Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A Method for Labeling Vasculature in Embryonic Mice
W tym Artykule
Podsumowanie
This article describes a method for labeling embryonic skin and thymus blood vessels.
Streszczenie
The establishment of a functional blood vessel network is an essential part of organogenesis, and is required for optimal organ function. For example, in the thymus proper vasculature formation and patterning is essential for thymocyte entry into the organ and mature T-cell exit to the periphery. The spatial arrangement of blood vessels in the thymus is dependent upon signals from the local microenvironment, namely thymic epithelial cells (TEC). Several recent reports suggest that disruption of these signals results in thymus blood vessel defects 1,2. Previous studies have described techniques used to label the neonatal and adult thymus vasculature 1,2. We demonstrate here a technique for labeling blood vessels in the embryonic thymus. This method combines the use of FITC-dextran or Griffonia (Bandeiraea) Simplicifolia Lectin I (GSL 1 - isolectin B4) facial vein injections and CD31 antibody staining to identify thymus vascular structures and PDGFR-β to label thymic perivascular mesenchyme 3-5. The option of using cryosections or vibratome sections is also provided. This protocol can be used to identify thymus vascular defects, which is critical for defining the roles of TEC-derived molecules in thymus blood vessel formation. As the method labels the entire vasculature, it can also be used to analyze the vascular networks in multiple organs and tissues throughout the embryo including skin and heart 6-10.
Protokół
1. Fluorescein labeled dextran and GSL I-isolectin B4 facial vein injections to label embryonic vasculature
- Prepare FITC-dextran (50ug/mL) in Phosphate Buffered Saline (PBS) or GSL 1 - isolectin B4 (20ug/200uL) in PBS in a 1.5mL Eppendorf tube and warm to 37°C. Add 100uL of stock 1.25mM Fast Green/PBS to the FITC-dextran solution (total volume 1mL) and 180uL of stock 1.25mM Fast Green/PBS to the GSL 1 - isolectin B4 (total volume 200uL), so that the solution is visibly blue.
- Dissect E14.5-E18.5 embryos and yolk sac together, leaving the allantoic stalk (umbilical artery and vein) intact.
- Transfer embryos to a new petrie dish (60 X 15 mm) and immerse them in PBS at room temperature.
- Position the embryo to provide a sagittal view of the head/face. Use micro dissecting forceps to gently grasp the embryo at the head.
- Using a 30G needle, inject 50uL FITC-dextran (50ug/mL) or GSL 1 - isolectin B4 (20ug in 200uL PBS) into the facial vein pointing the needle toward the back of the head.
- When the dye is visible in the umbilical vein, remove the needle and separate the embryo from the allantoic stalk (umbilical artery and vein).
- Following injections, allow embryo to remain in PBS at room temperature for 2-3 minutes so that the dye circulates throughout the embryo.
2. Whole-mount analysis of skin vasculature
- After allowing the dye to circulate throughout the embryo, remove skin samples from regions of the limbs, back, and stomach, etc. 8,9.
- Wash skin sample in cold PBS, and fix in 4% PFA/PBS for 2 hours 8,9. Wash 3 times for 10 minutes each in 4mL clear vial with 2mL cold PBS.
- Place skin sample on a microscope slide and add 100 μl of mounting media to each slide and a cover glass.
- Allow slides to dry in a dark storage area.
- Proceed to Step 2 'Image Acquisition' section.
3. Multi-color labeling of thymus and heart vasculature and perivascular cells for cryosections (Continue from Section 1, Step 7)
- 'Flash freeze' whole embryo in liquid nitrogen. Embryos can be and stored at -80°C until analysis.
- Alternatively, dissect out thymus, rinse in 4°C PBS, and fix in 2mL 4% Paraformaldehyde (PFA)/PBS for 2 hours. Wash 3 times for 10 minutes in cold PBS, place thymi in OCT, and freeze and store until use at -80°C.
- For cryosectioning, spread OCT on a section 'block' and mount the embryo or dissected organs/tissues for sectioning.
- Cut frozen tissue into 10 μm thick sections and collect on slides.
- Fix sections in acetone for 5-10 minutes. Wash 3 times in cold TBS.
- Block in 10% donkey serum/TBS in a humidity chamber at room temperature.
- Incubate sections for 1 hour-overnight with 100 μl of primary antibodies in a humidity chamber at 4°C: in this example, we use rat anti-mouse CD31 (1:100) to label endothelium, and goat anti-mouse PDGFR-β (1:100) to label perivascular cells. It is useful to cover slides with individually cut Parafilm strips to ensure that the antibody is uniformly spread across the section.
- Following incubation with primary antibody, wash sections 3 times in cold TBS. Incubate with 100 μl of appropriate secondary antibodies for 30 minutes minimum.
- Wash 3 times in cold TBS. Add 100 μl of mounting media to each slide and a cover glass.
- Allow slides to dry in a dark storage area.
- Proceed to 'Image Acquisition' section.
4. Multi-color labeling of thymus vasculature and perivascular cells for vibratome sections (Continue from Section 1, Step 7)
- Dissect out thymus lobes from embryo and rinse in cold PBS.
- Fix thymus in 4% PFA/PBS at room temperature for 2 hours.
- Wash in PBS-Triton X (0.15%) 3 times, 10 minutes and place thymi in a small plastic cartridge and submerge in 4% low melt agarose/PBS (˜4°C). The thymus should be in contact with the bottom of the cartridge.
- Allow agarose to solidify on ice (3-5 minutes). Use a razor blade to cut off excess agarose. Add glue to the vibratome block and adhere sample to the block.
- Add cold PBS to vibratome water bath until the sample and blade are immersed.
- Set speed and amplitude (high amplitude and low-moderate speed is ideal for soft thymus sections). The amplitude should be reduced if sections break up due to excess agitation.
- Cut 50 um sections.
- Using a paintbrush, collect sections in a 24-well microplate in cold PBS.
- Block sections in 500 μl of 10% donkey serum in PBS-Triton X (0.15%) for 30 minutes.
- Incubate sections for 8 hours to overnight with primary antibody, such as anti-CD31 and anti-PDGFR-β, in a covered 24-well microplate.
- Wash 3 times in PBS-Triton X (0.15%) over a total of 8 hours at 4°C.
- Block sections in 10% donkey serum in PBS-Triton X (0.15%) for 30 minutes.
- Incubate sections for 8 hours to overnight at 4°C with appropriate secondary antibodies.
- Wash 3 times in PBS-Triton X (0.15%) over a total of 8 hours at 4°C.
- Re-fix samples in 4% PFA/PBS for 30 minutes on ice.
- Wash 3 times in PBS-Triton X (0.15%) over 30 minutes on ice.
- Dehydrate samples through a graded MeOH/PBS-Triton X series: 25% MeOH, 50% MeOH, 75% MeOH, and 100% MeOH at 10 minutes for each step. Replace 100% MeOH with fresh MeOH after 10 minutes and incubate for 1 hour at room temperature.
- In a glass container, mix BABB (Benzyl Alcohol:Benzyl Benzoate) in a 1:2 ratio. Combine BABB with MeOH for a final concentration of 50% BABB and 50% MeOH. Incubate the sample in BABB:MeOH for 10-15 minutes.
- Transfer sample to a glass container with 100% BABB and incubate for 10-15 minutes or until cleared, at room temperature.
- Fill depression slide (0.7mm depth) with fresh 100% BABB and transfer sample to the side. Add cover glass (No. 1.5) and seal with 2-3 coats of nail polish. Allow nail polish to harden in the dark at room temperature, then store sample at 4°C.
Note: Slides must be completely sealed prior to confocal image acquisition. Images should be acquired within 12-24 hours, as fluorescent dyes can fade in BABB. - Proceed to 'Image Acquisition' section.
5. Image acquisition
- Image 10 μm frozen sections with a confocal microscope using the Plan-Apochromat 20X/0.8 objective (512 X 512 Pixels) with 488- (FITC-dextran/GSL 1 - isolectin B4), 543-, and 633-nm laser lines.
- Acquire confocal z-sections of whole mount skin and 50 μm agarose-embedded sections using the Plan-Apochromat 10X/0.4 objective (512 X 512 Pixels) with 488- (FITC-dextran/GSL 1 - isolectin B4), 543-, and 633-nm laser lines. Serial Z-sections should be collected sequentially at 1-micron for each respective channel.
- Reconstruct serial Z-sections using Zeiss Axiovision 4.6 or other image analysis software.
6. Representative Results:
Efficient labeling of the embryonic vasculature is critical for assessing blood vessel defects in embryonic mice. Figure 1 shows specific labeling of E16.5 thymus blood vessels (1A-B) and co-labeling with CD31 (1B), in addition to staining of the right and left ventricles (1E-F), respectively. The GSL I-isolectin B4 protocol for cryosections as described in sections 1, 3, and 5 was used in these experiments. Whole-mount labeling of the skin blood vessel network on E16.5 mice, using the protocols described in sections 1, 2, and 5 is shown in Figure 1C-D.
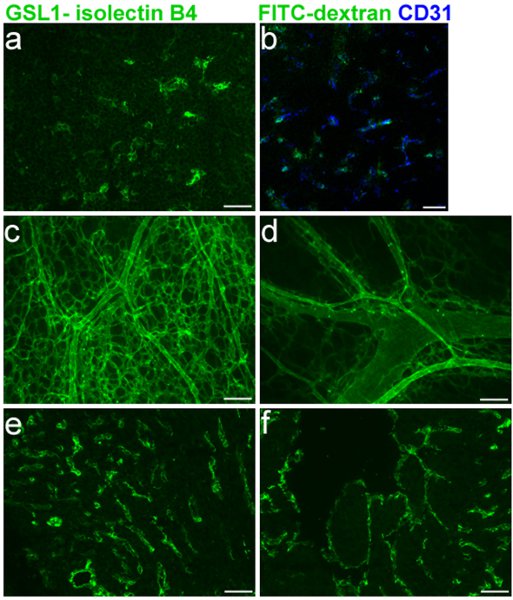
Figure 1 Legend. FITC GSL I - isolectin B4 facial vein injections into E16.5 mouse embryos. a. Cryosection of embryonic thymus following injection. b. Merge of CD31 co-labeling with isolectin B4. c. and d. Whole-mount of embryonic skin vasculature following injection. e. and f. Cryosection of embryonic heart e. (right ventricle) f. (left ventricle) following injection.
Dyskusje
Whole-mount and PECAM-1 (CD31) staining on sections are the conventional methods for labeling the vasculature in embryonic mice. These methods require the use of direct and/or indirect immunofluorescence, and detergents to permeabilize mouse tissue. This proves to be a rather timely process. Here, we have employed FITC-dextran or isolectin B4 facial vein injections to directly label the embryonic vasculature, thereby eliminating the requirement for antibody labeling steps. Furthermore, this method allows the a...
Ujawnienia
No conflicts of interest declared.
Podziękowania
This work was supported by grant numbers R01AI055001 and R01AI082127 from NIAID to NRM and SREB Dissertation Fellowship Award to JLB.
Materiały
| Name | Company | Catalog Number | Comments |
| FITC-dextran | Sigma-Aldrich | FD150S-1G | |
| Fluorescein labeled GSL 1 - isolectin B4 | Vector Laboratories | FL-1201 | |
| Fast Green | MP Biomedicals | 195178 | |
| PFA | Fluka | 76240 | |
| Fetal Bovine Serum | Atlanta Biologicals | S11550 | |
| Optimal Cutting Temperature Compound (O.C.T. | VWR international | 25608-930 | |
| Acetone | JT Baker | 9006-33 | |
| Donkey Serum | Jackson | 017-000-121 | |
| rat anti-mouse CD31, | BD Biosciences | 558736 | |
| goat anti-mouse PDGFR-β | R&D Systems | AF1042 | |
| donkey anti-rat CD31 Alexa 647 (Invitrogen) | Biolegend | 102516 | |
| donkey anti-goat Alexa 594 (Invitrogen) | Invitrogen | A11058 | |
| Triton X -100 | Sigma-Aldrich | X-100 | |
| Low melt agarose/PBS | Sigma-Aldrich | A9414-25G | |
| Methanol | Fisher Scientific | A413-4 | |
| Benzyl Alcohol | Acros Organics | 148390010 | |
| Benzyl Benzoate | Acros Organics | 105860010 | |
| Depression slides | Fisher Scientific | S175201 | |
| Fluorogel | Electron Microscopy Sciences | 17985-10 | |
| Cover Glass (22X22)-1.5 | Thermo Fisher Scientific, Inc. | 152222 | |
| Zeiss LSM 510 Meta Confocal Microscope | Carl Zeiss, Inc. | ||
| Micro dissecting forceps | Roboz Surgical Instruments Co. | RS-5135 | |
| Parafilm No. OM992 | Fisher Scientific | 13-374-16 | |
| 12 and 24 well microplates | Evergreen Scientific | 222-8044-01F | |
| Superfrost/PlusMicroscope Slides | Fisher Scientific | 12-550-15 | |
| 4mL clear vials | National Scientific Company | B7800-2 |
Odniesienia
- Cuddihy, A. R. VEGF-mediated cross-talk within the neonatal murine thymus. Blood. 113, 2723-2731 (2009).
- Muller, S. M. Gene targeting of VEGF-A in thymus epithelium disrupts thymus blood vessel architecture. Proc. Natl. Acad. Sci. U. S. A. 102, 10587-10592 (2005).
- Muller, S. M. Neural crest origin of perivascular mesenchyme in the adult thymus. J. Immunol. 180, 5344-5351 (2008).
- Foster, K. Contribution of neural crest-derived cells in the embryonic and adult thymus. J. Immunol. 180, 3183-3189 (2008).
- Liu, C. Coordination between CCR7- and CCR9-mediated chemokine signals in prevascular fetal thymus colonization. Blood. 108, 2531-2539 (2006).
- Lavine, K. J. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev. 20, 1651-1666 (2006).
- Lavine, K. J., Kovacs, A., Ornitz, D. M. Hedgehog signaling is critical for maintenance of the adult coronary vasculature in mice. J. Clin Invest. 118, 2404-2414 (2008).
- Mukouyama, Y. S., Gerber, H. P., Ferrara, N., Gu, C., Anderson, D. J. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 132, 941-952 (2005).
- Mukouyama, Y. S., Shin, D., Britsch, S., Taniguchi, M., Anderson, D. J. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 109, 693-705 (2002).
- Murphy, P. A. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc. Natl. Acad. Sci. U. S. A. 105, 10901-10906 (2008).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone