Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A Cell-to-cell Macromolecular Transport Assay in Planta Utilizing Biolistic Bombardment
W tym Artykule
Podsumowanie
Macromolecular trafficking between plant cells can be assessed by transiently expressing a fluorescently-tagged protein of interest and analyzing its intra- and intercellular distribution by confocal microscopy.
Streszczenie
Here, we present a simple and rapid protocol to detect and assess the extent of cell-to-cell macromolecular transport in planta. In this protocol, a fluorescently tagged-protein of interest is transiently expressed in plant tissue following biolistic delivery of its encoding DNA construct. The intra- and intercellular distribution of the tagged protein is then analyzed by confocal microscopy. We describe this technology in detail, providing step-by-step protocols to assay and evaluate the extent of symplastic protein transport in three plant species, Arabidopsis thaliana, Nicotiana benthamiana and N. tabacum (tobacco).
Protokół
Background
Symplastic transport of macromolecules through plant intercellular connections, the plasmodesmata, is of interest to many plant pathologists and biologists. For example, several viral proteins are known to regulate plasmodesmal size exclusion limits to enable viral movement1-3. Also, some endogenous proteins, among them important developmental regulators, are assumed to move from cell to cell, presumably through plasmodesmata, to function non-cell-autonomously4. Thus, a reliable methodology to identify and visualize macromolecular transport between plant cells is much in demand.
1)Grow the target plants
For high transformation efficiency, healthy, robust plants should be used.
1. Arabidopsis plants
Grow one Arabidopsis plant on Pro-Mix BX in a pot (10 cm x 10 cm x 10 cm) in an environment-controlled chamber with a short photoperiod (8 hr of 130-150 μE m-2 s-1 light at 23°C/16 hr dark at 20°C) and 40-65% relative humidity for 6 to 8 weeks5. Fertilize them occasionally with commercially available products as described5. Leaves with the size larger than 15 mm x 35 mm (the length measurement includes petiole) are selected for the experiments.
2. N. benthamiana and N. tabacum
Grow one plant on Pro-Mix BX in a pot (20 cm x 20 cm x 20 cm) in an environment-controlled chamber with a long photoperiod (16 hr of 130-150 μE m-2 s-1 light at 23°C/8 hr dark at 20°C) and 40-65% relative humidity for 7 to 10 weeks. Fertilize them occasionally with commercially available products as describe. Leaves with size larger than 50 mm x 70 mm for N. benthamiana, or 100 mm x 125 mm for N. tabacum (these length measurements do not include petiole) are selected for the experiments.
2) Preparation of the Gene Gun Cartridge with DNA-coated Gold Microparticles
The protocol for this experimental step has been described in detail previously6. It is very important to use well purified plasmid DNA at high concentrations (~1 μg/μl) to obtain the highest transformation efficiency for the ease of confocal microscopy analysis during the later stages of the experiment.
For this assay, it is important to ascertain that the prepared cartridge does not transform two or more adjacent cells simultaneously at high frequency, because the numbers of cells associating with a fluorescent signal cluster is used as an indicator of the extent of symplastic transport (i.e., a single cell containing the signal indicates no movement, whereas a multicell signal cluster indicates movement). The quality of the each cartridge can be checked by analyzing the expression of the protein 16-20 hours post bombardment. Our protocol6 produces cartridges that yield multicell expression clusters only in <3% of all expression events in this time frame, thus suitable for this experiment.
3) Biolistic Delivery of DNA-coated Microparticles
- Remove the leaves of the same developmental stage (i.e. with the same size and same age, see step 1) with a sharp razor blade and immediately place them with the abaxial sides facing up onto a flat Styrofoam surface. The abaxial side of the leaf represents a better substrate for bombardment due to its lower trichome density and thinner cuticle. Arabidopsis leaves, because of their relatively small size, should be covered with a piece of window screen mesh, and the mesh then secured with pushpins to the Styrofoam surface. Maintaining leaves flat increases the efficiency of the particle delivery and minimizes the damage to the tissue during microbombardment.
- Insert the cartridge with DNA-coated microparticles (prepared in step 1) into the gun. For Arabidopsis, the shooting is performed at pressures of 80-110 psi for 1-μm microparticles, and 140-160 psi for 0.6-μm microparticles. For N. benthamiana and tobacco, the shooting is performed at pressures of 100-120 psi for 1-μm microparticles, and 160-180 psi for 0.6-μm microparticles. For Arabidopsis, we usually utilize one cartridge per leaf, because each leaf has enough surface area only for one shot which spreads microparticles over an area of 10-12 mm in diameter. With larger N. benthamiana and tobacco leaves, multiple bombardments of the same leaf are possible, however, they must be done at the symmetrical positions on each side of the mid-rib to target the leaf areas at the same developmental stage (see Discussion).
- Remove the leaves from the Styrofoam surface and place them into a Petri dish on 3 layers of wet Whatman filter paper, seal the Petri dish with Parafilm, and incubate it at room temperature for 36-48 hr to allow expression of the delivered transgenes and potential cell-to-cell movement of their protein products.
4) Imaging of Protein Expression
The fluorescent signal of the transiently expressed tagged proteins is visualized by confocal microscopy. Care should be taken to find optimal microscopy settings for detection of each tested protein. For example, proteins that show limited intracellular accumulation with weak signal intensities, such as the plasmodesmal localization of the Tobacco mosaic virus movement protein (TMV MP)1-3,6, should be observed under a 40X objective lens, which affords higher resolution and sensitivity, whereas proteins that show cytoplasmic distribution with strong signal intensities, such as free YFP, can be visualized under a 10X objective lens with confocal zoom function for faster imaging (see Figure 1).
Symplastic transport is inferred from the appearance of multicell clusters that contain the fluorescent signal. The number of such clusters and number of cells in each cluster is indicative of the extent of cell-to-cell transport. To obtain reliable data, at least 100 expression clusters should be recorded per each experimental system. For example, if the cell-to-cell movement of a protein is compared in two different genetic backgrounds (e.g., wild type and transgenic plants), of a total 200 expression clusters should be recorded. Importantly, experiments, the results of which are to be compared to each other, should be carried out simultaneously.
5. Representative Results
The figure below illustrates representative experiments for detection of symplastic transport of fluorescently tagged proteins. Panels A and B show the typical confocal images that are obtained following microbombardment of a N. benthamiana leaf with a TMV MP-YFP-expressing construct. In panel A, symplastic movement of TMV MP-YFP is observed based on the appearance of multicell clusters of the YFP signal. Not all transiently expressed TMV MP-YFP is able to move as evidenced by single-cell signal in some microbombardments (panel B). Statistically, in <40% of the counted signal clusters, TMV MP-YFP is unable to move between cells whereas in >60% of the clusters, the protein moves between 2-5 cells, with the two-cell spread being the most frequent (ca. ~50% cases) (Figure 1C).
The proteins with relatively small molecular size without innate movement activity can diffuse through PD to move cell-to-cell. For example, free YFP, or 1xYFP (ca. 27 kDa, panel D), spreads between several cells in 30% of the counted clusters (panels C). This non-specific diffusion does not occur for a translational YFP dimmer, or 2xYFP (54 kDa, panel E), which is comparable in size to TMV MP-YFP fusion protein (57 kDa), and which is completely cell-autonomous (Figure 1C).
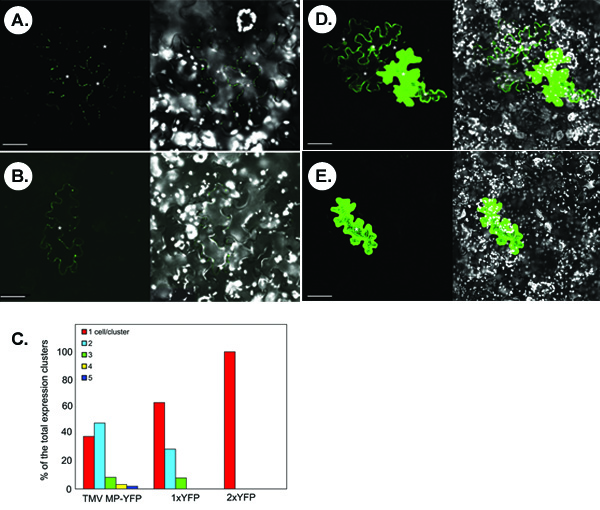
Figure 1.Typical results obtained from the symplastic transport assay in N. benthamiana leaf tissues. (A, B) Visualization of TMV MP-YFP. (C) Quantitation of signal clusters. 1xYFP and 2xYFP, free YFP and translational YFP dimer, respectively. (D) Visualization of 1xYFP. (E) Visualization of 2xYFP. In micrographs, the left panels show the YFP signal and the right panels show merged images of YFP (in green) and chloroplast autofluorescence (in white) signals. Images are single confocal sections. Asterisks in the micrographs show the epidermal cells showing YFP signal. Bars = 50 μm.
Dyskusje
The key for the success of the symplastic transport assay is to obtain high transformation efficiency, which allows production of statistically significant and easily detectible signal clusters. This can be achieved using the leaves harvested from healthy, robust plants, and preparing gold particles coated by a pure and concentrated DNA preparation.
Using the leaves at the same growth stage is also vital for the assay reliability. Plasmodesmal aperture is known to be differentially regulated,...
Ujawnienia
No conflicts of interest declared.
Podziękowania
Our work is supported by grants from NIH/NIGMS, NSF, USDA/NIFA, and BARD to VC.
Materiały
| Name | Company | Catalog Number | Comments |
| Gold microparticles, 1.0 μm in diameter | Bio-Rad | 165-2262 | |
| Gold microparticles, 0.6 μm in diameter | Bio-Rad | 165-2263 | |
| Spermidine | Sigma-Aldrich | S0266-1G | |
| Tefzel tubing | Bio-Rad | 165-2441 | |
| Helios cartridge preparatory station | Bio-Rad | 165-2420 | |
| Tubing cutter | Bio-Rad | 165-2422 | |
| Helios gene gun | Bio-Rad | 165-2432 | |
| Helium gas regulator | Bio-Rad | 165-2413 |
Odniesienia
- Waigmann, E., Ueki, S., Trutnyeva, K., Citovsky, V. The ins and outs of non-destructive cell-to-cell and systemic movement of plant viruses. . Crit Rev Plant Sci. 23, 195-250 (2004).
- Lucas, W. J. Plant viral movement proteins: agents for cell-to-cell trafficking of viral genomes. Virology. 344, 169-184 (2006).
- Epel, B. L. Plant viruses spread by diffusion on ER-associated movement-protein-rafts through plasmodesmata gated by viral induced host beta-1,3-glucanases. Semin Cell Dev Biol. 20, 1074-1081 (2009).
- Zambryski, P. C., Crawford, K. Plasmodesmata: gatekeepers for cell-to-cell transport of developmental signals in plants. Annu Rev Cell Dev Biol. 16, 393-421 (2000).
- Rivero-Lepinckas, L., Crist, D., Scholl, R. Growth of plants and presercation of seeds. Methods Mol Biol. 323, 3-12 (2006).
- Ueki, S., Lacroix, B., Krichevsky, A., Lazarowitz, S. G., Citovsky, V. Functional transient genetic transformation of Arabidopsis leaves by biolistic bombardment. Nat Protoc. 4, 71-77 (2009).
- Oparka, K. J. Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell. 97, 743-754 (1999).
- Imlau, A., Truernit, E., Sauer, N. Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell. 11, 309-322 (1999).
- Gisel, A., Barella, S., Hempel, F. D., Zambryski, P. C. Temporal and spatial regulation of symplastic trafficking during development in Arabidopsis thaliana apices. Development. 126, 1879-1889 (1999).
- Kim, I., Cho, E., Crawford, K. M., Hempel, F. D., Zambryski, P. C. Cell-to-cell movement of GFP during embryogenesis and early seedling development in Arabidopsis. Proc Natl Acad Sci USA. 102, 2227-2231 (2005).
- Crawford, K. M., Zambryski, P. C. Non-targeted and targeted protein movement through plasmodesmata in leaves in different developmental and physiological states. Plant Physiol. 125, 1802-1812 (2001).
- Roberts, A. G. Phloem unloading in sink leaves of Nicotiana benthamiana: comparison of a fluorescent solute with a fluorescent virus. Plant Cell. 9, 1381-1396 (1997).
- Guenoune-Gelbart, D., Elbaum, M., Sagi, G., Levy, A., Epel, B. L. Tobacco mosaic virus (TMV) replicase and movement protein function synergistically in facilitating TMV spread by lateral diffusion in the plasmodesmal desmotubule of Nicotiana benthamiana. Mol Plant Microbe Interact. 21, 335-345 (2008).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone