 96웰 플레이트 #1을 사용하는 경우 Corning, plate, 96, N/A, 3603, 96웰 Corning(Blk/Wht), 마이크로플레이트를 선택합니다.
- 96웰 플레이트 #2를 사용하는 경우 Nunc, 플레이트, 96, N/A, 152028, 96웰 Nunc opt bottom(Blk/Wht), Microplates를 선택합니다.
- Drawer(서랍)에서 용기의 위치를 지정하고 Next(다음)를 클릭합니다. Scan Pattern(스캔 패턴) 섹션에서 스캔해야 하는 웰을 선택하고, 웰당 이미지 수를 선택하고(대표적인 개요를 얻기 위해 웰당 4개의 이미지를 스캔함) Next(다음)를 클릭합니다.
알림: 자동 초점에 영향을 미치므로 빈 웰을 선택하지 마십시오. - vessel notebook 섹션에 플레이트 이름을 삽입하여 vessel에 대한 정보를 제공하고 Next(다음)를 클릭합니다. Analysis Setup(분석 설정) 섹션에서 Defer analysis until later up(나중까지 분석 연기)을 선택하고 Next(다음)를 클릭합니다.
- Scan Schedule(스캔 일정) 섹션에서 선박에 대한 검사 일정을 정의합니다. 다음 간격으로 검사를 사용하여 새 일정 만들기를 선택하고 1시간을 선택합니다. 검색 중지 및 첫 번째 검색 후 00:05시간을 선택합니다. 다음(Next)을 클릭하고 스캔 정보가 올바르면 일정에 추가(Add to Schedule)를 클릭합니다.
- 스캔 일정을 편집해야 하는 경우 화면 상단의 스캔 일정을 두 번 클릭합니다. Scanning Schedule(스캔 일정 )을 마우스 오른쪽 버튼으로 클릭하고 원하는 대로 편집 및 조정합니다. Floppy disk(플로피 디스크 ) 아이콘을 클릭하여 스캔 일정을 저장합니다.
참고: 동일한 실험에서 여러 용기(플레이트)를 이미징하는 경우 더하기 버튼(Launch add vessel)을 클릭하고 Scan on Schedule을 선택한 후 Next를 클릭하여 기존 스캔 일정에 추가 vessel을 추가할 수 있습니다. 처음부터 새 vessel을 생성하거나(이 프로토콜의 5.2단계 참조) 기존 vessel을 복사하거나 이전에 스캔한 vessel을 사용합니다. 그 결과 이전 플레이트와 동일한 빈도와 설정에서 순차적 스캔이 가능합니다.
- 스캔 일정을 편집해야 하는 경우 화면 상단의 스캔 일정을 두 번 클릭합니다. Scanning Schedule(스캔 일정 )을 마우스 오른쪽 버튼으로 클릭하고 원하는 대로 편집 및 조정합니다. Floppy disk(플로피 디스크 ) 아이콘을 클릭하여 스캔 일정을 저장합니다.
6. 라이브 이미징 현미경 NET 분석 라이브 셀 현미경 분석 소프트웨어를 사용한 분석
참고: 생체 세포 현미경 분석 소프트웨어를 사용하여 위상차 및 면역형광 이미지(Table of Materials)를 분석했습니다. 이 시스템을 사용하지 않는 경우 공용 도메인 소프트웨어 패키지 4,18,19,20을 사용하여 유사한 NET 분석을 수행할 수 있습니다.
- 라이브 셀 현미경 분석 소프트웨어를 열고 장치에 연결을 클릭합니다. 사용자 이름과 비밀번호를 입력하고 최근 스캔 보기를 클릭합니다. 실험의 용기 이름을 두 번 클릭하여 분석할 실험을 엽니다.
- 분석 시작을 클릭하고 새 분석 정의 생성을 선택합니다. 다음을 클릭합니다.
- 분석 유형 섹션에서 Basic Analyzer 를 선택하고 Next를 클릭합니다. 이미지 채널 섹션에서 녹색을 선택하고 위상을 선택 취소한 후 다음을 클릭합니다.
- Image Layers 창을 열고 Green을 선택하고 Phase를 선택 해제합니다. 녹색 섹션에서 자동 크기 조정을 끄고 녹색 채널의 최소값 및 최대값을 수동으로 설정하여 NET을 배경과 구분합니다.
알림: 위상차는 필요할 때 켜고 끌 수 있습니다. 예를 들어, NET이 세포 외 환경에서 추방되는지 여부를 확인합니다. - Vessel Scan Times(선박 스캔 시간) 창을 열고 NET이 있어야 하는 적절한 선박 스캔 시간을 선택합니다. 실험 내의 변동성을 나타내는 이미지 세트를 선택합니다(NET이 있는 positive control well의 이미지 및 NET이 없는 negative control well의 이미지). 이러한 이미지는 분석을 미리 보고 구체화하는 데 사용됩니다. 다음을 클릭합니다.
- 분석 정의 설정 창을 열고 거짓 긍정 또는 거짓 부정 NET을 생성하지 않고 NET 구조가 윤곽이 그려지도록 녹색 채널에 대한 분석 설정을 조정하기 시작합니다. 다음 설정을 사용하고 현재 이미지 미리 보기(Preview Current) 또는 모두 미리 보기(Preview All)를 클릭하여 현재 이미지 또는 모든 이미지에 각각 설정을 적용합니다.
- 개체 이름으로 NET을 선택합니다.
- 세분화의 경우 No Background Subtraction - Adaptive를 선택합니다.
- 임계값 GCU의 경우 3 에서 5 사이(실험에 따라 다름)를 선택합니다.
- 가장자리 분할의 경우 ON(-10과 0 사이)을 선택합니다.
- 정리를 위해 구멍 채우기: 100μm2 및 크기 조정: -1픽셀을 선택합니다.
- 필터의 경우 실험에 따라 선택하지만 권장되는 컷오프 값은 면적: >200μm2, 평균 강도: <24.6, 통합 강도: >7000입니다.
참고: 사용된 설정에 따라 선택된 구조체는 자홍색으로 윤곽선이 표시됩니다. 이러한 구조 위로 마우스를 가져가면 개체의 매개변수(예: 면적, 편심, 통합 강도 및 평균 강도)에 대한 자세한 정보가 제공되어 NET 매개변수에 대한 포함 또는 제외 기준과 관련된 분석을 최적화하는 데 도움이 됩니다. 여러 이미지(positive 및 negative control well에서)를 확인하여 분석 설정이 올바른지 테스트합니다.
- NET 분석에 대한 설정이 올바르면 다음을 클릭합니다. Scan times and wells(스캔 시간 및 웰) 섹션에서 분석할 시점과 웰을 선택하고 Next(다음)를 클릭합니다. Save and Apply analysis definition(분석 정의 저장 및 적용) 섹션에 정의 이름을 삽입하고 Next(다음)를 클릭합니다. 분석 정보가 확인되고 정확해지면 Finish(마침 )를 클릭합니다.
참고: 여러 플레이트를 분석하거나 이전 실험의 분석을 사용하는 경우 분석을 시작하고 기존 분석 정의 복사 또는 기존 분석 정의 사용을 선택하고 다음을 클릭합니다. 적절한 기존 분석 정의를 선택하고 다음을 클릭합니다. 후속 분석 절차의 경우 기존 분석 정의 복사 를 선택한 경우 6.4단계로 이동하거나 기존 분석 정의 사용을 선택한 경우 6.7단계로 이동합니다. - 관심 있는 용기 내의 분석을 클릭하여 실험 분석을 엽니다. 그래프 메트릭 창을 엽니다.
- 더하기 버튼(지표 만들기)을 클릭합니다. 데이터는 NET confluency(NET으로 표시된 이미지 영역의 백분율) 또는 NETTING 호중구 백분율(NET 수를 세포 수로 나눈 값)으로 표시할 수 있습니다.
- 데이터를 NET fluencent 로 표시할 때 Metric 섹션에서 Area를 선택하고 Value 섹션에서 Confluence를 선택합니다. 데이터를 네팅 호중구의 백분율로 표시할 때 Metric 섹션에서 Object Count를 선택하고 Value 섹션에서 Per Image를 선택합니다.
- 확인을 클릭합니다. 사용자 정의 메트릭 섹션에서 NETs Area Confluence (%) 또는 NETs Object Count Per Image (Per Image)(이미지당)를 선택합니다. Select Scans(스캔 선택) 섹션에서 분석해야 하는 모든 시점을 선택합니다. Select Wells 섹션에서 분석해야 하는 모든 Well을 선택하고 Export Data를 클릭합니다.
- Graphing Export(그래프 내보내기) 섹션에서 다음 설정을 선택합니다.
- 각 스캔을 자체 테이블로 표시를 선택합니다(열: 1, 2... 행: A, B...).
- 행 및 열 레이블 표시를 선택합니다.
- 하나의 파일에 있는 모든 스캔을 선택하고 찾아보기를 클릭하여 파일 위치와 이름을 조정합니다.
- 헤더에 실험 세부 정보 포함을 선택합니다.
- 데이터를 개별 이미지로 분할을 선택합니다.
- 내보내기를 클릭하여 데이터를 ".txt" 파일로 내보내면 스프레드시트에서 가져와서 추가로 분석할 수 있습니다.
참고: 그물 호중구의 백분율을 결정하려면 t = 0에서 호중구의 수를 결정해야 합니다. 이렇게 하려면 프로토콜의 다음 단계를 계속 진행합니다. - 6.1단계로 이동하여 t = 0에서 이미지의 호중구 수를 계산하는 데 필요한 추가 분석을 시작합니다.
- 6.3단계에서 위상 을 선택하고 이미지 채널 섹션에서 녹색 을 선택 취소합니다.
- 6.4단계의 vessel scan times(용기 스캔 시간) 섹션에서 t = 0 을 선택합니다.
- 6.5단계에서 녹색을 선택 취소하고 위상을 선택한 다음 필요한 경우 위상 채널 설정을 조정합니다.
- 6.6단계에서 개체 이름을 추가하고, 세분화 섹션에서 클래식 컨플루언스를 선택하고, 세분화 조정을 0(배경)으로 설정하고, 구멍 채우기를 100μm2로, 영역을 50μm2로 조정합니다.
- 6.7단계의 스캔 선택 섹션에서 t = 0 을 선택합니다.
- 6.8단계에서 호중구 계수 분석을 엽니다.
- 6.9단계의 User Defined Metrics(사용자 정의 메트릭) 섹션에서 Object Count Per Image (Per Image)(이미지당)를 선택합니다.
- 마지막으로 다음 계산을 사용하여 그물 호중구의 백분율을 생성합니다: (그물 수 / t = 0에서의 호중구 수) x 100%.
결과
불침투성 DNA 염료와 함께 실시간 고처리량 현미경 검사를 통해 NETosis의 역학, 특성 및 기본 경로를 연구할 수 있으며 NET 방출의 잠재적 억제제를 평가할 수 있습니다. 이 접근법을 통해 NET은 건강한 호중구의 표면적에 비해 훨씬 더 큰 표면적을 가진 DNA 염료에 양성인 구조로 정의되었으며(그림 1A), 이는 염색질이 세포 외 환경으로 배출되었음을 나타냅니다18. 표면적 기반 분석을 통해 NET과 원형질막 무결성이 손상된 활성 호중구를 구별할 수 있었으며, 밝은 세포 내 DNA 염색을 보여주었습니다(그림 1A).
칼슘 이오노포어(A23187) 및 PMA는 일반적으로 체외에서 NETosis를 유도하는 데 사용됩니다. 비생리적 자극임에도 불구하고 뚜렷한 NETosis 경로를 활성화하고 기증자 간의 낮은 변동성으로 일관된 NETosis 유도를 보장하기 때문에 가치가 있습니다. A23187은 칼슘 유입을 유발하여 PAD4 활성화 및 시트룰린 히스톤이 풍부한 NET의 방출로 이어지며, PMA는 NADPH 산화효소 복합체를 활성화하여 반응성 산소종(ROS) 생산과 낮은 수준의 시트룰린 히스톤 수치가 낮은 NET의 방출을 초래합니다 5,16,21. NETosis 반응의 속도와 크기는 각 자극의 농도에 따라 달라지며(그림 1B, C), A23187은 NETosis를 더 빠르게 유도하고 PMA는 NET을 방출하는 호중구의 비율이 더 높습니다(그림 1D-F). A23187 자극으로 인한 그물(그림 1D, 빨간색 화살표)은 호중구 원형질막 너머로 더 확산되는 반면, PMA 유도 그물은 호중구 원형질막에 더 인접해 있다는 점에서 PMA 유도 NET(그림 1D, 노란색 화살표)과 구별되었습니다. 또한 A23187 자극은 투과성 비그물 DNA 염료 양성 호중구(그림 1D, 파란색 화살표)를 생성했으며, 이는 DNA를 세포 외 공간으로 배출하지 않았습니다. 투과성 비그물 DNA 염료 양성 호중구의 검출은 A23187의 농도에 따라 달라졌으며(그림 1G) NETosis를 자극하는 데 사용되는 PMA의 농도에 관계없이 거의 없었습니다(그림 1D, H). 비생리학적 NETosis 자극 A23187 및 PMA를 사용하는 것 외에도 이 분석은 NETosis의 질병 관련 유발 요인을 연구하는 데에도 적합합니다. 예를 들어, 칼슘 피로인산 변위병(CPPD)에 존재하는 용해성 면역 복합체(sIC) 또는 결정으로 활성화된 호중구는 자극이 없는 경우와 비교하여 순 방출이 유의하게 증가한 것으로 나타났습니다. 호중구가 코팅된 면역 복합체(cIC), fMLP 및 요산나트륨(MSU) 결정으로 활성화될 때 NET 수치가 상승하는 추세가 관찰되었습니다(그림 1I). 그러나 이러한 자극에 대해 우리는 상당한 기증자 간 변동을 관찰했습니다.
NETosis 경로 및 NETosis-associated enzymes의 약리학적 억제는 NETosis-targeting therapeutics가 NET 축적이 병리학을 현저하게 유발하는 질병에 대한 효과적인 치료법이 될 수 있음을 보여주었다 14,17,22,23,24. 이 Real-time microscopy NET assay는 고처리량 방식으로 NETosis 길항제를 연구하기 위한 쉽고 신뢰할 수 있으며 재현 가능한 접근 방식입니다. 이를 예시하기 위해, 우리는 높은 친화력15,16으로 시트룰린 화 히스톤 H2A 및 H4를 표적으로 하는 동급 최초의 인간화 단일클론 항체인 CIT-013을 사용했습니다. A23187은 NETosis 경로를 활성화하여 시트룰린 히스톤을 포함하는 NET을 생성하고, 이 히스톤은 CIT-013에 의해 NETosis 억제 기능16을 수행하기 위해 표적화됩니다. 실제로, A23187에 대한 NET 방출은 CIT-013(그림 2A 및 보충 비디오 1)에 의해 완전히 금지되었으며, IC50은 4.6nM(그림 2B)이었습니다. CIT-013의 NETosis-inhibitory capacity는 다른(비) 시트룰린 히스톤을 표적으로 하는 다른 상용 항체가 NET 방출을 억제할 수 없었기 때문에 독특합니다(그림 2C).
이전에, 우리는 CIT-013의 매우 유사한 전구체 분자(두 개의 아미노산은 다르지만 동일한 친화력으로 유사한 항원결정기를 결합)가 활성화된 혈소판, 통풍 활액 및 RA 활액액과 같은 생리학적 자극에 대한 반응으로 NETosis를 차단한다는 것을 보여주었습니다17. 여기에서는 sIC에 의해 유도된 NET 방출이 CIT-013에 의해 억제될 수 있음을 보여줍니다(그림 2D). 이러한 자극에 의해 유도된 NETosis를 억제하는 치료적 타당성은 SLE, RA 및 기타 자가면역 질환에 의해 강조되는데, 혈청 또는 활액의 자가항체가 NETosis를 유발하는 IC의 형성을 지원한다25,26.
이러한 데이터는 이 실시간 고처리량 현미경 접근 방식이 NET 방출의 역학 및 특성을 연구하는 데 적합하며 NETosis의 억제제를 연구할 수 있음을 보여줍니다. 이 방법은 인간 호중구의 사용에 최적화되어 있지만 수정을 통해 다른 종의 호중구를 연구하는 데에도 적합할 수 있습니다. 이 분석으로 생성된 데이터는 CIT-013이 NET 기반 질병에 대한 강력하고 효과적인 치료법이라는 근거의 초석입니다.
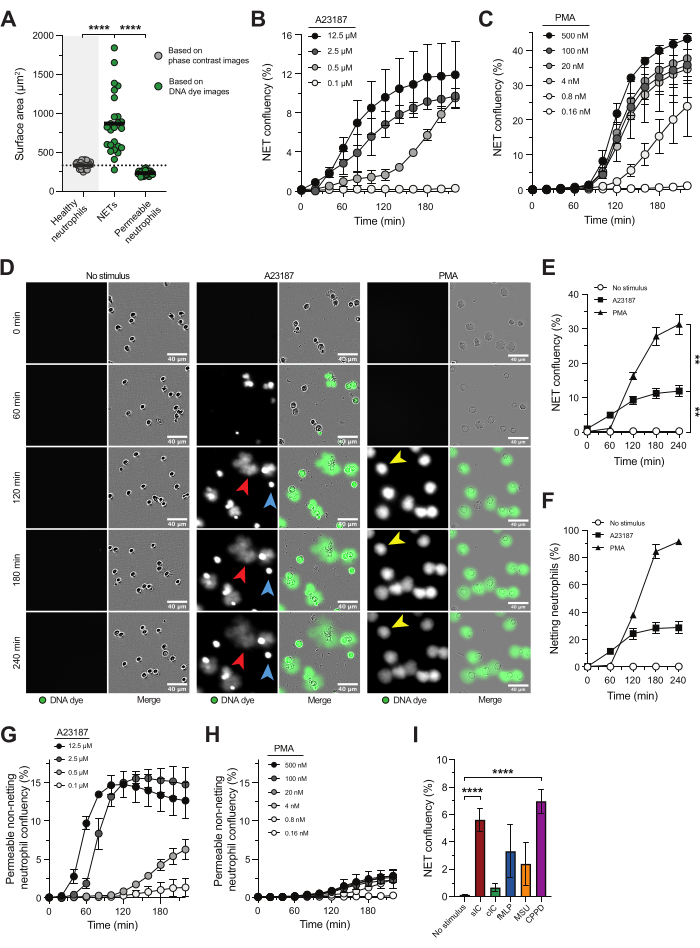
그림 1: NET 방출 연구를 위한 실시간 고처리량 현미경 검사. 건강한 지원자의 혈액에서 분리된 호중구를 A23187 또는 PMA로 자극하여 NETosis 경로를 유발했습니다. NET 릴리스는 원형질막 불투과성 DNA 염료를 사용하여 실시간 고처리량 현미경으로 시각화하고 라이브 셀 현미경 분석 시스템 소프트웨어를 사용하여 표면적을 기반으로 정량화했습니다. (A) 비자극 건강한 호중구(회색), 세포외 NET 및 세포 내 DNA 염색(녹색)을 통한 투과성 비그물 호중구의 표면적 분석. (B,C) 표시된 A23187 또는 PMA 농도(n = 2)로 자극된 호중구로부터 시간 경과에 따른 NET 방출의 정량화. (D) 서로 다른 시점에서 A23187(빨간색 화살표) 및 PMA(노란색 화살표)에 대한 반응으로 릴리스되는 NET의 대표 이미지. 투과성 비그물 호중구의 예는 파란색 화살표로 표시됩니다. (E) NET confluency의 백분율로 표시되는 시간 경과에 따른 NET 릴리스의 수량화(n = 5). 통계는 t = 240분에 대해 수행되었습니다. (F) 시간 경과에 따른 순 방출의 정량화는 그물 호중구의 백분율(n = 2)로 제시되었습니다. (지, H) 표시된 A23187 또는 PMA 농도(n = 2)로 자극된 호중구로부터 시간 경과에 따른 투과성 비그물 호중구의 정량화. (I) 가용성 면역 복합체(sIC), 코팅된 IC(cIC), fMLP, 요산나트륨(MSU) 결정 및 칼슘 피로인산염 처분병(CPPD)에 존재하는 결정에 의해 유도된 t = 240분에서 NET 방출의 정량화(n = 8-28). 결과는 평균의 표준 오차± 평균으로 보고됩니다. **P < 0.01, ****P < 0.0001, 반복은 Dunnett의 다중 비교 검정(B)을 사용한 일원 분산 분석, Dunn의 다중 비교 검정(I)을 사용한 Kruskal-Wallis 검정을 측정합니다. 패널 A-F는 van der Linden et al.16의 허가를 받아 수정되었습니다. 이 그림의 더 큰 버전을 보려면 여기를 클릭하십시오.
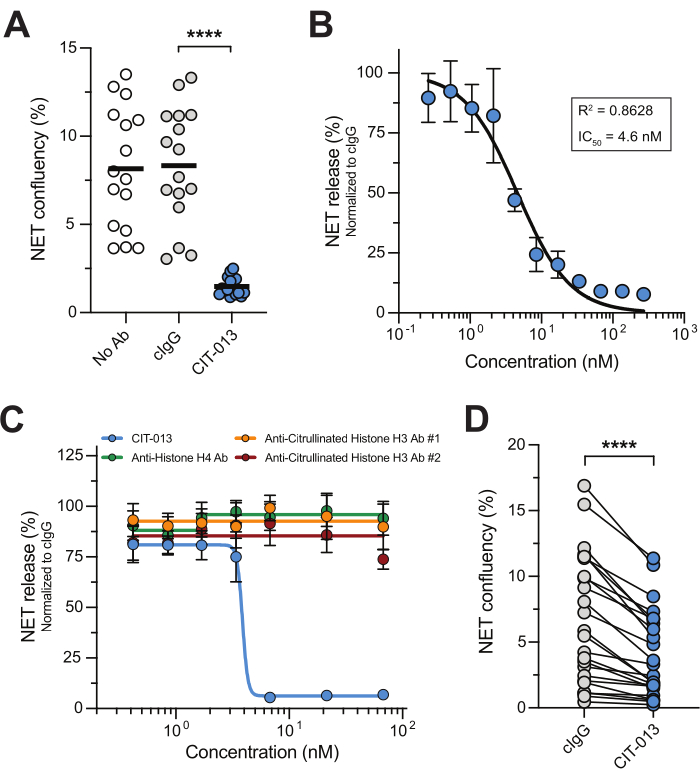
그림 2: CIT-013은 NET 릴리스를 금지합니다. (A) CIT-013 또는 동형 대조 항체(cIgG)의 부재(Ab 없음) 또는 존재 시 t = 240분에서 A23187 유도 NET 방출의 정량화. (B) t = 240분(n = 3)에서 CIT-013을 사용한 A23187 유도 NET 방출의 용량 의존적 억제. 데이터는 cIgG로 정규화되었습니다(100% NET 릴리스로 설정). (C) CIT-013, 항-히스톤 H4 항체, 항-시트룰린 히스톤 H3 항체 #1 또는 항-시트룰린 히스톤 H3 항체 #2(n = 6)의 표시된 농도가 존재하는 경우 t = 240분에서 A23187 유도 NET 방출의 정량화. (D) CIT-013 또는 cIgG의 존재 하에서 용해성 면역 복합체(IC)에 의해 유도된 t = 240분에서 NET 방출의 정량화. 결과는 평균의 표준 오차± 평균으로 보고됩니다. P < 0.0001, 반복은 Tukey의 다중 비교(A) 또는 양측 Wilcoxon 일치 쌍 부호 순위 검정(D)을 사용하여 일원 분산 분석을 측정합니다. 패널 A, B 및 D 는 van der Linden et al.16의 허가를 받아 수정되었습니다. 이 그림의 더 큰 버전을 보려면 여기를 클릭하십시오.
| 96웰 플레이트 #1 | 96웰 플레이트 #2 | |
| 웰당 부피 0.001% 폴리-L-라이신 용액 | 50 마이크로L | 100 마이크로L |
| well당 호중구 현탁액의 부피 | 50 마이크로L | 87.5 μL |
| 웰당 호중구의 수 | 2 x 104 셀 | 3.5 x 104 셀 |
| 웰당 NET 분석 버퍼에 4배 농축된(= 80 nM) DNA 염료 부피 | 50 마이크로L | 87.5 μL |
| 부피: 웰당 NET 분석 버퍼에서 4배 농축된 NETosis 자극 | 50 마이크로L | 87.5 μL |
| 부피 4x 농축 NETosis 길항제는 웰당 NET 분석 버퍼에 농축되어 있습니다. | 50 마이크로L | 87.5 μL |
표 1: 다양한 96-well 이미징 플레이트에 최적화된 부피 및 세포 수.
| 농축 작업 용액 (4x 농축) | 최종 집중 | |
| 칼슘 이오노단(A23187) | 50 마이크로미터 | 12.5 μm |
| PMA는 | 16nM | 4nM |
| 에프MLP | 4 μM | 1 μM |
| 요산나트륨(MSU) 결정 | 400 μg/mL | 100 μg/mL |
| 칼슘 피로인산 치환병(CPPD) 결정 | 400 μg/mL | 100 μg/mL |
| 수용성 면역 복합체(sIC) | 1. DPBS의 5μg/mL 인간 혈청 알부민(HSA)을 DPBS의 282.5μg/mL 다클론 토끼 항-HSA 항체에 추가합니다. | |
| 2. 37 ° C에서 90 분 이상 배양합니다. | ||
| 3. 와류에 의하여 균질화하고 대응 wells에 50 μL sIC 해결책을 추가하십시오. | ||
| 코팅된 면역 복합체(cIC) | 1. 96웰 플레이트의 해당 웰에 DPBS의 10μg/mL HSA를 추가합니다. | |
| 2. 4 °C에서 밤새 배양합니다. | ||
| 3. DPBS에서 200μL 0.05% Tween-20(이하 PBS/0.05%Tween이라고 함)으로 웰을 3회 세척합니다. | ||
| 4. PBS/0.05% Tween의 200μL 1%(w/v) 소 혈청 알부민(이하 차단 버퍼라고 함)으로 웰을 차단합니다. | ||
| 5. 실온에서 120분 동안 배양하고 부드럽게 교반(400rpm)합니다. | ||
| 6. 200 μL PBS/0.05% Tween으로 우물을 3번 세척합니다. | ||
| 7. 해당 웰에 차단 완충액에 50μL polyclonal rabbit anti-HSA 항체를 추가합니다. | ||
| 8. 실온에서 60분 동안 배양하고 부드럽게 교반(400rpm)합니다. | ||
| 9. 200 μL PBS/0.05% Tween으로 우물을 3회 세척합니다. | ||
| 10. 마지막으로 200μL DPBS로 웰을 3회 세척합니다. Wells는 이제 프로토콜의 4.7단계를 수행할 준비가 되었습니다. | ||
표 2: NETosis 자극에 대한 권장 농도.
| 농축 작업 용액 (4x 농축) | 최종 집중 | |
| 항암탉 난자 라이소자임 항체(대조 항체, cIgG) | 80nM | 20nM |
| CIT-013 【무수정유출】 | 80nM | 20nM |
표 3: NETosis 길항제에 대한 권장 농도.
보충 비디오 1. cIgG(왼쪽) 또는 CIT-013(오른쪽)이 있는 상태에서 A23187로 호중구를 자극하고, 원형질막 불투과성 DNA 염료를 사용하여 시간이 지남에 따라 NET 방출을 시각화했습니다. NET 릴리스는 CIT-013이 있는 경우 금지됩니다. 영화는 DNA 염료(녹색)와 위상 대비의 오버레이입니다. 이 비디오는 van der Linden et al.16의 허가를 받아 입수했습니다. 이 비디오를 다운로드하려면 여기를 클릭하십시오.
토론
2004년 NET이 발견된 이후, NET in vitro 방출을 실험적으로 조사하기 위한 많은 전략이 개발되었으며, 면역형광 현미경 검사는 NETosis를 정량화하는 가장 일반적인 기술입니다27,28. 현미경은 NET 릴리스를 시각화하는 데 유용하지만 고정된 시점 이미지의 자동화되지 않은 현미경 정량화는 다소 부정확하고 관찰자 편향으로 인해 어려움을 겪을 수 있기 때문에 한계가 있습니다. NET 방출을 연구하는 데 사용되는 또 다른 기술은 다중분광 이미징 유세포 분석 29,30으로, 많은 수의 그물 호중구를 측정하고 편향되지 않은 분석을 채택하지만 NETosis의 초기 단계에 있는 호중구에 초점을 맞추고 NET을 방출한 호중구를 정량화하지 않습니다. NETosis kinetics에 대한 많은 연구에서는 형광 플레이트 리더와 함께 NET 방출을 정량화하기 위해 DNA 염료를 사용합니다31. 이 기술은 세포 내 DNA 염색으로 활성화되거나 죽어가는 호중구와 NET을 구별할 수 없으므로 NET 방출의 정량화 및 NETosis 길항제 연구에 적합하지 않습니다. 위의 내용은 NETosis를 연구하기 위해 현재 사용되고 있는 접근 방식이 가치가 있지만 한계가 있음을 강조합니다.
이 연구에서 설명한 실시간 현미경 방법은 이전에 보고된 기술에서 발견된 많은 문제를 해결합니다. 편향되지 않고, 반자동이며, 처리량이 많고, 재현 가능하며, 정확한 NET 정량화를 제공합니다. 실제로, 이 접근법은 NETs18의 늘어난 형태와 같은 피펫팅 아티팩트를 최소화하며, DNA 방출의 뚜렷한 형태 및 동역학을 통해 NETosis를 괴사 및 세포사멸과 구별할 수 있습니다18,32. 또한, 이 접근법은 NET의 기능적 및 표현형 기준을 충족하는 구조를 생성하는 괴사 호중구의 NET 형성과 같이 조절된 세포 사멸을 위한 다양한 생화학적 경로를 연구할 수 있는 기회를 제공합니다33.
이 프로토콜에는 NET의 성공적인 정량화를 위해 따라야 하는 몇 가지 중요한 단계가 있습니다. 첫째, 웰당 정확한 세포 수는 정확한 NET 정량화를 위해 중요합니다. 호중구 밀도가 너무 높으면 세포와 NET이 인접한 세포 및 NET과 겹쳐 구별하기 어려워지고 결과적으로 정량화가 부정확해집니다. 둘째, 저농도의 무독성 원형질막 불투과성 DNA 결합 염료를 사용하여 세포외 환경으로 분비될 때 그물을 염색하는 것이 전제 조건입니다. 원형질막 투과성 DNA 결합 염료는 호중구 활성화 또는 세포 사멸을 쉽게 유도할 수 있습니다. 셋째, 이질적인 호중구 집단 간의 NET 방출에 대한 대표적인 개요를 위해 웰당 여러 이미지를 스캔해야 합니다. 넷째, t = 0에서 호중구의 위상차 이미지는 이미지당 호중구의 수를 수정하고 그물 호중구의 비율을 계산하는 데 필요합니다.
이 실시간 고처리량 현미경 접근법은 다른 NET 검출 분석에 비해 많은 장점이 있지만, 우리가 아는 한 NET 방출을 확인하기 위해 추가 NET 성분을 검출할 수 있는 형광 염료가 없기 때문에 이 방법에는 한계가 있습니다. 추가 NET 성분을 검출하기 위한 형광 표지 항체를 사용할 수 있지만 항체 면역 복합체가 호중구 활성화에 영향을 미치고 NETosis에 영향을 미칠 수 있기 때문에 원치 않는 영향을 미칠 수 있습니다. 따라서 이 분석 설정에서 추가 항체를 사용하지 않는 것이 좋으며 대신 잘 알려진 NETosis 유도 자극을 사용하는 것이 좋습니다. 아직 확립되지 않은 NETosis 자극이 사용되는 경우, 당사는 라이브 이미징 분석 전에 시트룰린 히스톤과 같은 NET 특이적 단백질과 복합체가 있는 DNA를 검출하기 위해 ELISA를 사용하는 것을 지지합니다. 둘째, 분석 내 및 분석 간 변동은 건강한 인구 집단 내의 이질성으로 인해 건강한 기증자의 호중구가 다양한 NETosis 자극에 다르게 반응하는 기증자 간 변동성으로 인해 발생할 수 있습니다. 분석 변동을 최소화하기 위해 호중구는 수명이 짧고 동결 보존할 수 없기 때문에 갓 채취한 혈액에서 1시간 이내에 호중구를 분리하고 실험에 즉시 사용해야 합니다. 또한, 호중구 활성화를 최소화하기 위해 호중구 분리 프로토콜을 검증하고 채택하는 것이 중요합니다. 호중구는 민감한 세포이며 기계적 스트레스 및 기타 유형의 스트레스로 인해 정화 과정에서 자극에 대한 반응성을 변경할 수 있습니다. 호중구 활성화 상태 및 NET 방출은 바늘의 유형, 사용된 채혈 튜브, 배양 온도, 원심분리 속도 및 혈액 채취에서 호중구 분리까지의 시간에 영향을 받을 수 있습니다 34,35,36,37. 고려할 수 있는 추가적인 호중구 분리 방법은 Krémer et al.36에 의해 적혈구 용해 없이 음성 면역자기 선택 방법을 사용하여 설명됩니다. 이 방법은 전혈의 손대지 않은 호중구와 유사하며 정제 과정에서 호중구 활성화를 방지하는 데 적합할 수 있습니다. 위의 모든 사항은 서로 다른 연구 그룹의 데이터를 매우 신중하게 비교해야 한다는 점을 이 분야에 경고하는 데 도움이 되어야 합니다.
전반적으로, 설명된 실시간 고처리량 현미경 방법은 재현 가능하고 효율적인 방식으로 NET을 정확하게 정량화할 수 있으며 NET 방출의 특성, 크기 및 역학을 연구하는 데 사용할 수 있으며 NETosis 길항제의 활성을 조사할 수 있습니다. 후자의 예로, 현재 임상 개발 중인 인간화 항시트룰린 히스톤 H2A 및 H4 단클론 항체 CIT-013을 사용했습니다.
공개
저자는 시트릴의 직원이며 재정적 이해관계가 있습니다.
감사의 말
저자는 실시간 이미징 현미경 방법과 관련된 프로젝트의 일부를 관리해 준 Paul Vink에게 감사를 표합니다.
자료
| Name | Company | Catalog Number | Comments |
| A-23187 Free Acid (Calcimycin) | Thermo Fisher | A1493 | |
| Ammonium chloride (NH4Cl) | Sigma Aldrich | A9434 | |
| Anti-Hen egg lysozyme (control antibody; cIgG) | CrownBio | C0001 | |
| APC-Cy7-conjugated mouse anti-human CD45 antibody (Clone 2D1) | Biolegend | 368515 | Use at 1 µg/mL final concentration |
| BD FACSCantoTM II system + FACSDiva software (version 8.0.1) | BD | n/a | Flow cytometry system |
| Bovine Serum Albumin (BSA) Fraction V | Roche | 10735108001 | |
| CaCl2 (1 M) | VWR | E506 | |
| Calcium pyrophosphate disposition disease (CPPD) crystals | InvivoGen | lrl-cppd | |
| Cellometer Auto T4 Bright Field Cell Counter + analysis software (version 3.3.9.5) | Nexcelom Bioscience | n/a | Bright field cell counter |
| CIT-013 | Citryll B.V. | n/a | |
| Costar black 96-well plate, clear bottom | Corning | 3603 | 96-well plate #1 |
| Dextran T500 | Pharmacosmos | 551005009006 | |
| DPBS (1x) | Gibco | 14190-144 | |
| Fetal Bovine Serum (FBS) | Serena | S-FBS-SA-015 | |
| Ficoll | GE Healthcare | 17-1440-02 | Density gradient solution |
| FITC-conjugated mouse anti-human CD66b antibody (Clone G10F5) | eBioscience | 17-0666-42 | Use at 1.5 µg/mL final concentration |
| Fixable viability dye eFluor 506 | eBioscience | 65-0866-14 | |
| Flow cytometry analysis software | FLowJo | Version 10.8.0 | |
| fMLP | Sigma Aldrich | 47729-10MG-F | |
| HEPES (1M) | Gibco | 15630-080 | |
| Human serum albumin (HSA) | VWR | 31020 | |
| Human TruStain FcX | Biolegend | 422302 | Fc receptor block |
| IBIDI 96-well plate, clear bottom | IBIDI | 89626 | 96-well plate #2 |
| IncuCyte SX3 + analysis software (version 2022B Rev1) | Satorius | n/a | Live cell microscopic analysis system |
| Monosodium urate crystals | InvivoGen | tlrl-msu | |
| Mouse anti-histone H3 (citrulline R2 + R8 + R17) antibody | Cayman | 17939 | Clone 11D3; referred to as #1 |
| Mouse anti-histone H4 (K8Ac + K12Ac + K16Ac) antibody (Clone KM-2) | Absolute Antibodies | Ab01681-2.0 | |
| Na2EDTA | Sigma Aldrich | E5134 | |
| NaCl (0.9%) | B. Braun | 25900 | |
| Penicillin (5000 U/mL) - Streptomycin (5000 µg/mL) | Gibco | 15070-063 | |
| PerCP-Cy5.5-conjugated mouse anti-human CD16 antibody (Clone 3G8) | Biolegend | 302027 | Use at 0.33 µg/mL final concentration |
| PMA | Sigma Aldrich | P1585 | |
| Polyclonal rabbit anti-HSA antibody | Sigma Aldrich | A0433-2ml | |
| Poly-L-Lysine (0.01%) | Sigma Aldrich | P4832 | |
| Potassium bicarbonate (KHCO3) | Sigma Aldrich | 237205 | |
| Rabbit anti-histone H3 (citrulline R2 + R8 + R17) antibody | Abcam | ab281584 | Multiclonal; referred to as #2 |
| RPMI 1640 with GlutaMAXTM supplement | Gibco | 61870-010 | Culture medium containing L-glutamine |
| RPMI 1640 without phenol red | Gibco | 11835-030 | Culture medium without phenol red |
| Sodium azide (NaN3) | Sigma Aldrich | S2002 | |
| Sterile H2O | Gibco | 15230204 | |
| Sytox Green Nucleid Acid Stain | Thermo Fisher | S7020 | DNA dye |
| Trypan blue solution (0.4%) | Gibco | 15250-061 | |
| Tween-20 | Sigma Aldrich | P1379 | |
| Vacutainer blood tubes Li-Heparin (17 IU/mL) | BD | 367526 |
참고문헌
- Brinkmann, V., et al. Neutrophil extracellular traps kill bacteria. Science. 303 (5663), 1532-1535 (2004).
- De Bont, C., Pruijn, G. J. M. Citrulline is not a major determinant of autoantibody reactivity to neutrophil extracellular traps. Philos Trans R Soc Lond B Biol Sci. 378 (1890), 20220249 (2023).
- Urban, C. F., et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 5 (10), e1000639 (2009).
- Hoppenbrouwers, T., et al. In vitro induction of NETosis: Comprehensive live imaging comparison and systematic review. PLoS One. 12 (5), e0176472 (2017).
- Kenny, E. F., et al. Diverse stimuli engage different neutrophil extracellular trap pathways. ELife. 6, e24437 (2017).
- Parker, H., Dragunow, M., Hampton, M. B., Kettle, A. J., Winterbourn, C. C. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol. 92 (4), 841-849 (2012).
- Neeli, I., Radic, M. Opposition between PKC isoforms regulates histone deimination and neutrophil extracellular chromatin release. Front Immunol. 4, 38 (2013).
- Bosmann, M., Ward, P. A. Protein-based therapies for acute lung injury: targeting neutrophil extracellular traps. Expert Opin Ther Targets. 18 (6), 703-714 (2014).
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 18 (2), 134-147 (2018).
- Shiratori-Aso, S., Nakazawa, D. The involvement of NETs in ANCA-associated vasculitis. Front Immunol. 14, 1261151 (2023).
- Byrd, A. S., et al. Neutrophil extracellular traps, B cells, and type I interferons contribute to immune dysregulation in hidradenitis suppurativa. Sci Transl Med. 11 (508), eaav5908 (2019).
- Kimball, A. S., Obi, A. T., Diaz, J. A., Henke, P. K. The emerging role of NETs in venous thrombosis and immunothrombosis. Front Immunol. 7, 236 (2016).
- O'Neil, L. J., et al. Neutrophil extracellular trap-associated carbamylation and histones trigger osteoclast formation in rheumatoid arthritis. Ann Rheum Dis. 82 (5), 630-638 (2023).
- Li, M., et al. A novel peptidylarginine deiminase 4 (PAD4) inhibitor BMS-P5 blocks formation of neutrophil extracellular traps and delays progression of multiple myeloma. Mol Cancer Ther. 19 (7), 1530-1538 (2020).
- Chirivi, R. G. S., et al. Anti-citrullinated protein antibodies as novel therapeutic drugs in rheumatoid arthritis. J Clin Cell Immunol. S6, 006 (2013).
- Van der Linden, M., et al. Anti-citrullinated histone monoclonal antibody CIT-013, a dual action therapeutic for neutrophil extracellular trap-associated autoimmune diseases. MAbs. 15 (1), 2281763 (2023).
- Chirivi, R. G. S., et al. Therapeutic ACPA inhibits NET formation: a potential therapy for neutrophil-mediated inflammatory diseases. Cell Mol Immunol. 18 (6), 1528-1544 (2021).
- Van der Linden, M., Westerlaken, G. H. A., Van Der Vlist, M., Van Montfrans, J., Meyaard, L. Differential signalling and kinetics of neutrophil extracellular trap release revealed by quantitative live imaging. Sci Rep. 7 (1), 6529 (2017).
- Hoffmann, J. H. O., Schaekel, K., Gaiser, M. R., Enk, A. H., Hadaschik, E. N. Interindividual variation of NETosis in healthy donors: introduction and application of a refined method for extracellular trap quantification. Exp Dermatol. 25 (11), 895-900 (2016).
- Silva, L. M., Moutsopoulos, N., Bugge, T. H., Doyle, A. Live imaging and quantification of neutrophil extracellular trap formation. Curr Protoc. 1 (7), e157 (2021).
- De Bont, C. M., Koopman, W. J. H., Boelens, W. C., Pruijn, G. J. M. Stimulus-dependent chromatin dynamics, citrullination, calcium signalling and ROS production during NET formation. Biochim Biophys Acta Mol Cell Res. 1865 (11 Pt A), 1621-1629 (2018).
- Gajendran, C., et al. Alleviation of arthritis through prevention of neutrophil extracellular traps by an orally available inhibitor of protein arginine deiminase 4. Sci Rep. 13 (1), 3189 (2023).
- Biron, B. M., Chung, C. -. S., O'Brien, X. M., Chen, Y., Reichner, J. S., Ayala, A. Cl-Amidine prevents histone 3 citrullination and neutrophil extracellular trap formation, and improves survival in a murine sepsis model. J Innate Immun. 9 (1), 22-32 (2017).
- Perdomo, J., et al. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat Commun. 10 (1), 1322 (2019).
- Lood, C., et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 22 (2), 146-153 (2016).
- Kraaij, T., et al. A novel method for high-throughput detection and quantification of neutrophil extracellular traps reveals ROS-independent NET release with immune complexes. Autoimmun Rev. 15 (6), 577-584 (2016).
- De Buhr, N., Von Köckritz-Blickwede, M. How neutrophil extracellular traps become visible. J Immunol Res. 2016, 1-13 (2016).
- Brinkmann, V., Laube, B., Abu Abed, U., Goosmann, C., Zychlinsky, A. Neutrophil extracellular traps: How to generate and visualize them. J Vis Exp. (36), e1724 (2010).
- Zhao, W., Fogg, D. K., Kaplan, M. J. A novel image-based quantitative method for the characterization of NETosis. J Immunol Methods. 423, 104-110 (2015).
- Gavillet, M., et al. Flow cytometric assay for direct quantification of neutrophil extracellular traps in blood samples. Am J Hematol. 90 (12), 1155-1158 (2015).
- Sil, P., Yoo, D., Floyd, M., Gingerich, A., Rada, B. High throughput measurement of extracellular DNA release and quantitative NET formation in human neutrophils in vitro. J Vis Exp. (112), e52779 (2016).
- Gupta, S., Chan, D. W., Zaal, K. J., Kaplan, M. J. A high-throughput real-time imaging technique to quantify NETosis and distinguish mechanisms of cell death in human neutrophils. J Immunol. 200 (2), 869-879 (2018).
- D'Cruz, A. A., et al. The pseudokinase MLKL activates PAD4-dependent NET formation in necroptotic neutrophils. Sci Signal. 11 (546), eaao1716 (2018).
- Nash, G., Abbitt, K., Tate, K., Jetha, K., Egginton, S. Changes in the mechanical and adhesive behaviour of human neutrophils on cooling in vitro. Pflugers Arch. 442 (5), 762-770 (2001).
- Hundhammer, T., Gruber, M., Wittmann, S. Paralytic impact of centrifugation on human neutrophils. Biomedicines. 10 (11), 2896 (2022).
- Krémer, V., Godon, O., Bruhns, P., Jönsson, F., De Chaisemartin, L. Isolation methods determine human neutrophil responses after stimulation. Front Immunol. 14, 1301183 (2023).
- Freitas, M., Porto, G., Lima, J. L. F. C., Fernandes, E. Isolation and activation of human neutrophils in vitro. The importance of the anticoagulant used during blood collection. Clin Biochem. 41 (7-8), 570-575 (2008).
재인쇄 및 허가
JoVE'article의 텍스트 или 그림을 다시 사용하시려면 허가 살펴보기
허가 살펴보기This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. 판권 소유