Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A Simple and Efficient Protocol for the Catalytic Insertion Polymerization of Functional Norbornenes
W tym Artykule
Podsumowanie
We describe the catalytic insertion polymerization of 5-norbornene-2-carboxylic acid and 5-vinyl-2-norbornene to form functional polymers with a very high glass transition temperature.
Streszczenie
Norbornene can be polymerized by a variety of mechanisms, including insertion polymerization whereby the double bond is polymerized and the bicyclic nature of the monomer is conserved. The resulting polymer, polynorbornene, has a very high glass transition temperature, Tg, and interesting optical and electrical properties. However, the polymerization of functional norbornenes by this mechanism is complicated by the fact that the endo substituted norbornene monomer has, in general, a very low reactivity. Furthermore, the separation of the endo substituted monomer from the exo monomer is a tedious task. Here, we present a simple protocol for the polymerization of substituted norbornenes (endo:exo ca. 80:20) bearing either a carboxylic acid or a pendant double bond. The process does not require that both isomers be separated, and proceeds with low catalyst loadings (0.01 to 0.02 mol%). The polymer bearing pendant double bonds can be further transformed in high yield, to afford a polymer bearing pendant epoxy groups. These simple procedures can be applied to prepare polynorbornenes with a variety of functional groups, such as esters, alcohols, imides, double bonds, carboxylic acids, bromo-alkyls, aldehydes and anhydrides.
Wprowadzenie
Norbornene, NBE, the Diels-Alder adduct of ethylene and cyclopentadiene (obtained by "cracking" of dicyclopentadiene (DCPD)), is readily polymerized using either free-radical polymerization,1 cationic polymerization,2 ring-opening metathesis polymerization3 and catalytic insertion polymerization.4,5,6,7 Unlike the other mechanisms, the catalytic insertion polymerization leads to the formation of a very high glass-transition temperature (Tg) polymer whereby the bicyclic backbone of NBE is conserved. A variety of catalysts such as metallocene catalysts and late transition metal catalysts can be used to promote the polymerization of NBE.4,5,6,7 However, due to its low solubility and due to difficulties associated with the processing of a very high Tg polymer, the PNBE homopolymer has, to our knowledge, never found any use.
Functional polynorbornenes (PNBEs) have been the object of considerable scrutiny for the last 20 years, because they combine the high Tg imparted by the bicyclic rigid repeat unit as well as desirable properties endowed by their functionalities.8,9,10 NBE monomers are obtained from rather simple and inexpensive feedstocks, using a one-step Diels-Alder reaction between cyclopentadiene and a functionalized dienophile. However, the Diels-Alder reaction leads to two stereoisomers, endo and exo, which have very different reactivities.11,12 In fact, the endo stereoisomer is less reactive than exo form and deactivates the catalyst.11,12 Thus, in the past, the preparation of functional polynorbornenes usually required the separation of the endo and exo stereoisomers, and only the exo stereoisomer was used. Such a separation procedure was time-consuming, and led to the accumulation of unreacted endo stereoisomers as undesirable waste.
Recently we have shown that the polymerization of functionalized NBEs containing both stereoisomers is in fact feasible.13 We have thus been able to prepare a variety of substituted PNBEs, containing functional groups such as esters, anhydrides, aldehydes, imides, alcohols and double bonds. Due to their high Tg and functionality, these polymers show desirable properties. We describe here two methods to prepare functional polymers. The first one leads to the synthesis of the water soluble polymer poly(5-norbornene-2-carboxylic acid), PNBE(CO2H), using a cationic Pd catalyst (Figure 1).13,14 The same polymerization method can be used to prepare functional PNBEs with various pendant functionalities, such as esters, alcohols, imides, bromo-alkyls, aldehydes and anhydrides. In our hands, this cationic Pd catalyst cannot be used for NBEs containing pendant double bonds such as 5-vinyl-2-norbornene. In this case, a partial insertion of the pendant double bond during the polymerization leads to the formation of a cross-linked material. Therefore, we present here a second method dedicated to the formation of poly(5-vinyl-2-norbornene), PNBE(vinyl), using Pd2(dba)3:AgSbF6:PPh3 as an in situ catalyst.14 The pendant vinyl groups of the polymer are then further epoxidized, to lead to the formation of PNBE(epoxy) (Figure 1). Both PNBE(CO2H) and PNBE(epoxy) have been found to lead to the formation of thermoset resins with a Tg as high as 350 °C.14 Thus, the simple method described here allows one to efficiently prepare polymers with a very high Tg and having a variety of functional groups, which can be used for numerous applications.
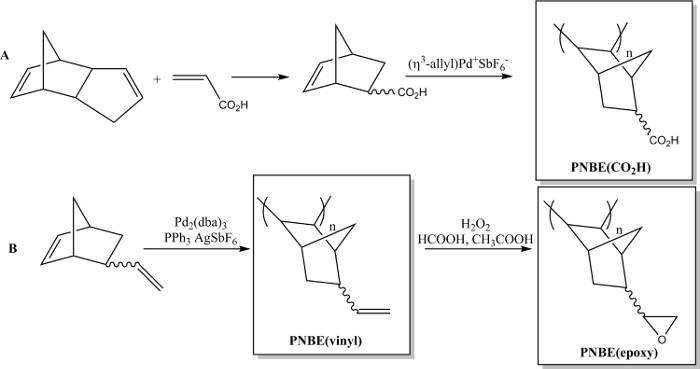
Figure 1: Functional PNBEs prepared by Pd catalyzed polymerization. (A) preparation of PNBE(CO2H), (B) preparation of PNBE(vinyl) and PNBE(epoxy). The dashed bond indicates a mixture of endo and exo isomers. Please click here to view a larger version of this figure.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Preparation of Poly(5-norbornene-2-carboxylic acid), PNBE(CO2H)
- Preparation of the monomer NBE(CO2H)
- Weigh out acrylic acid (AA) (327 g, 4.5 mol, 2 eq.) and hydroquinone (4.9 g, 4.5 x 10-2 mol, 0.02 eq.) and add them to a 2 L round-bottom flask equipped with a condenser and a magnetic stir bar. Heat the flask at 150 °C using a silicone oil bath.
- Once reflux is settled, add DCPD (300 g, 2.3 mol, 1 eq.) in a single portion, and then increase the temperature to 170 °C.
- Leave the reaction at this temperature for 16 h. Observe the color change from clear to yellow-brown.
- Take a sample by extraction with a Pasteur pipet, and analyze it by 1H NMR (using CDCl3 as the solvent)15. Observe the apparition of NBE (double bond signals between 6.0 and 6.5 ppm, Figure 2 top).16
- Purification of NBE(CO2H)
- Replace the condenser with a simple distillation setup (one plateau) connected to a condenser in which cold water is circulated.
- Put the reaction setup under a vacuum set to approx. 1 mmHg. Heat the mixture at 100 °C, and collect a clear liquid (ca. 40 mL) that can be discarded.
- Replace the collection flask with a 500 mL round bottom flask. Heat the oil bath to 155 °C, and observe the dropwise distillation of NBE(CO2H) (317 g, 2.3 mol. Yield = 98%). The distillation takes over 7 h.
- Analyze the colorless liquid by 1H NMR to assess purity as well as endo:exo proportions (Figure 2, bottom).15 The endo:exo ratio changes with the time used for distillation as well as with the heating time used for the preparation of the crude NBE(CO2H). Typically, endo:exo ratios between 50:50 and 80:20 are obtained (60:40 in this case).
- Polymerization of NBE(CO2H)
- Place 300 g (2.3 mol, 5,000 eq.) of NBE(CO2H) in a 500 mL round bottom flask equipped with a magnetic stir bar. Degas the liquid by bubbling nitrogen for 30 min.
- Weigh allylpalladium(II) chloride dimer, [PdCl(C3H5)]2 (76 mg, 4.2 x 10-1 mmol, 1 eq. of Pd) and add it to the solution. Add silver antimonate AgSbF6 (180 mg, 5.2 x 10-1 mmol, 1.2 eq.).
- Under stirring, dissolve the Pd salt by heating at 70 °C, and maintain the temperature at 70 °C under a slight nitrogen flux. After 7 to 8 h, the stirring stops due to a viscosity increase.
- Stop the reaction after 36 h.
- Cool the round bottom flask with liquid nitrogen. With a spatula, break the polymer into small pieces.
- In a 2 L beaker equipped with a magnetic stir bar, add 750 mL of ethyl acetate. Add the polymer chunks to the ethyl acetate under vigorous stirring. Continue stirring for 2 h.
- Filter the solution over a 15 cm diameter Buchner funnel equipped with a paper filter (grade 413, 15 cm diameter).
- Wash the polymer with ethyl acetate three times (500 mL each washing). Dry the polymer (123 g, 9.4 x 10-1 mol, yield = 41%) in a vacuum oven set at 50 °C for 12 h.
2. Preparation of PNBE(vinyl)
- Polymerization of NBE(vinyl)
- Degas toluene (ca. 200 mL) and NBE(vinyl) (ca. 200 mL) by bubbling with N2 for 30 min and place them in a glove box.
- Within the glove box, load toluene (100 g) in a 250 mL round bottom flask.
- Add Pd2(dba)3 (76 mg, 1.6 x 10-1 mmol, 1 eq. of Pd), AgSbF6 (68 mg, 2.0 x 10-1 mmol, 1.2 eq.) and triphenylphosphine, PPh3 (43 mg, 1.6 x 10-1 mmol, 1 eq.) successively to the toluene solution.
- Heat the mixture to 70 °C until complete dissolution occurs. It occurs within 10 min.
- Add 100 g (8.0 x 10-1 mol, 5,000 eq.) of NBE(vinyl) to this purple solution.
- Stir at 70 °C for 72 h.
- Remove the solution from the glovebox, and transfer the viscous black solution to a 1 L glass bottle containing a magnetic stir bar.
- Add toluene (200 mL) and stir.
- Add silica powder (silica gel 40-63 µm, 10 g). Stir at room temperature for 16 h.
- Stop stirring and let the powder settle for at least 2 h in order for the silica particles to sedimentate.
- Filter the solution over a 15 cm diameter Buchner funnel equipped with a paper filter (grade 413, 15 cm diameter). Avoid pouring sedimented silica particles in the Buchner funnel.
- Rinse the silica particles with toluene (50 mL) and filter it through the Buchner funnel.
- Add methanol (1.2 L) to a 4 L beaker equipped with a magnetic stir bar.
- Add all of the toluene solution containing the polymer to the methanol gradually under vigorous stirring, and continue stirring for 30 min.
- Filter the polymer over a 15 cm diameter Buchner funnel equipped with a paper filter (grade 413, 15 cm diameter). Wash the polymer with three aliquots of methanol (400 mL each). Change the filter paper between each washing.
- Assess the polymer purity by 1H NMR in CDCl3, in order to see if the residual monomer is present (double bond signals between 6.0 and 6.3 ppm).15,16 If this is the case, continue washing with methanol.
- Dry the polymer (75 g, 6.3 x 10-1 mol, yield = 78%) under vacuum at room temperature overnight.
3. Preparation of PNBE(epoxy)
- Epoxidation of PNBE(vinyl)
- Add 150 g of dichloromethane to a 500 mL round bottom flask equipped with a magnetic stirrer and a condenser.
- Add PNBE(vinyl) (15 g, 1.3 x 10-1 mol, 1 eq.) with stirring until complete dissolution.
- Place the flask in an ice-bath and let it cool for 15 min.
- In a separate container, mix together formic acid (30 g, 6.5 x 10-1 mol, 5 eq.) and acetic acid (5 g, 8.3 x 10-2 mol, 0.6 eq.). Add the combined acids to the polymer solution.
- Let it cool for 15 min.
- Add hydrogen peroxide aqueous solution (30%) (75 g, 6.5 x 10-1 mol, 5 eq.) to the polymer solution.
- Stir for 18 h. The ice bath does not need to be removed, as the temperature will gradually increase to ambient temperature.
- Take a small sample, precipitate the polymer with acetone, and analyze it by 1H NMR in CDCl3.15 If the signal for the double bond (δ = 4.5-6.0 ppm) is sufficiently decreased (Figure 3), pass to the next step. Typically, the ratio of the integral of the double bonds to the other protons is less than 1:20 (1:83 in Figure 3). Otherwise, continue the reaction.
- Add acetone (750 mL) to a 4 L beaker equipped with a magnetic stir bar.
- Add the polymer solution to the acetone gradually under vigorous stirring for 15 min.
- Filter the polymer over a 15 cm diameter Buchner funnel equipped with a paper filter (grade 413, 15 cm diameter).
- Wash the polymer four times with acetone (200 mL each time).
- Change the filter paper between each washing.
- Dry the polymer (7.5 g) under vacuum at room temperature overnight.

Figure 2: 1H NMR spectra of crude (top) and purified (bottom) NBE(CO2H). The purified product is obtained by simple distillation. Note the peaks which are used to assess the endo:exo ratio. Please click here to view a larger version of this figure.
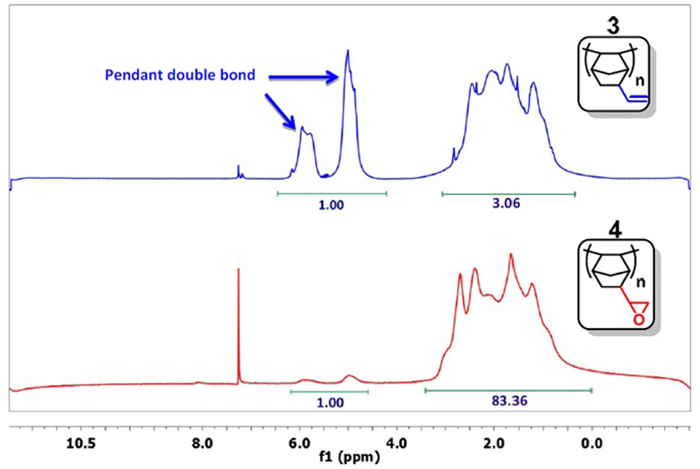
Figure 3: 1H NMR spectra of PNBE(vinyl) (blue) and PNBE(epoxy) (red). Note the ratio 1:3 between the integrals of the vinyl group (δ = 4.5-6.0 ppm) and the other protons in the PNBE(vinyl) spectrum. After reaction with H2O2, the ratio decreases to 1:83, thus confirming that epoxidation of vinyl group has occurred. Please click here to view a larger version of this figure.
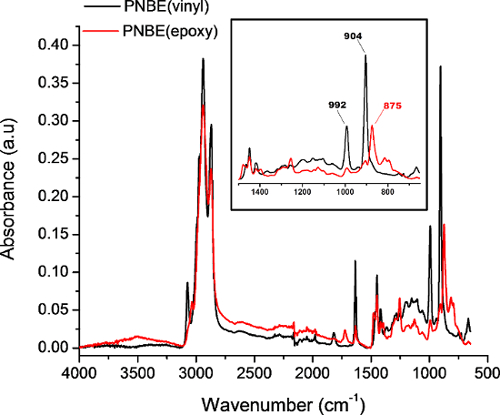
Figure 4: FTIR spectra of PNBE(vinyl) (black) and PNBE(epoxy) acquired in attenuated total reflectance mode. Insert shows a zoom of the characteristic bands of PNBE(vinyl) and PNBE(epoxy). The 902 cm-1 and 992 cm-1 bands correspond to the C=C-H out-of-plane bending, whereas the 875 cm-1 band corresponds to the epoxide ring deformation. Please click here to view a larger version of this figure.
Access restricted. Please log in or start a trial to view this content.
Wyniki
The NBE monomers are prepared by simple Diels-Alder reaction of DCPD and a suitable dienophile, for example acrylic acid (AA). Normally, DCPD is cracked to yield cyclopentadiene (CPD) before reaction.17 Freshly cracked CPD is then engaged in the Diels-Alder reaction. However, in this protocol, both cracking and Diels-Alder steps are performed concomitantly, in a one-pot reaction. Thus, as soon as CPD is formed, it reacts with AA to yield 5-norbornene-2-carboxylic a...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
The method proposed here is simple, and readily amenable to scale-up. All chemicals could be used as received without any purification. Note that performing the reaction at a lower scale (e.g. scales ≤1 g) usually yields lower yields due to an unavoidable loss of material during the handling and the collection.
The catalysts are formed in situ upon the reaction of commercial Pd compounds with cationizing agents. In our hands, the yield of the reaction as well as the cha...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors acknowledge funding from Fonds de Recherche du Québec - Nature et Technologies, from Conseil Recherches en Sciences Naturelles et Génie (program INNOV) and PrimaQuébec.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| acrylic acid | Sigma-Aldrich | 147230 | |
| hydroquinone | Sigma-Aldrich | H9003 | |
| dicyclopendadiene | Sigma-Aldrich | 454338 | |
| palladium allyl dichloride dimer | Sigma-Aldrich | 222380 | |
| silver hexfluoro antimonate | Sigma-Aldrich | 227730 | |
| liquid nitrogen | Local Facility | NA | |
| ethyl acetate | Fischer Scientific | E14520 | |
| 5-vinyl-2-norbornene | Sigma-Aldrich | 148679 | |
| toluene | Fischer Scientific | T290-4 | |
| palladium dba | Sigma-Aldrich | 227994 | |
| triphenyl phosphine | Sigma-Aldrich | 93090 | |
| silica gel 40-63 microns | Silicycle | Siliaflash | |
| methanol | Fischer Scientific | BPA412-20 | |
| dichloromethane | EMD Millipore | DX08311 | |
| formic acid | Sigma-Aldrich | F0507 | |
| acetic acid | Sigma-Aldrich | 320099 | |
| hydrogen peroxide solution | Sigma-Aldrich | 216763 | |
| acetone | Fischer Scientific | A18-200 |
Odniesienia
- Gaylord, N. G., Mandal, B. M., Martan, M. Peroxide-induced polymerization of norbornene. J. Polym. Science, Polym. Lett. Ed. 14 (9), 555-559 (1976).
- Janiak, C., Lassahn, P. G. The vinyl homopolymerization of norbornene. Macromol. Rapid Comm. 22 (7), 479-493 (2001).
- Bielawski, C. W., Grubbs, R. H. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 32 (1), 1-29 (2007).
- Blank, F., Janiak, C. Metal catalysts for the vinyl/addition polymerization of norbornene. Coord. Chem. Rev. 253 (7-8), 827-861 (2009).
- Kaminsky, W., Boggioni, L., Tritto, I. Cycloolefin polymerization. Polymer Science: A Comprehensive Reference, 10 Volume Set. 3, 843-873 (2012).
- Boggioni, L., Tritto, I. State of the art of cyclic olefin polymers. MRS Bull. 38 (3), 245-251 (2013).
- Goodall, B. Cycloaliphatic polymers via late transition metal catalysis. Late Transition Metal Polymerization Catalysis. Rieger, B., Baugh, L., Kacker, S., Striegler, S. , Wiley-VCH Verlag GmbH. 101-154 (2003).
- Zhou, W., He, X., Chen, Y., Chen, M., Shi, L., Wu, Q. Vinyl-addition copolymerization of norbornene and polar norbornene derivatives using novel bis(β-ketoamino)Ni(II)/B(C6F5)3/AlEt3 catalytic systems. J. Appl. Polym. Sci. 120 (4), 2008-2016 (2011).
- Müller, K., Jung, Y., Yoon, D. Y., Agarwal, S., Greiner, A. Vinyl-type polymerization of alkylester-substituted norbornenes without endo/exo separation. Macromol. Chem. Phys. 211 (14), 1595-1601 (2010).
- Boffa, L. S., Novak, B. M. Copolymerization of polar monomers with olefins using transition-metal complexes. Chem. Rev. 100 (4), 1479-1494 (2000).
- Funk, J. K., Andes, C. E., Sen, A. Addition Polymerization of Functionalized Norbornenes: The Effect of Size Stereochemistry, and Coordinating Ability of the Substituent. Organometallics. 23 (8), 1680-1683 (2004).
- Hennis, A. D., Polley, J. D., et al. Novel, efficient, palladium-based system for the polymerization of norbornene derivatives: Scope and mechanism. Organometallics. 20 (13), 2802-2812 (2001).
- Commarieu, B., Claverie, J. P. Bypassing the lack of reactivity of endo-substituted norbornenes with the catalytic rectification-insertion mechanism. Chem. Sci. 6 (4), 2172-2182 (2015).
- Commarieu, B., Potier, J., et al. Ultrahigh Tg epoxy thermosets based on insertion polynorbornenes. Macromoecules. 49 (3), 920-925 (2016).
- Pirrung, M. C. The Synthetic Organic Chemist's Companion. , John Wiley & Sons, Inc. (2007).
- Kanao, M., Otake, A., Tsuchiya, K., Ogino, K. Stereo-selective synthesis of 5-norbornene-2-exo-carboxylic acid-Rapid isomerization and kinetically selective hydrolysis. Int. J. Org. Chem. 2 (1), 26-30 (2012).
- Huertas, D., Florscher, M., Dragojlovic, V. Solvent-free Diels-Alder reactions of in situ generated cyclopentadiene. Green Chem. 11 (1), 91-95 (2009).
- Pierre, F., Commarieu, B., Tavares, A. C., Claverie, J. High Tg sulfonated insertion polynorbornene ionomers prepared by catalytic insertion polymerization. Polymer. 86, 91-97 (2016).
- Woo, H. G., Li, H. Advanced functional materials, Chapter 1.6.8,30. 1, Zheijiang University Press. Hangzhou. (2011).
- Kim, D. -G., Bell, A., Register, R. a Living vinyl addition polymerization of substituted norbornenes by a t-Bu3P-Ligated Methylpalladium Complex. ACS Macro Letters. 4 (3), 327-330 (2015).
- Seung, H., S, A., Baek, K., Sang, S. Low Dielectric Materials for Microelectronics. Dielectric Material. Intech, S. ilagui,M. .A. .,ed., , 59-76 (2012).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone