Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
In vitro Electroporation of the Lower Rhombic Lip of Midgestation Mouse Embryos
W tym Artykule
Podsumowanie
This study describes the development of an in vitro electroporation technique that allows for the manipulation of gene expression in the lower rhombic lip of midgestation embryos.
Streszczenie
The rhombic lip is an embryonic neuroepithelium located in the hindbrain at the junction between the neural tube and the roofplate of the fourth ventricle (reviewed in 1). The rhombic lip can be subdivided into the upper rhombic lip (URL) which encompasses rhombomere 1 (r1) and generates neurons of the cerebellum and the lower rhombic lip (LRL) which gives rise to diverse neuronal brainstem lineages 2-4. LRL derivatives include the auditory neurons of the cochlear nuclei and those of the precerebellar nuclei that are involved in regulating balance and motor control 5-8. Neurogenesis from the LRL occurs over a large temporal window that encompasses embryonic days (E) 9.5-16.55, 9. Different neuronal lineages emerge from the LRL as postmitotic cells (or are born) during distinct developmental days during this neurogenic window.
Electroporation of gene expression constructs can be used to manipulate gene expression in LRL progenitors and can potentially change the fate of the neurons produced from this region 10-12. Altering gene expression of LRL progenitors in the mouse via in utero electroporation has been highly successful for manipulating lineages born on embryonic day E12.5 or later 10, 12-14. In utero electroporations prior to E12.5 have been unsuccessful primarily due to the lethality associated with puncturing the fourth ventricle roofplate, a necessary step in delivering exogenous DNA that is electroporated into the LRL. However, many LRL derived lineages arise from the LRL earlier than E12.5 9. These earlier born lineages include the neurons that comprise the lateral reticular, external cuneate, and inferior olivary nuclei of the precerebellar system which function to connect inputs from the spinal cord and cortex to the cerebellum 5. In order to manipulate expression in the LRL of embryos younger than E12.5, we developed an in vitro system in which embryos are placed into culture following electroporation.
This study presents an efficient and effective method for manipulating the gene expression of LRL progenitors at E11.5. Embryos electroporated with green fluorescent protein (GFP) driven from the broadly active CAG promoter reproducibly expressed GFP after 24 hours of culture. A critical aspect of this assay is that gene expression is only altered because of the expression of the exogenous gene and not because of secondary effects that result from the electroporation and culturing techniques. It was determined that the endogenous gene expression patterns remain undisturbed in electroporated and cultured embryos. This assay can be utilized to alter the fate of cells emerging from the LRL of embryos younger than E12.5 through the introduction of plasmids for overexpression or knock down (through RNAi) of different pro-neural transcription factors.
Protokół
1. Preparations Prior to Electroporation
- Amplify the DNA for electroporation by a maxi prep (Prime-It or Qiagen). The concentration of the DNA should be a minimum of 1 mg/mL for efficient uptake.
- Remove 495 μL of DNA and mix with 5 μl of 0.01% Fast Green in 1 X PBS (phosphate buffered saline) in a microcentrifuge tube.
2. Embryonic Harvest
- Establish timed matings of CD-1 mice (Harlan). Check for the presence of vaginal plugs and regard the date a vaginal plug is observed as embryonic day (E) 0.5. Embryos will be harvested 11 days after visualizing plug (E11.5).
- Place sterilized tools in 70% ethanol. Treat a laminar flow hood with UV light for at least one hour prior to use. Spray hood, dissecting tray, and dissecting scope with 70% ethanol. Pre-heat 1 X PBS to 37 °C.
- On E11.5 euthanize the female according to conditions approved by the Institutional Committee for the Care and Use of Animals (ICCUA). Place dissecting tray in the laminar flow hood. Spray down the abdomen of the female with 70% ethanol.
- Open the peritoneal cavity and pin the walls to the dissecting tray. Pull out the uterine horns so that it rests within the peritoneal cavity.
- Carefully cut open the wall of the uterine horn. Use a 20 mm spoon (Fine Scientific Tools) to gently remove the embryo in the yolk sac away from the placenta. Place embryos into a 100 mm tissue culture dish prefilled with 10 mL of sterile 1 X PBS that was preheated in 2.2.
- Repeat for all embryos present.
3. Electroporation of E11.5 Embryos (Figure 1)
- Using a 20 mm spoon, transfer the first embryo to a new 100 mm dish prefilled with 10 mL of sterile 1XPBS that was preheated to 37 °C.
- Use 11 cm forceps with a 0.05 x 0.02 mm tip (Fine Scientific Tools) to carefully remove and discard the yolk sac.
- Position embryo so that its dorsal side is facing up so that it resembles the cartoon in Figure 1A. Use 7 mm electrode paddles (Harvard Apparatus) to gently hold the embryo. The electrodes should be positioned at either side of the neural tube at the level of the hindbrain fourth ventricle. The ventricle is visible to the naked eye but using a dissection microscope can facilitate accurate paddle placement. Positioning of the paddles is critical to determining the region that receives the electroporated DNA. If the lower rhombic lip (LRL) of the dorsal embryonic hindbrain is desired, paddles must be place such that they are directly flanking the widest part of the fourth ventricle opening.
- Use a 1 cc syringe to draw up the plasmid DNA mixed with 0.01% Fast Green. 500 μL of the mixture should be sufficient for the electroporation of at least 8-10 embryos (see 3.5).
- Gently puncture the roofplate overlying the fourth ventricle with a 25G 5/8 tuberculin needle attached to the 1 cc syringe and inject the DNA-dye mixture into the ventricle. Successful injections are characterized by the DNA-dye mixture filling the entire ventricular system (Figure 1C). The amount of DNA-dye mixture typically injected is less than 50 μL. Exact amounts are variable between embryos as a portion of the mixture tends to leak out of ventricle into the PBS surrounding the embryo. An alternate means of delivering the DNA-dye mixture would be through accessing the ventricular system by puncturing the velum overlying the midbrain. Again, successful injections are characterized by the DNA-dye mixture filling the entire ventricular system.
- Deliver five square pulses using an electric pulse generator (BTX) and 7 mm electrode paddles. Each pulse is 50 V lasting 5 ms per pulse with 500 ms between each pulse. The tissue closest to the positively charged electrode will then take up the plasmid.
4. Culture of Embryos
- In a laminar flow hood, fill the outer wells of a 12-well culture dish with 2 mL of DMEM/F12 media supplemented with 10% fetal bovine serum, 5% equine serum, 1% glutamine, 1% penicillin/streptomycin that was preheated to 37 °C. The culture conditions were adapted from de Diego and colleagues.15
- In laminar flow hood pinch embryos at midsection (below the heart) with forceps and remove posterior portion of embryo. Place the anterior portion into one of the filled wells of the 12-well culture dish.
- Repeat for all embryos. Fill only the outer wells of the 12-well plate to avoid contamination of cultures.
- Culture embryos in a 37 °C incubator with 5% CO2. Expression of the electroporated plasmid should be observable within 24 hr.
- Should longer culture times be desired, fill the out wells of a new 12 well plate with 2 mL of the media used in 4.1. With a sterilized spoon transfer the embryos to a well in the new plate. Place back into the 37 °C incubator. Culture for up to 48 hr is possible.
- When the desired culture time is reached, fix embryos for analysis (see below).
5. Preparation of Embryos for Analysis
- Rinse embryos in 1 X PBS at 4 °C for five min. Repeat.
- Fix embryos for analysis in 2% paraformaldehyde (PFA) in 1XPBS for 2 hours at 4 °C.
- Rinse embryos in 1 X PBS at 4 °C for five min. Repeat.
- Equilibrate embryos in 30% sucrose in 1 X PBS overnight at 4 °C.
- Embed embryos in Optimal Cutting Temperature (O.C.T) compound using a dry ice/ethanol bath. Embryos can be stored at -20 °C.
- Section embryos on a cryostat (Leica) into 30 μm sections and mount on slides (VWR, Superfrost Plus). Store at -20 °C.
6. Immunohistochemistry Analysis
- Immunohistochemistry was performed as described in 16. Primary antibody dilutions used for this study include rabbit α-GFP (Invitrogen) 1:2500; mouse α-Mash1 (BD Biosciences) 1:100; rabbit α-Ngn1 (Jane Johnson) 1:5000; rabbit α-Ptf1a (Jane Johnson) 1:2500; rabbit -Math1 (Jane Johnson) 1:100. Incubate slide flat, specimen side up on a staining tray at 4 °C overnight.
- Slides were analyzed on a compound microscope (Olympus BX51).
7. Representative Results
A schematic in Figure 1A depicts the electroporation experiment. Figure 1B shows a sagittal view of an E11.5 embryo prior to manipulation. The same embryo following injection of the plasmid containing CAG::GFP in 0.01% Fast Green is shown in Figure 1C and a representative unfixed embryo exhibiting unilateral GFP expression in the dorsal hindbrain 24 hr following culture is shown in Figure 1D. The extent of the area of the LRL that is successfully electroporated is variable and appears to be highly dependent on positioning of the electrodes. In our studies it was found that 52 out of 65 (80%) of the electroporated embryos successfully expressed GFP. Tissue was deemed to be successfully electroporated if it was positive for GFP in localized regions over several sections following fixation and immunohistochemical analysis (see below). Embryos that failed to meet this criteria were scored as unsuccessful attempts at electroporation.
Further assessment of electroporation efficiency can be ascertained by performing immunohistochemistry against GFP on transverse sections of electroporated embryos at the level of the fourth ventricle. Figure 2 shows representative serial sections from an embryo displaying unilateral GFP expression. Figure 2A shows an idealized schematic that illustrates that the left side of the embryo was toward the positive electrode. Transverse sections (at levels represented by the middle cartoon of Figure 2A) reveal localized GFP expression exclusively on the left side of the hindbrain tissue (Figures 2C and 2D). The electroporated area of the LRL did not extend the entire anterior-posterior axis of the neural tube as examination of sections 300 μm rostral or greater to that shown in Figure 2C does not express GFP (Figure 2B).
The utility of this assay for manipulation of gene expression is dependent upon the stability of the expression domains for the endogenous proteins. The LRL has been characterized as possessing unique progenitor domains characterized by the differential expression of proneural transcription factors (reviewed in 1). A subset of these factors (Mash1, Math1, Ngn1, and Ptf1a) were chosen for analysis due to their proposed and/or characterized roles in the specification of precerebellar neural subtypes in the LRL, a subject of future studies 16-18. All four proteins have highly characteristic expression domains in the caudal hindbrain at E11.5 16-18. We observed that embryos that were placed cultures failed to increase in size and also failed to initiate production of choroid plexus epithelium and invagination of the LRL and roofplate, morphological events that occur between E11.5 and E12.5 5. Based on these observations it was determined that normal development in these embryos was halted or grossly delayed and the comparable control for the cultured embryos should be uncultured embryos at E11.5.
To ensure that culture and electroporation do not disturb levels of endogenous proteins, we analyzed four different proteins by immunohistochemistry (IHC) in 34 different embryos that were electroporated and then cultured for at least 24 hr. Table I shows the number of embryos analyzed for each marker and the percentage of the embryos analyzed that retained normal protein levels. Figure 3 shows representative IHC data from two of the proteins analyzed, Mash1 (Figures 3A and 3B) and Math1 (Figures 3C and 3D). We observed that the majority of the embryos retained normal levels of expression following electroporation and culture (Figures 3B and 3D) as compared to the control embryos at E11.5 (Figures 3A and 3C). Importantly, the characteristic expression domains of these proteins were not perturbed.
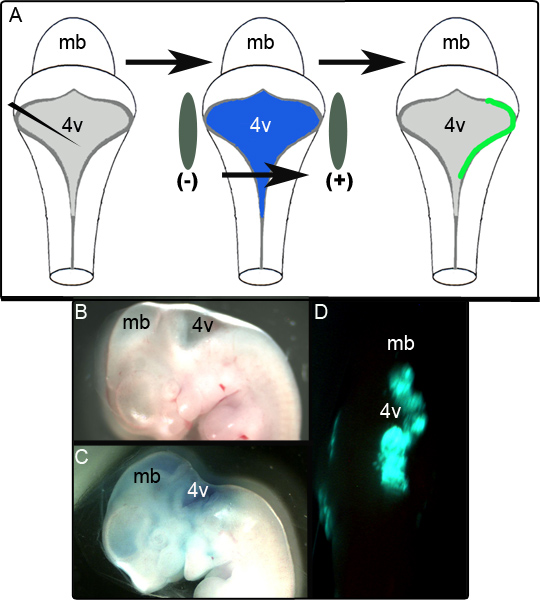
Figure 1. Electroporation of Embryos at E11.5. (A) Schematic of the electroporation experiment. An E11.5 embryo is isolated and the expression plasmid in 0.01% Fast Green is injected into the fourth ventricle. The embryo is then flanked by electrode paddles and subjected to a 50 V pulse prior to being placed into culture (B) Sagittal view of an E11.5 embryo prior to injection. (C) The same E11.5 embryo following injection of plasmid in 0.01% Fast Green. (D) Unilateral hindbrain expression of GFP observed in E11.5 embryo following 24 hr of culture. mb- midbrain; 4v- fourth ventricle.
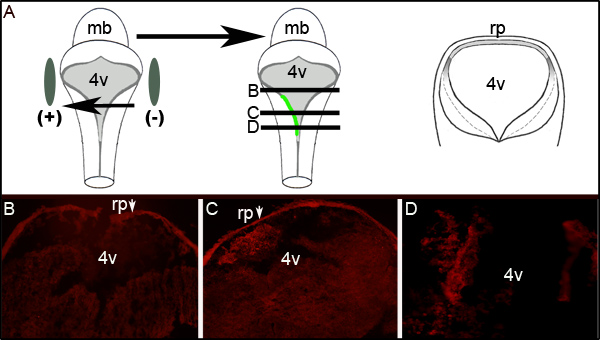
Figure 2. Expression of GFP in Electroporated Tissue. (A) The cartoon on left depicts the placement of electrodes around an E11.5 embryo. Middle cartoon depicts uptake and expression of plasmid encoding GFP on the left side of the embryo. Cartoon on right is schematic of an idealized transverse section taken through the embryo at levels denoted by black lines in middle cartoon. (B−D) Immunohistochemistry for GFP on transverse sections through an electroporated E11.5 embryo after 24 hr of culture. Arrows indicate the roofplate (rp) which traps the secondary antibody. Images are taken at 10X magnification. The relative levels of the sections shown are depicted by the horizontal lines through the middle cartoon in (A). mb- midbrain;l 4v- fourth ventricle; rp- roofplate.
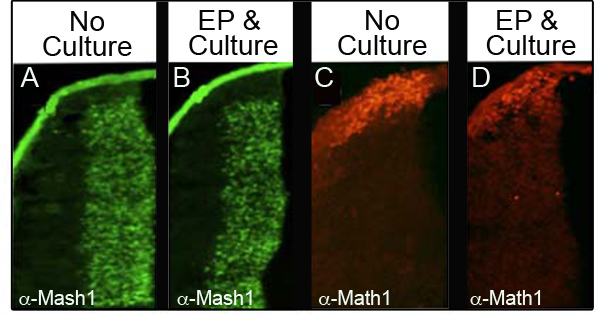
Figure 3. Expression of Endogenous Proteins in the Lower Rhombic Lip. Immunohistochemistry for Mash1 (A and B) or Math1 (C and D) comparing transverse sections of E11.5 embryos that were not cultured (A, C) with embryos that were electroporated with CAG::GFP and cultured for 24 hr (B and D). Images taken at 10X magnification.
| Proneural Transcription Factor Analyzed | Number of Embryos Analyzed | Percentage Retaining Normal Expression Patterns |
| Math1 | 15 | 86.7% |
| Mash1 | 12 | 83.3% |
| Ngn1 | 7 | 71.4% |
| Ptf1a | 6 | 100% |
Table I. Percentage of Electroporated and Cultured Embryos Retaining Normal Proneural Transcription Factor Domains in the LRL.
Dyskusje
The in vitro electroporation technique presented in this study is a novel methodology that can be efficiently utilized to manipulate gene expression in embryos younger than 12 days of gestation. Placement of the embryos into culture permits expression of the introduced gene and circumvents the lethality observed when electroporated embryos are allowed to remain in vivo. This technique allows for the manipulation of gene expression in embryonic progenitors that were previously inaccessible for e...
Ujawnienia
No conflicts of interest declared.
Podziękowania
The authors would like to thank Jane Johnson for the Math1, Ngn1, and Ptf1a antibodies and Connie Cepko for the pCAG::GFP plasmid. This work was funded by NIH R15 1R15HD059922-01.
Materiały
| Name | Company | Catalog Number | Comments |
| Cryostat | Leica Microsystems | CM-1850 | |
| Biologie tip Dumoxel treated DUMONT forceps | Fine Science Tools | 11252-30 | |
| 20 mm MORIA perforated spoon | Fine Science Tools | 10370-17 | |
| ECM 830 Square Wave Electroporation Generator | BTX (VWR) | 47745-928 | |
| Harvard Apparatus 7 mm Tweezertrodes* Electrodes | BTX (Fisher) | BTX450165 | |
| Fisher Isotemp CO2 Incubator | Fisher Scientific | 1325525 | |
| NAPCO CO2 Gas Regulator | Fisher Scientific | 15497020 | |
| 12 Well Tissue Culture Plates | BD Falcon (Fisher) | 877229 | |
| HyClone Liquid Media DMEM/F-12 (1:1); With L-Glutamine and HEPES; 500mL | Thermo Scientific (Fisher) | SH3002301 | |
| HyClone* Donor Equine Serum | Thermo Scientific (Fisher) | SH3007402 | |
| Fetal Bovine Serum, Qualified, Heat Inactivated | Invitrogen | 16140-063 | |
| cellgro* 10,000 IU Penicillin, 10,000μg/mL Streptomycin | Mediatech (Fisher) | MT-30-002-CI | |
| HyClone* L-Glutamine L-Glutamine; 200mM in 0.85% NaCl | Thermo Scientific (Fisher) | SH3003401 | |
| Fast-Green | Fisher Scientific | AC41053-0250 | 0.01% |
Odniesienia
- Ray, R. S., Dymecki, S. M. Rautenlippe Redux -- toward a unified view of the precerebellar rhombic lip. Current opinion in cell biology. 21, 741-747 (2009).
- Machold, R., Fishell, G. Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron. 48, 17-24 (2005).
- Wingate, R. J. The rhombic lip and early cerebellar development. Curr. Opin. Neurobiol. 11, 82-88 (2001).
- Wingate, R. J., Hatten, M. E. The role of the rhombic lip in avian cerebellum development. Development (Cambridge, England). 126, 4395-4404 (1999).
- Altman, J., Bayer, S. A. . Development of Cerebellar System: In relation to its evolution, structure, and function. , (1997).
- Farago, A. F., Awatramani, R. B., Dymecki, S. M. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron. 50, 205-218 (2006).
- Wang, V. Y., Rose, M. F., Zoghbi, H. Y. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 48, 31-43 (2005).
- Rodriguez, C. I., Dymecki, S. M. Origin of the precerebellar system. Neuron. 27, 475-486 (2000).
- Taber-Pierce, E. Histogenesis of the nuclei griseum ponitis, corporis pontobulbaris and reticularis tegmenti pontis (bechterew) in mouse. J. Comp. Neurol. 126, 219-240 (1966).
- Dipietrantonio, H. J., Dymecki, S. M. Zic1 levels regulate mossy fiber neuron position and axon laterality choice in the ventral brain stem. Neuroscience. 162, 560-573 (2009).
- Takahashi, M., Sato, K., Nomura, T., Osumi, N. Manipulating gene expressions by electroporation in the developing brain of mammalian embryos. Differentiation. 70, 155-162 (2002).
- Taniguchi, H., Kawauchi, D., Nishida, K., Murakami, F. Classic cadherins regulate tangential migration of precerebellar neurons in the caudal hindbrain. Development (Cambridge, England). 133, 1923-1931 (2006).
- Kawauchi, D., Taniguchi, H., Watanabe, H., Saito, T., Murakami, F. Direct visualization of nucleogenesis by precerebellar neurons: involvement of ventricle-directed, radial fibre-associated migration. Development (Cambridge, England). 133, 1113-1123 (2006).
- Okada, T., Keino-Masu, K., Masu, M. Migration and nucleogenesis of mouse precerebellar neurons visualized by in utero electroporation of a green fluorescent protein gene. Neuroscience research. 57, 40-49 (2007).
- de Diego, I., Kyriakopoulou, K., Karagogeos, D., Wassef, M. Multiple influences on the migration of precerebellar neurons in the caudal medulla. Development (Cambridge, England). 129, 297-306 (2002).
- Landsberg, R. L. Hindbrain rhombic lip is comprised of discrete progenitor cell populations allocated by Pax6. Neuron. 48, 933-947 (2005).
- Hoshino, M. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 47, 201-213 (2005).
- Yamada, M. Origin of climbing fiber neurons and their developmental dependence on. Ptf1a. J. Neurosci. 27, 10924-10934 (2007).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone