Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Pharmacologic Induction of Epidermal Melanin and Protection Against Sunburn in a Humanized Mouse Model
W tym Artykule
Podsumowanie
Epidermal melanin is induced by topical application of forskolin in a murine model of the fair-skinned UV-sensitive human. Pharmacologic manipulation of cAMP levels in the skin and epidermal darkening strongly protect against UV-mediated inflammation (sunburn) as measured by the minimum erythematous dose (MED) assay.
Streszczenie
Fairness of skin, UV sensitivity and skin cancer risk all correlate with the physiologic function of the melanocortin 1 receptor, a Gs-coupled signaling protein found on the surface of melanocytes. Mc1r stimulates adenylyl cyclase and cAMP production which, in turn, up-regulates melanocytic production of melanin in the skin. In order to study the mechanisms by which Mc1r signaling protects the skin against UV injury, this study relies on a mouse model with "humanized skin" based on epidermal expression of stem cell factor (Scf). K14-Scf transgenic mice retain melanocytes in the epidermis and therefore have the ability to deposit melanin in the epidermis. In this animal model, wild type Mc1r status results in robust deposition of black eumelanin pigment and a UV-protected phenotype. In contrast, K14-Scf animals with defective Mc1r signaling ability exhibit a red/blonde pigmentation, very little eumelanin in the skin and a UV-sensitive phenotype. Reasoning that eumelanin deposition might be enhanced by topical agents that mimic Mc1r signaling, we found that direct application of forskolin extract to the skin of Mc1r-defective fair-skinned mice resulted in robust eumelanin induction and UV protection 1. Here we describe the method for preparing and applying a forskolin-containing natural root extract to K14-Scf fair-skinned mice and report a method for measuring UV sensitivity by determining minimal erythematous dose (MED). Using this animal model, it is possible to study how epidermal cAMP induction and melanization of the skin affect physiologic responses to UV exposure.
Wprowadzenie
The incidence of melanoma, the most deadly form of skin cancer, has increased dramatically over the last several decades in United States, particularly among fair-skinned individuals. Strong molecular and epidemiologic evidence implicates UV radiation as a major causative environmental factor 2-5. Increased UV exposure in the form of sun exposure and tanning bed use is likely to be responsible for much of increases in melanoma incidence 6-7. Melanoma risk seems particularly linked with sunburns 8, especially those early in life 9-10. Risk of sunburn is linked not only to dose and intensity of UV exposure, but also by inherited factors that influence cutaneous response to UV radiation. Skin pigmentation is one of the most important determinants of UV sensitivity, risk of sunburn and cancer risk. Melanoma occurs roughly twenty times more frequently in light-skinned persons compared to dark-skinned individuals 11-13.
Melanin, a pigment produced by melanocytes in the epidermis, is the main determinant of skin complexion. Melanin comes in two major varieties: (1) eumelanin, a dark brown/black pigment effective at absorbing the energy of UV radiation, and (2) pheomelanin, a reddish/blonde pigment less effective at preventing penetration of UV photons into the skin. Skin color, UV sensitivity and melanoma risk are largely determined by epidermal eumelanin content 14-15. The more eumelanin in the epidermis, the less UV photons can penetrate into the skin. Because of low innate levels of eumelanin, fair-skinned individuals are much more prone to acute and chronic effects of UV radiation 16-18.
Skin pigmentation, melanoma risk and the ability to "tan" after UV exposure all correlate with the signaling ability of the melanocortin 1 receptor (Mc1r), a Gs-coupled seven transmembrane surface receptor on melanocytes 19-22. When Mc1r binds its cognate high-affinity ligand, α-melanocyte stimulating hormone (α-MSH), there is activation of adenylyl cyclase and production of the second messenger cAMP 23. The normal physiologic response of the skin after UV exposure includes epidermal production of α-MSH by keratinocytes 24-29. We and others hypothesize that keratinocyte-derived α-MSH binds to Mc1r on epidermal melanocytes, initiating downstream production of the cAMP second messenger through activation of adenylyl cyclase 30. cAMP levels control many aspects of melanocyte differentiation, including survival pathways, DNA repair and pigment synthesis. Mc1r signaling and cAMP clearly induce pigment enzyme levels and eumelanin production. When Mc1r signaling is intact and melanocytic cAMP levels are robust, eumelanin is produced and the skin darkens. However, if Mc1r signaling is defective and cytoplasmic cAMP levels remain low, pheomelanin is produced instead 1. Eumelanin synthesis can be stimulated pharmacologically by agents that raise cAMP levels 1,14,31-35.
Since the Mc1r protein is a major regulator of melanoma risk in humans 36-46, we are interested in mechanisms by which Mc1r protects melanocytes against UV-induced carcinogenesis. As a foundation for our studies, we generated a transgenic Mc1r-variant murine model on a pure C57BL/6 genetic background 1. In this model, stem cell factor (Scf) is constitutively expressed in the basal epidermis and epidermal interfollicular melanocytes are retained in the skin throughout life 47, in contrast to the non-transgenic mice in which melanocytes localize to the dermis in hair follicles. With the K14-Scf transgene incorporated, the epidermis becomes pigmented with the particular melanin pigments characteristic of the pigment strain of the animal 1. K14-Scf mice on the C57BL/6 genetic background with wild type Mc1r signaling have jet-black skin characterized by very high levels of eumelanin pigment. Not surprisingly, these animals are highly UV-resistant. In contrast, genetically matched K14-Scf C57BL/6 animals that harbor a mutant inactive Mc1r have almost no eumelanin in the epidermis. Instead, these "extension" animals (Mc1re/e) have a fair skin complexion caused by deposition of pheomelanin pigment (Figure 1A) and are much more UV-sensitive 48-49.
Pharmacologic compounds with chemical properties that allow penetration into the skin have been shown to potently induce eumelanin in the extension (Mc1re/e) K14-Scf animal model by directly manipulating cAMP levels in epidermal melanocytes in the skin. Melanin upregulation in this model has been reported by adenylyl cyclase activation 1 as well as phosphodiesterase 4 inhibition 35. In this article, we demonstrate the preparation and topical application of forskolin in extension (Mc1re/e) K14-Scf animals which model the fair-skinned UV-sensitive human. We show that twice daily application of the drug promotes accelerated melanization, that skin darkening is due to epidermal deposition of melanin pigment and that induced epidermal melanin protects against UV-induced sunburn through measurement of "minimal erythematous dose" (MED) 48.
Protokół
1. Preparation of Forskolin for Topical Administration from a Crude Root Extract of the Plectranthus barbatus (Cohleus forskohlii) Plant
- Protocols for murine experiments followed the guidelines for ethical conduct in the care and use of animals and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky (Protocol # 00768M2004). The root extract is made up at 40% weight/volume in a standard dermatologic base of 70% ethanol, 30% propylene glycol.
- Weigh 200 g of crude forskolin root extract and transfer it to a beaker. To make 500 ml of a 40% (w/v) solution, resuspend 200 g of crude forskolin root extract by adding most but not all of the volume of the vehicle (70% ethanol, 30% propylene glycol) and bring the solution to roughly 450 ml.
- Stir for an hour at room temperature. The solution will be somewhat viscous and may require manual agitation to "lift" the extract into solution before the stir bar is able to take over.
- After an hour of stirring, pour the mixture into a graduated cylinder and bring the volume to 500 ml using vehicle that has been used to "rinse out" the beaker that was used to stir the slurry (to maximize recovery of forskolin from the beaker).
- Transfer the slurry to 50 ml polypropylene centrifuge tubes. Centrifuge (1,500 x g, room temperature, 15 min) using a table-top centrifuge. At this point, the insoluble material will be fairly compacted, allowing the supernatant to be easily poured off.
- Filter the solution through a 0.22 μm cellulose acetate membrane to remove any residual insoluble material from the extract. We use a bottle-top system designed for cell culture, along with the use of pre-filters that come with the unit to prevent premature clogging of the membrane from insoluble components of the root extract. When making large volumes of the extract, filter roughly 100 ml at a time, changing the pre-filter between each added volume.
- When stored at room temperature, the extract maintains biologic activity up for up to one year.
2. Preparation of C57Bl/6 K14-Scf Mice for Topical Treatments
- Remove dorsal fur from the animals by electric shearing. Briefly anesthetize the animals with inhalational isoflurane to facilitate shearing of dorsal fur with electric shears outfitted with a 0.25 mm surgical preparatory head (Fisher Scientific). Preferably only use one type of anesthesia (e.g. ketamine/xylazine) to minimize risk of anesthetic overdose. The saturated inhalation chamber carries occupational risk when used outside a fume hood and delivers unknown amounts of anesthetic to animals. Ideally a precision vaporizer should be used.
- To remove residual hair stubble, treat the animals with a chemical depilatory. Administer anesthesia to the animal with an i.p. injection of ketamine 40 mg/kg and xylazine 4 mg/kg
- Once animals are adequately anesthetized (as judged by toe pinch), apply a fingertip-sized amount of depilatory cream to sheared dorsal skin using a gloved finger. Rub the cream into the skin for 30-60 sec or until hairs can clearly be seen in the cream as it is being moved around. Leave the cream on only for the minimum amount of time required for hair removal as prolonged exposure leads to chemical burning of the skin, epidermal breakdown and death from loss of epidermal integrity.
- Wipe the dorsal skin with water-soaked gauze pads repeatedly until all cream has been removed. Dry animals using soft paper towels, and allow them to recover in a warm secluded location (e.g. clean cages placed on a warming pad). Depilate the animals one-by-one and monitor closely throughout the procedure.
3. Topical Administration of Forskolin or Vehicle Control
- Animals should be treated one at a time. Briefly anesthetize with inhaled isoflurane by placing the mouse on top of a form-fitted nylon air-permeable filter under which has been placed an isoflurane-saturated paper towel in an isoflurane-saturated lidded clear glass jar in a fume hood. Expose the mouse to isoflurane for a sufficient time as to suppress voluntary muscular movements but to preserve spontaneous respiration (typically 10-20 sec). Leaving the animal in isoflurane too long will result in respiratory suppression and death. It is better to err on the side of "going light" and having to re-expose the mouse briefly to more isoflurane rather than to over-expose the animal to the drug and risk death.
- Remove the animal from the isoflurane chamber and place on a clean absorbent bench pad.
- Using a 1,000 μl micropipette outfitted with a disposable polypropylene tip, draw up 400 μl of 40% crude forskolin extract (vehicle control animals will receive 70% ethanol, 30% propylene glycol alone).
- Transfer the extract onto the back of the animal by dripping it onto the skin and then, using the side of the pipette tip, smear the extract over the dorsal skin until all the skin has been covered. There is no need to blot the skin after application.
- Return the mouse to its cage, and carefully observe until it recovers from anesthesia.
- In order that non-pigment cAMP effects should not confound UV sensitivity experiments, discontinue all topical treatments 2 days prior to UV exposure (pigment effect lasts several days beyond last topical treatment).
4. Skin Color Measurement by Reflective Colorimetry
- Briefly anesthetize the mouse with inhaled isoflurane (see above).
- Calibrate a Minolta colorimeter by placing the portable head on the standardized white surface provided with the colorimeter.
- Place the portable measuring head of the colorimeter flush with the dorsal skin of the animal ensuring that the 1 cm2 round aperture is completely pressed onto the skin. Take at least three separate measurements in different areas of dorsal skin.
- Calculate mean L* score ± SD per animal and per treatment group. Reflective colorimetry can be done at any point in the experiment.
5. Determination of UV Sensitivity by Calculation of "Minimal Erythematous Dose" (MED)
- Use animals that have been pre-treated with either vehicle or forskolin as described above. Anesthetize animals with intraperitoneal injection of a standard mixture of ketamine and xylazine (see above).
- Prepare a piece of UV-occlusive tape for MED testing. To generate holes in the tape, use a heavy-duty hole punch with a 1 cm2 circular cutout (Figures 2A and B). Having holes of a defined size and symmetric arrangement in the tape facilitates recognition of skin changes after irradiation. Over each hole in the tape, apply a small but easily detachable piece of tape that can be removed at defined times during UV exposure to allow administration of different UV doses.
- Once animals are adequately sedated, place the tape on the dorsal surface. Eye lubricant should always be utilized under anesthesia.
- Turn on the UV source consisting of two Westinghouse F15T8UV-B lamps with a peak output of 313 nm and a range of 280-370 nm. Allow the lamp to equilibrate to a constant UV output as measured by a UV photometer with a UVB sensor (generally takes a few minutes for the lamps to warm up).
- Based on the UV transmission rate as measured by the UV Photometer, calculate UV exposure time for each desired dose. For example, our lamp's UVB output measures 2.4 mW/cm2. Therefore, to administer 5 kJ/m2, the skin would need to be exposed to 208 sec (which is 3 min and 28 sec) of UVB radiation, as calculated below:
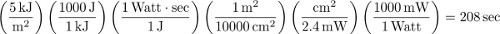
- Place sedated animals (each with occlusive tape in place) ventral surface down to ensure even UV exposure. To administer the chosen doses of UV radiation, sequentially remove the small occlusive tapes covering the holes to expose 1 cm2 areas of skin to the correct doses of radiation. Therefore, using the above example, if 40 kJ/m2 is the largest dose in the experiment, then the animal would be under the lamp for 27 min and 47 sec total and the skin in the 40 kJ/m2 condition would have no overlying tape the entire time. However, tape overlying the 5 kJ/m2 condition would be removed when there is 208 sec remaining in the exposure. Timing of tape removal should be done so that each condition ends simultaneously.
- After UV exposure, peel off the tape from the dorsal skin carefully, taking care not to rip the skin with sudden or overly-forceful movements. Place animals in a warm quiet place to allow recovery from anesthesia.
- Monitor mice for 24-48 hr to look for discreet areas of erythema (redness) or edema (swelling) corresponding to the anatomic sites exposed to the specific dose of UV irradiation. Document skin findings photographically.
- MED value corresponds to the minimum dose of UV that causes inflammation as defined by erythema and/or edema of the entire exposed circle of skin. Note that pigmentation of the skin can challenge determination of MED, however, erythema and edema can still generally be accurately assessed, thanks in part to the defined shape of the apertures in the tape during UV exposure.
6. Statistical Analysis
Analyze data between cohorts of mice by one way ANOVA with Bonferroni post test (Graph Pad PRISM). p values <0.05 are considered statistically significant.
Wyniki
C57BL/6 mice were generated on eumelanotic, pheomelanotic or amelanotic backgrounds incorporating the K14-Scf transgene as described (Figure 1A). Cohorts of fair-skinned extension (Mc1re/e, Tyr+/+) mice were treated topically with twice daily doses of either vehicle (70% ethanol, 30% propylene glycol) or 40% crude Coleus forskohlii root extract (80 μM per dose) for 5 days (Figure 2B). Effects of topical treatments on epidermal pigmentation were ...
Dyskusje
Using an animal model of the fair-skinned human, we find that topical application of a forskolin-rich crude root extract robustly darkens the epidermis by stimulating melanin production in the skin. Epidermal melanization is dependent on the expression of stem cell factor in the basal epidermis, as occurs in human skin but not in genetically-unmodified mouse skin. The dorsal skin of genetically-unmodified mice lacks sufficient numbers of interfollicular melanocytes to impart pigment to the skin. Only in the setting of co...
Ujawnienia
The authors declare that they have no competing financial interests.
Podziękowania
The authors wish to thank Malinda Spry for technical assistance. We also acknowledge current and past funding sources: the National Cancer Institute (R01 CA131075, R01 CA131075-02S1), the Wendy Will Case Cancer Research Fund, the Markey Cancer Foundation, the Children's Miracle Network and the Jennifer and David Dickens Melanoma Research Foundation.
Materiały
| Name | Company | Catalog Number | Comments |
| Reagents | |||
| Coleus Forskoli extract 20% | Buckton Scott USA Inc. | n/a | Princeton, NJ |
| Isothesia, Isoflurane , USP | Butler Schein | NCD 11695-6776-1 | Dublin, OH, USA |
| Xylazine | Anased Injection | LA04612 | Shenandoah, Iowa, USA |
| Ketamine HCl, USP | Putney | NDC 26637-411-01 | St. Joseph, MO, USA |
| Ethanol | Decon Labs. | 2705 | |
| Propylene glycol | Adesco | 05751L | Solon, OH, USA |
| Depilatory cream, Nair | Church Dwight | JF-11 4381322 | Priceton, NJ |
| EQUIPMENT | |||
| Germicidal Hg Lamp UV-B | Westinghouse | F15T8UV-B | |
| Radiometer photometer | International light | 1LT400A | Peabody, MA,USA |
| Chromameter | Konica Minolta | CR-400 | Ramsey, NJ, USA |
| Data Processor for Chromameter CR-400 | Konica Monilta | DR-400 | Ramsey, NJ, USA |
Odniesienia
- D'Orazio, J. A., et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 443, 340-344 (2006).
- Gallagher, R. P., et al. Suntan, sunburn, and pigmentation factors and the frequency of acquired melanocytic nevi in children. Similarities to melanoma: the Vancouver Mole Study. Arch Dermatol. , 126-770 (1990).
- Kraemer, K. H., Lee, M. M., Andrews, A. D., Lambert, W. C. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch Dermatol. 130, 1018-1021 (1994).
- Wang, Y., et al. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc. Natl. Acad. Sci. U.S.A. 106, 6279-6284 (2009).
- Pleasance, E. D., et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 463, 191-196 (2009).
- Weinstock, M. A., Fisher, D. E. Indoor ultraviolet tanning: what the data do and do not show regarding risk of melanoma and keratinocyte malignancies. J. Natl. Compr. Canc. Netw. 8, 867-873 (2010).
- Fisher, D. E., James, W. D. Indoor tanning--science, behavior, and policy. N. Engl. J. Med. 363, 901-903 (2010).
- Pfahlberg, A., Kolmel, K. F., Gefeller, O. Timing of excessive ultraviolet radiation and melanoma: epidemiology does not support the existence of a critical period of high susceptibility to solar ultraviolet radiation- induced melanoma. Br. J. Dermatol. 144, 471-475 (2001).
- Lew, R. A., Sober, A. J., Cook, N., Marvell, R., Fitzpatrick, T. B. Sun exposure habits in patients with cutaneous melanoma: a case control study. J. Dermatol. Surg. Oncol. 9, 981-986 (1983).
- Autier, P., Dore, J. F. Influence of sun exposures during childhood and during adulthood on melanoma risk. EPIMEL and EORTC Melanoma Cooperative Group. European Organisation for Research and Treatment of Cancer. Int. J. Cancer. 77, 533-537 (1998).
- Udayakumar, D., Mahato, B., Gabree, M., Tsao, H. Genetic determinants of cutaneous melanoma predisposition. Semin. Cutan. Med. Surg. 29, 190-195 (2010).
- Psaty, E. L., Scope, A., Halpern, A. C., Marghoob, A. A. Defining the patient at high risk for melanoma. Int. J. Dermatol. 49, 362-376 (2010).
- Tucker, M. A. Melanoma epidemiology. Hematol. Oncol. Clin. North Am. 23, 383-395 (2009).
- Abdel-Malek, Z. A., Knittel, J., Kadekaro, A. L., Swope, V. B., Starner, R. The melanocortin 1 receptor and the UV response of human melanocytes--a shift in paradigm. Photochem. Photobiol. 84, 501-508 (2008).
- Suzuki, I., et al. Participation of the melanocortin-1 receptor in the UV control of pigmentation. J. Investig. Dermatol. Symp. Proc. 4, 29-34 (1999).
- Gibson, G. E., Codd, M. B., Murphy, G. M. Skin type distribution and skin disease in Ireland. Ir. J. Med. Sci. 166, 72-74 (1997).
- Evans, R. D., et al. Risk factors for the development of malignant melanoma--I: Review of case-control studies. J. Dermatol. Surg. Oncol. 14, 393-408 (1988).
- Pack, G. T., Davis, J., Oppenheim, A. The relation of race and complexion to the incidence of moles and melanomas. Ann. N.Y. Acad. Sci. 100, 719-742 (1963).
- Valverde, P., Healy, E., Jackson, I., Rees, J. L., Thody, A. J. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat. Genet. 11, 328-330 (1995).
- Rees, J. L., Healy, E. Melanocortin receptors, red hair, and skin cancer. J. Investig. Dermatol. Symp. Proc. 2, 94-98 (1997).
- Beaumont, K. A., et al. Melanocortin MC(1) receptor in human genetics and model systems. Eur. J. Pharmacol. 660, 103-110 (2011).
- Palmer, J. S., et al. Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype?. Am. J. Hum. Genet. 66, 176-186 (2000).
- Haskell-Luevano, C., et al. Compounds that activate the mouse melanocortin-1 receptor identified by screening a small molecule library based upon the beta-turn. J. Med. Chem. 42, 4380-4387 (1999).
- Yamaguchi, Y., Hearing, V. J. Physiological factors that regulate skin pigmentation. Biofactors. 35, 193-199 (2009).
- Eves, P. C., MacNeil, S., Haycock, J. W. alpha-Melanocyte stimulating hormone, inflammation and human melanoma. Peptides. 27, 444-452 (2006).
- Slominski, A., Wortsman, J., Luger, T., Paus, R., Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 80, 979-1020 (2000).
- Slominski, A., Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 21, 457-487 (2000).
- Luger, T. A., et al. Role of epidermal cell-derived alpha-melanocyte stimulating hormone in ultraviolet light mediated local immunosuppression. Ann. N.Y. Acad. Sci. 885, 209-216 (1999).
- Chakraborty, A. K., et al. UV light and MSH receptors. Ann. N.Y. Acad. Sci. 885, 100-116 (1999).
- Cui, R., et al. Central Role of p53 in the Suntan Response and Pathologic Hyperpigmentation. Cell. 128, 853-864 (2007).
- Imokawa, G., Yada, Y., Hori, Y. Induction of melanization within hair bulb melanocytes in chinchilla mutant by melanogenic stimulants. J Invest Dermatol. 91, 106-113 (1988).
- Siegrist, W., et al. Interactions of alpha-melanotropin and agouti on B16 melanoma cells: evidence for inverse agonism of agouti. J. Recept. Signal Transduct Res. 17, 75-98 (1997).
- Abdel-Malek, Z., et al. The melanocortin-1 receptor is a key regulator of human cutaneous pigmentation. Pigment Cell Res. 13, 156-162 (2000).
- Wood, J. M., Gibbons, N. C., Schallreuter, K. U. Melanocortins in human melanocytes. Cell Mol Biol (Noisy-le-grand). 52, 75-78 (2006).
- Khaled, M., Levy, C., Fisher, D. E. Control of melanocyte differentiation by a MITF-PDE4D3 homeostatic circuit. Genes Dev. 24, 2276-2281 (2010).
- Ghiorzo, P., et al. MC1R variation and melanoma risk in relation to host/clinical and environmental factors in CDKN2A positive and negative melanoma patients. Exp. Dermatol. , (2012).
- Cust, A. E., et al. MC1R genotypes and risk of melanoma before age 40 years: a population-based case-control-family study. Int. J. Cancer. 131, E269-E281 (2012).
- Ibarrola-Villava, M., et al. Genetic analysis of three important genes in pigmentation and melanoma susceptibility: CDKN2A, MC1R and HERC2/OCA2. Exp Dermatol. 19, 836-844 (2010).
- Scherer, D., et al. Melanocortin receptor 1 variants and melanoma risk: A study of 2 European populations. Int. J. Cancer. , (2009).
- Hoiom, V., et al. MC1R variation and melanoma risk in the Swedish population in relation to clinical and pathological parameters. Pigment Cell Melanoma Res. 22, 196-204 (2009).
- Galore-Haskel, G., et al. MC1R variant alleles and malignant melanoma risk in Israel. Eur. J. Cancer. 45, 2015-2022 (2009).
- Sturm, R. A. Skin colour and skin cancer - MC1R, the genetic link. Melanoma Res. 12, 405-416 (2002).
- Kennedy, C., et al. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J. Invest. Dermatol. 117, 294-300 (2001).
- Box, N. F., et al. MC1R genotype modifies risk of melanoma in families segregating CDKN2A mutations. Am. J. Hum. Genet. 69, 765-773 (2001).
- Rees, J. L. The melanocortin 1 receptor (MC1R): more than just red hair. Pigment Cell Res. 13, 135-140 (2000).
- Valverde, P., et al. The Asp84Glu variant of the melanocortin 1 receptor (MC1R) is associated with melanoma. Hum. Mol. Genet. 5, 1663-1666 (1996).
- Kunisada, T., et al. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development. 125, 2915-2923 (1998).
- Vanover, J. C., et al. Stem cell factor rescues tyrosinase expression and pigmentation in discreet anatomic locations in albino mice. Pigment Cell Melanoma Res. 22, 827-838 (2009).
- Spry, M. L., et al. Prolonged treatment of fair-skinned mice with topical forskolin causes persistent tanning and UV protection. Pigment Cell Melanoma Res. 22, 219-229 (2009).
- Takayama, H., La Rochelle, W. J., Anver, M., Bockman, D. E., Merlino, G. Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc. Natl. Acad. Sci. U.S.A. 93, 5866-5871 (1996).
- Kunisada, T., et al. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J. Exp. Med. , 187-1565 (1998).
- Takeuchi, T., Kobunai, T., Yamamoto, H. Genetic control of signal transduction in mouse melanocytes. J. Invest. Dermatol. 92, 239S-242S (1989).
- Ozeki, H., Ito, S., Wakamatsu, K., Hirobe, T. Chemical characterization of hair melanins in various coat-color mutants of mice. J. Invest. Dermatol. 105, 361-366 (1995).
- Lamoreux, M. L., Wakamatsu, K., Ito, S. Interaction of major coat color gene functions in mice as studied by chemical analysis of eumelanin and pheomelanin. Pigment Cell Res. 14, 23-31 (2001).
- Barbini, P., et al. Instrumental measurement of skin colour and skin type as risk factors for melanoma: a statistical classification procedure. Melanoma Res. 8, 439-447 (1998).
- Takiwaki, H. Measurement of skin color: practical application and theoretical considerations. J. Med. Invest. 44, 121-126 (1998).
- Anderson, R. R., Parrish, J. A. The optics of human skin. J. Invest. Dermatol. 77, 13-19 (1981).
- Rubegni, P., et al. Relationship between skin color and sun exposure history: a statistical classification approach. Photochem. Photobiol. 65, 347-351 (1997).
- Chen, J., Hammell, D. C., Spry, M., D'Orazio, J. A., Stinchcomb, A. L. In vitro skin diffusion study of pure forskolin versus a forskolin-containing Plectranthus barbatus root extract. J. Nat. Prod. 72, 769-771 (2009).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone