Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Characterization and Application of Passive Samplers for Monitoring of Pesticides in Water
W tym Artykule
Podsumowanie
A protocol about the characterization and application of five different passive sampling devices is presented.
Streszczenie
Five different water passive samplers were calibrated under laboratory conditions for measurement of 124 legacy and current used pesticides. This study provides a protocol for the passive sampler preparation, calibration, extraction method and instrumental analysis. Sampling rates (RS) and passive sampler-water partition coefficients (KPW) were calculated for silicone rubber, polar organic chemical integrative sampler POCIS-A, POCIS-B, SDB-RPS and C18 disk. The uptake of the selected compounds depended on their physicochemical properties, i.e., silicone rubber showed a better uptake for more hydrophobic compounds (log octanol-water partition coefficient (KOW) > 5.3), whereas POCIS-A, POCIS-B and SDB-RPS disk were more suitable for hydrophilic compounds (log KOW < 0.70).
Wprowadzenie
Pesticides are continuously introduced to the aquatic environment and may pose a risk to aquatic organisms1. Monitoring of pesticides in the aqueous environment is typically performed using grab sampling, however, this sampling technique does not fully account for temporal variations in concentrations due to fluctuations in flow or episodic inputs (e.g., precipitation, combined sewer overflows, sewage lagoon release)2,3. Thus, monitoring methods need to be improved for a better estimation of environmental risks associated with pesticides. Passive sampling allows continuous monitoring over an extended period of time with minimal infrastructure and low contaminant concentrations4,5.
Passive samplers have been shown to be a valuable tool for the monitoring in groundwater6, fresh water7-10, wastewater11 and marine waters12. Besides monitoring purposes13,14, passive samplers have also been used for non-target analysis15, toxicology testing16,17, and as an alternative to sediment- and biomonitoring18. Passive samplers accumulate chemicals continuously from water and provide time weighted average (TWA) concentrations14. The uptake of the contaminant depends on the sampling rate (RS) and passive sampler-water partition coefficient (KPW), which depends on the passive sampler design, sampler material, physicochemical properties of the contaminant, and environmental conditions (e.g., water turbulence, temperature)13,14,19,20.
The detailed video aims to show how to calibrate and apply passive samplers for pesticides in water. The specific objectives included i) to perform preparation, extraction and instrumental analysis for 124 individual pesticides using five different types of passive samplers, including silicone rubber, polar organic chemical integrative sampler (POCIS)-A, POCIS-B, SDB-RPS and C18 disk, ii) to assess RS and KPW for the pesticides in a laboratory uptake study, and iii) to demonstrate how to select the appropriate passive sampler of the target compound of interest and how to calculate TWA concentrations for the respective passive sampler.
Reference standards and passive sampler devices
Target compounds included 124 legacy and currently used pesticides including herbicides, insecticides and fungicides (Table 1). Internal standard mixture (IS mixture) included fenoprop (2,4,5-TP), clothianidin-D3, ethion and terbuthylazine-D5. Other used chemicals included methanol (MeOH), acetonitrile (ACN), acetone (ACE), dichloromethane (DCM), cyclohexane (CH), ethyl acetate (EA), petroleum ether (PE), 2-propanol, 25% ammonia solution, acetic acid (HAc) and formic acid (FA). Five different passive sampling devices were characterized, including silicone rubber, POCIS-A and POCIS-B, SDB-RPS, and C18 disk1,21.
Table 1. Passive sampler sampling rate (R'S, L day-1), sampler-water partition coefficients (K'PW, L kg-1) and equations (Eq.) used for the calculation of concentrations in field samples for individual pesticidesa. (Reprinted from Journal of Chromatography A, 1405, Lutz Ahrens, Atlasi Daneshvar, Anna E. Lau, Jenny Kreuger, Characterization of five passive sampling devices for monitoring of pesticides in water, 1-11, Copyright (2015), with permission from Elsevier.)22 Please click here to download this file.
Protokół
1. Passive Sampler Design and Preparation
- Silicone rubber sheets
- Cut the silicone rubber sheets (600 mm x 600 mm, 0.5 mm thick) into stripes of 2.5 mm x 600 mm and 2.5 mm x 314 mm using a stainless steel cutter and connect them using a stainless steel blind rivet (3.2 mm x 10 mm) with a rivet gun to obtain a total sampler stripe size of 2.5 mm x 914 mm (surface area = 457 cm2, sorbent mass = 15.6 g, volume = 22.9 cm3).
- Place the silicone rubbers in an extraction chamber of a Soxhlet apparatus. Add 50 ml EA in the extraction chamber and add 250 ml EA and three boiling stones in a 500 ml round bottle flask.
- Connect the extraction chamber with the bottle flask and a condenser. Clean the silicone rubbers by Soxhlet extraction for 96 hr at approximately 80 °C, and dry them thereafter under gentle nitrogen gas.
- Attach the silicone rubber stripe to a stainless steel spider sample holder by wrapping the silicone rubber stripe around the rods on the holder (Figure 1). Attach each end of the silicone rubber stripe to a rod on the holder using cable ties.

Figure 1. Schematic of silicone rubber. Passive sampler schematic for silicone rubber showing the attachment of the silicone rubber stripe to a stainless steel spider sample holder A) from the top and B) the side view. Please click here to view a larger version of this figure.
- POCIS-A and POCIS-B
- For POCIS-A, place 220 mg of HLB bulk sorbent (surface area = 1.78 x 106 cm2) between two 9.0 cm by 9.0 cm square polyethersulfone (PES) membranes (Figure 2).
- For POCIS-B, place 220 mg of a sorbent mixture (i.e., hydroxylated polystyrene-divinylbenzene resin (80%) and a carbonaceous adsorbent dispersed on a styrene divinylbenzene copolymer (20%)) (surface area = 2.82 x 106 cm2) between two PES membranes (Figure 2).
- Compress the sorbent and two PES between two stainless steel rings manually (inner Ø = 5.4 cm) and secure it on a stainless steel sample holder (Figure 2).

Figure 2. Schematic of passive sampler disks. Passive sampler schematic for POCIS A, POCIS B, SDB-RPS disk and C18 disk showing A) the assembling of the passive sampler using stainless steel rings, polyethersulfone (PES) membranes, and the receiving phase, and B) the assembling on a stainless steel sample holder. Please click here to view a larger version of this figure.
- SDB-RPS disk and C18 disk
- Place the SDB-RPS (surface area = 35 cm2, sorbent mass = 0.34 g, volume = 1.7 cm3) and C18 disks (surface area = 35 cm2, sorbent mass = 0.58 g, volume = 1.7 cm3) between two PES membranes (Figure 2). Compress the disks and two PES between two stainless steel rings manually (inner Ø = 5.4 cm) and secure it on a stainless steel sample holder (Figure 2).
2. Laboratory Uptake Experiments
NOTE: The Laboratory uptake experiments were performed to quantitatively characterize the uptake kinetics for 124 individual pesticides for five different passive sampler devices under controlled conditions.
- Conduct the uptake study in rectangular glass containers (each ~95 L): Tank 1) silicone rubber (n = 16), tank 2) POCIS-A (n = 16), POCIS-B (n = 16), and tank 3) SDB-RPS disk (n = 16), C18 disk (n = 16). Fill natural water into the three tanks.
- Perform all experiments at a constant water temperature (~20 °C) and under turbulent water conditions (~10 cm sec-1) using two electric pumps attached to the wall on each side. Perform the experiments in the dark to minimize the effect of photodegradation.
- Spike each glass container with a pesticide standard mixture containing 124 pesticides using a glass syringe (c ≈ 400 ng L-1 for individual pesticides in the water tank). Take out the passive samplers manually from the tanks, at time intervals of 5, 11, 20, and 26 days, to determine the sampling rates of the pesticides.
- Monitor the concentrations of the pesticides in each tank by collecting 100 ml water samples at day 0, 5, 11, 20, and 26. The analysis of the water samples is performed as described elsewhere21.
- For quality control, expose blank samples to room air for 1 hr at day 0 and then store and treat them as real samples. Store all extracts as well as the 100 ml water samples collected from the tanks at -18 °C until further analysis.
3. Sample Extraction
- Silicone rubber
- Prior to extraction, dry the silicone rubber stripe under a stream of high purity nitrogen gas.
- For gas chromatography-mass spectrometry (GC-MS) analysis, carry out the solid-liquid extraction using Soxhlet extraction22.
- Place the silicone rubber into the Soxhlet extractor. Add 250 ml PE/ACE (50/50, v/v) and 3 boiling stones into the round bottle flask.
- Spike the silicone rubber with 100 µl of an IS mixture (c = 5 ng ml-1) using a glass syringe. Add 50 ml PE/ACE (50/50, v/v) into the Soxhlet extractor. Switch on the heater and run the Soxhlet extraction for 19 hr and then switch off the heater.
- Concentrate the extracts by rotary evaporation followed by gentle nitrogen blow-down to 1 ml. Exchange the solvent to CH/ACE (90/10, v/v) by adding three times 1 ml CH/ACE (90/10, v/v) during the nitrogen blow-down to 1 ml.
- For liquid chromatography−tandem mass spectrometry (LC-MS/MS) analysis, carry out the extraction using Soxhlet extraction22.
- Place the silicone rubber into the Soxhlet extractor. Add 250 ml MeOH and 3 boiling stones into the round bottle flask and 50 ml MeOH into the Soxhlet extractor. Spike the silicone rubber with 100 µl of an IS mixture (c = 5 ng ml-1) using a glass syringe.
- Switch on the heater and run the Soxhlet extraction for 19 hr and then switch off the heater. Concentrate the extracts by rotary evaporation followed by gentle nitrogen blow-down to 1 ml. Exchange the solvent to ACN by adding 1 ml ACN during the nitrogen blow-down to 1 ml.
- POCIS-A and POCIS-B
- Open the POCIS sampler carefully and transfer the sorbent with ultrapure water using a funnel into a pre-cleaned empty polypropylene solid phase extraction (SPE) cartridge (6 ml) containing two polyethylene (PE) frits. Dry the sorbent by vacuum to remove water. Record the weight of the empty and packed SPE cartridge to control the weight of the sorbent material. Please note that different cartridges are used for GC-MS and LC-MS/MS analysis.
- Prior to elution, spike the sorbent with 100 µl of an IS mixture (c = 5 ng ml-1) using a glass syringe. Elute POCIS-A and POCIS-B sorbents using 5 ml EA for GC-MS22.
- Concentrate the extracts to 1 ml by gentle nitrogen blow-down. Exchange the solvent to CH/ACE (90/10, v/v) by adding three times 1 ml CH/ACE (90/10, v/v) during the nitrogen blow-down to 1 ml.
- Elute POCIS-A and POCIS-B cartridges using 1.5 ml MeOH followed by 8 ml DCM/MeOH (80/20, v/v) for LC-MS/MS analysis22. Concentrate the extracts to 1 ml by gentle nitrogen blow-down. Exchange the solvent to ACN by adding 1 ml ACN during the nitrogen blow-down to 1 ml.
- SDB-RPS and C18 disk
- Transfer individual disks of SDB-RPS and C18 disk into a glass beaker and dry them under nitrogen gas. Spike the disks with 100 µl of an IS mixture (c = 5 ng ml-1) using a glass syringe and sonicate them two times in a glass beaker at room temperature, first with 5 ml of EA for 10 min and then with 3 ml of EA for 10 min.
- Transfer both extracts into one glass tube, concentrate them to 2 ml by gentle nitrogen blow down, and split them into two 1 ml fractions (for GC-MS and LC-MS/MS analysis, respectively).
- Concentrate the extracts to 0.5 ml by gentle nitrogen blow-down and exchange the solvent to CH/ACE (90/10, v/v) for GC-MS analysis22. Concentrate the extracts to 0.5 ml by gentle nitrogen blow-down and exchange the solvent to ACN for LC-MS/MS analysis22.
4. Water Samples
- Spike 20 ml water sample with 100 µl of an IS mixture (c = 5 ng ml-1) using a glass syringe, add 3 ml of DCM, vortex for 3 min, and decant into a phase separator for GC-MS analysis 22.
- After the two phases are separated, percolate the DCM phase into a glass tube. Repeat the extraction using 3 ml DCM, and rinse the tube with 2 ml DCM. Finally, concentrate the extracts to 0.5 ml by gentle nitrogen blow-down and exchange the solvent to CH/ACE (90/10, v/v).
- Analyze the water samples using large volume injection, similar to the method described elsewhere by LC-MS/MS 21.
5. Instrumental Analysis
- GC-MS analysis
- Perform the instrumental analysis of the CH/ACE extracts using GC-MS systems in electron ionization (EI) and negative chemical ionization (NCI) mode, respectively22.
- For the GC-MS method using EI, inject aliquots of 1 µl with splitless injection method on a HP-5MS UI column (30 m, 0.25 mm inner diameter, 0.25 µm film).
- For the GC-MS method using CI, inject aliquots of 3 µl on a HP-5MS UI column (30 m, 0.25 mm inner diameter, 0.25 µm film).
- HPLC-MS/MS analysis
- Perform the instrumental analysis of the ACN extracts using HPLC-MS/MS interfaced with an electrospray ionization source in negative ((-)ESI) and positive-ion mode ((+)ESI)22.
- For (+)ESI, dilute 100 µl of the ACN extracts with 900 µl ultrapure water adjusted to pH 5 using FA .
- For (-)ESI, dilute 100 µl of the ACN extracts with 900 µl solution of 1% FA in ultrapure water.
- For (+)ESI, use a binary gradient consisting of 2-propanol/methanol/10 mM ammonium formate (6/2/92, v/v/v) and MeOH at a flow rate of 0.3 ml min-1.
- For (-)ESI, use a binary gradient consisting of ACN/ultrapure water 0.1% HAc and ACN + 0.1% HAc at a flow rate of 0.3 ml min-1.
- Inject all samples using a large volume injection of 500 µl using two online SPE columns (both 20 x 2 mm id and 20-25 µm particle size), and an analytical column (C18, 100 x 3 mm, 3.5 µm)21.
6. Theory on Passive Sampling
NOTE: The uptake profile of the chemical to the passive sampler medium (PSM) is divided into three sections: Linear, curvilinear and equilibrium (Figure 3).
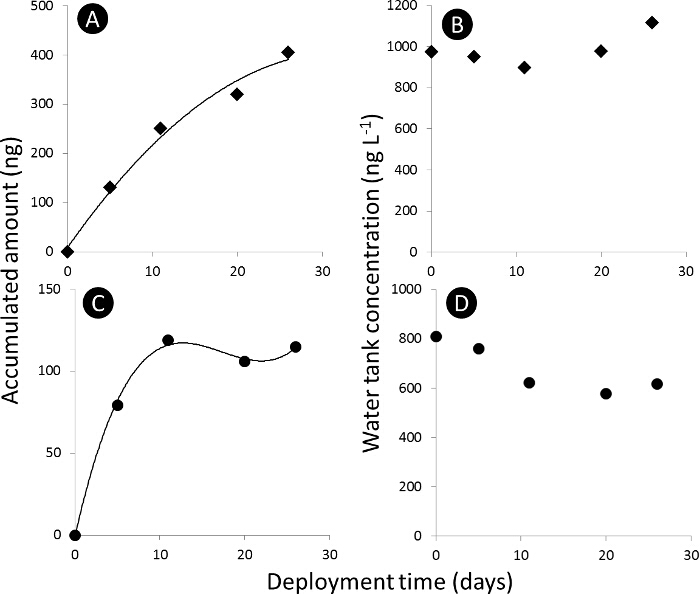
Figure 3. Passive sampler uptake curve. A) and C) uptake curve for the accumulated amount of acetamiprid and dimethoate, respectively, in the passive samplers (Nt) in ng absolute, and B) and D) water tank concentration of acetamiprid and dimethoate, respectively, in ng L-1. Please click here to view a larger version of this figure.
- Calculate the equivalent water volume (Veq L) for a passive sampler by dividing the accumulated amount of target compounds in the passive sampler after t days of exposure (N't, ng) by the concentration in the water phase using grab and time integrated active sampling (cw, ng L-1).
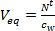 (1)
(1) - Derive the sampling rate (RS, L day-1) from the linear uptake phase of the uptake profile, by taking the slope of Veq versus deployment time.
- Calculate the KPW (L kg-1) for individual pesticides using Eq. 2.
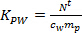 (2)
(2)
where mp is the sorbent mass per sampler (ng). - In the linear uptake phase, calculate the TWA concentration of the analyte in water derived by the passive sampler (cTWA, ng L-1) using Eq. 3.
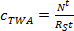 (3)
(3)
where RS is the sampling rate (L day-1), and t is the deployment time (days). - In the curvilinear phase, calculate cTWA using Eq. 4.
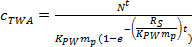 (4)
(4) - In the equilibrium phase, calculate cTWA using Eq. 5.
 (5)
(5)
7. Statistical Data Analysis
- Test non-normal distribution of the data using a Shapiro-Wilk test23. Use non-parametric Spearman's rank correlation for KPW and RS vs physicochemical properties of the tested pesticides (Spearman's rho ranging from -1 to 1)24.
Wyniki
Five different passive sampler techniques were compared for the uptake of 124 legacy and current used pesticides including silicone rubber (Figure 1), and POCIS A, POCIS B, SDB-RPS and C18 disk (Figure 2). The performance of the extraction method and instrumental analysis was optimized. The outcome of the laboratory uptake experiments can be used to calculate the R'S and log K'PW values (T...
Dyskusje
For quality control, as standard procedure, laboratory blanks, limits of detection (LOD), recoveries, and repeatability were examined23. A few pesticides were detected in the blank samples at low concentration levels. LODs were set as the value of the lowest point on the calibration curve which meets the criteria of a signal to noise ratio of 3. The average LODs were 8.0 pg absolute injected on column for silicone rubber, 1.7 pg absolute for POCIS-A, 1.6 pg absolute for POCIS-B, 3.0 pg absolute for SDB-RPS dis...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The Swedish EPA (Naturvårdsverket) (agreement 2208-13-001) and Centre for Chemical Pesticides (CKB) are gratefully acknowledged for funding this project. We thank Märit Peterson, Henrik Jernstedt, Emma Gurnell and Elin Paulsson at the OMK-lab, SLU, for skillful assistance with analytical support and supply of pesticide standards.
Materiały
| Name | Company | Catalog Number | Comments |
| Methanol | Merck Millipore | 1.06035.2500 | |
| Acetonitrile | Merck Millipore | 1.00029.2500 | |
| Acetone | Merck Millipore | 1.00012.2500 | |
| 2-propanol | Merck Millipore | 1.00272.2500 | |
| Dichloromethane | Merck Millipore | 1.06054.2500 | |
| Ammoniak | Merck Millipore | 1.05428.1000 | Purity 25% |
| Formic acid | Sigma-Aldrich | 94318-50ML-F | Purity ~98% |
| Ethyl acetate | Sigma-Aldrich | 31063-2.5L | for pesticide residue analysis |
| Petroleum ether | Sigma-Aldrich | 34491-4X2.5L | for pesticide residue analysis |
| Acetic acid | Sigma-Aldrich | 320099-500ML | Purity ≥99.7% |
| Cyclohexane | Fisher Chemicals | C/8933/17 | for residue analysis |
| Empty polypropylene SPE Tube with PE frits, 20 μm porosity, volume 6 ml | Supelco | 57026 | |
| Empore SPE Disks, C18, diam. 47 mm | Supelco | 66883-U | Passive sampler |
| Empore SPE Disks, SDB-RPS (Reversed-Phase Sulfonate), diam. 47 mm | Supelco | 66886-U | Passive sampler |
| POCIS-A | EST | POCIS-HLB | Passive sampler |
| POCIS-B | EST | POCIS-Pesticide | Passive sampler |
| Polyethersulfone (PES) membranes | EST | PES | |
| Silicone rubber sheet | Altec | 03-65-4516 | Passive sampler |
| Agilent 5975C | Agilent Technologies | 5975C | GC-MS |
| HP-5MS UI | J&W Scientific | HP-5MS | Analytical column for GC-MS |
| Agilent 6460 | Agilent Technologies | 6460 | HPLC-MS/MS |
| Strata C18–E, 20 x 2 mm id and 20–25 μm particle size | Phenomenex | Strata C18–E | Online SPE column for LC-MS/MS |
| Strata X, 20 x 2 mm id and 20–25 μm particle size | Phenomenex | Strata X | Online SPE column for LC-MS/MS |
| Zorbax Eclipse Plus C18 | Agilent Technologies | Zorbax Eclipse Plus C18 | Analytical column for LC-MS/MS |
| Isolute phase separator, 25 ml | Biotage | 120-1907-E | |
| Stainless steel blind rivet, 3.2x10 mm | Ejot & Avdel | 951222 |
Odniesienia
- Rodney, S. I., Teed, R. S., Moore, D. R. J. Estimating the toxicity of pesticide mixtures to aquatic organisms: A review. Hum. Ecol. Risk Assess. 19 (6), 1557-1575 (2013).
- Kreuger, J. Pesticides in stream water within an agricultural catchment in southern Sweden, 1990-1996. Sci. Total Environ. 216 (3), 227-251 (1998).
- Carlson, J. C., Challis, J. K., Hanson, M. L., Wong, C. S. Stability of pharmaceuticals and other polar organic compounds stored on polar organic chemical integrative samplers and solid-phase extraction cartridges. Environ. Toxicol. Chem. 32 (2), 337-344 (2013).
- Alvarez, D. A., et al. Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environ. Toxicol. Chem. 23 (7), 1640-1648 (2004).
- Vrana, B., et al. Passive sampling: An effective method for monitoring seasonal and spatial variability of dissolved hydrophobic organic contaminants and metals in the Danube river. Environ. Pollut. 184, 101-112 (2014).
- Dougherty, J. A., Swarzenski, P. W., Dinicola, R. S., Reinhard, M. Occurrence of herbicides and pharmaceutical and personal care products in surface water and groundwater around Liberty Bay, Puget Sound, Washington. J. Environ. Qual. 39 (4), 1173-1180 (2010).
- Muñoz, I., Martìnez Bueno, M. J., Agüera, A., Fernández-Alba, A. R. Environmental and human health risk assessment of organic micro-pollutants occurring in a Spanish marine fish farm. Environ. Pollut. 158 (5), 1809-1816 (2010).
- Wille, K., et al. Rapid quantification of pharmaceuticals and pesticides in passive samplers using ultra high performance liquid chromatography coupled to high resolution mass spectrometry. J. Chromatogr. A. 1218 (51), 9162-9173 (2011).
- Poulier, G., et al. Estimates of pesticide concentrations and fluxes in two rivers of an extensive French multi-agricultural watershed: application of the passive sampling strategy. Environ. Sci. Pollut. Res. 22 (11), 8044-8057 (2015).
- Moschet, C., Vermeirssen, E. L. M., Singer, H., Stamm, C., Hollender, J. Evaluation of in-situ calibration of chemcatcher passive samplers for 322 micropollutants in agricultural and urban affected rivers. Water Res. 71, 306-317 (2015).
- Petty, J. D., et al. An approach for assessment of water quality using semipermeable membrane devices (SPMDs) and bioindicator tests. Chemosphere. 41 (3), 311-321 (2000).
- Metcalfe, C. D., et al. Contaminants in the coastal karst aquifer system along the Caribbean coast of the Yucatan Peninsula, Mexico. Environ. Pollut. 159 (4), 991-997 (2011).
- Allan, I. J., et al. Field performance of seven passive sampling devices for monitoring of hydrophobic substances. Environ. Sci. Technol. 43 (14), 5383-5390 (2009).
- Vrana, B., et al. Passive sampling techniques for monitoring pollutants in water. TrAC - Trend. Anal. Chem. 24 (10), 845-868 (2005).
- Allan, I. J., Harman, C., Ranneklev, S. B., Thomas, K. V., Grung, M. Passive sampling for target and nontarget analyses of moderately polar and nonpolar substances in water. Environ. Toxicol. Chem. 32 (8), 1718-1726 (2013).
- Escher, B. I., et al. Evaluation of contaminant removal of reverse osmosis and advanced oxidation in full-scale operation by combining passive sampling with chemical analysis and bioanalytical tools. Environ. Sci. Technol. 45, 5387-5394 (2011).
- Pesce, S., Morin, S., Lissalde, S., Montuelle, B., Mazzella, N. Combining polar organic chemical integrative samplers (POCIS) with toxicity testing to evaluate pesticide mixture effects on natural phototrophic biofilms. Environ. Pollut. 159 (3), 735-741 (2011).
- Booij, K., Smedes, F., Van Weerlee, E. M., Honkoop, P. J. C. Environmental monitoring of hydrophobic organic contaminants: The case of mussels versus semipermeable membrane devices. Environ. Sci. Technol. 40 (12), 3893-3900 (2006).
- Harman, C., Allan, I. J., Vermeirssen, E. L. M. Calibration and use of the polar organic chemical integrative sampler-a critical review. Environ. Toxicol. Chem. 31 (12), 2724-2738 (2012).
- Jonker, M. T. O., Der Heijden, S. A. V. a. n., Kotte, M., Smedes, F. Quantifying the effects of temperature and salinity on partitioning of hydrophobic organic chemicals to silicone rubber passive samplers. Environ. Sci. Technol. 49 (11), 6791-6799 (2015).
- Jansson, C., Kreuger, J. Multiresidue analysis of 95 pesticides at low nanogram/liter levels in surface waters using online preconcentration and high performance liquid chromatography/tandem mass spectrometry. J. AOAC Int. 93 (6), 1732-1747 (2010).
- Ahrens, L., Daneshvar, A., Lau, A. E., Kreuger, J. Characterization of five passive sampling devices for monitoring of pesticides in water. J. Chromatogr. A. 1405, 1-11 (2015).
- Royston, P. Approximating the Shapiro-Wilk W-test for non-normality. Stat. Comput. 2 (3), 117-119 (1992).
- Gauthier, T. D. Detecting trends using Spearman's rank correlation coefficient. Environ. Forensics. 2 (4), 359-362 (2001).
- Morin, N., Miège, C., Coquery, M., Randon, J. Chemical calibration, performance, validation and applications of the polar organic chemical integrative sampler (POCIS) in aquatic environments. TrAC - Trend. Anal. Chem. 36, 144-175 (2012).
- . Water Quality - Sampling - Part 23: Guidance on Passive Sampling in Surface Waters. ISO 5667-23:2011. , (2011).
- Morin, N., Camilleri, J., Cren-Olivé, C., Coquery, M., Miège, C. Determination of uptake kinetics and sampling rates for 56 organic micropollutants using "pharmaceutical" POCIS. Talanta. 109, 61-73 (2013).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone