Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Purification of Biotinylated Cell Surface Proteins from Rhipicephalus microplus Epithelial Gut Cells
W tym Artykule
Podsumowanie
A modified density centrifugation gradient-based methodology was utilized to isolate epithelial cells from Rhipicephalus microplus gut tissue. Surface-bound proteins were biotinylated and purified through streptavidin magnetic beads for utilization in downstream applications.
Streszczenie
Rhipicephalus microplus – the cattle tick – is the most significant ectoparasite in terms of economic impact on livestock as a vector of several pathogens. Efforts have been dedicated to the cattle tick control to diminish its deleterious effects, with focus on the discovery of vaccine candidates, such as BM86, located on the surface of the tick gut epithelial cells. Current research focuses upon the utilization of cDNA and genomic libraries, to screen for other vaccine candidates. The isolation of tick gut cells constitutes an important advantage in investigating the composition of surface proteins upon the tick gut cells membrane. This paper constitutes a novel and feasible method for the isolation of epithelial cells, from the tick gut contents of semi-engorged R. microplus. This protocol utilizes TCEP and EDTA to release the epithelial cells from the subepithelial support tissues and a discontinuous density centrifugation gradient to separate epithelial cells from other cell types. Cell surface proteins were biotinylated and isolated from the tick gut epithelial cells, using streptavidin-linked magnetic beads allowing for downstream applications in FACS or LC-MS/MS-analysis.
Wprowadzenie
Rhipicephalus microplus, the cattle tick, is the most significant ectoparasite in terms of economic impact on the cattle industry of tropical and sub-tropical regions as it vectors bovine tick fever (babesiosis), anaplasmosis and equine piroplasmosis1,2,3,4. Efforts have been dedicated to cattle tick control, to diminish the deleterious effect, however conventional methods such as the use of chemical acaricides have implicit drawbacks, such as the presence of chemical residues in milk and meat, and the increase in prevalence of chemically resistant ticks5,6,7. Consequently, the development of alternative methods of tick control have been studied, such as the use of natural resistance cattle, biological control (biopesticides) and vaccines4,5,6,7,8,9.
In the pursuit of proteins capable of being utilized as vaccine candidates, current research is focused upon the tick gut. The midgut wall is built from a single layer of epithelial cells resting on a thin basal lamina, with the outside of the basal lamina forming a network of muscle. Light and electron microscope observations indicate that the midgut consists of three types of cells: reserve (undifferentiated), secretory, and digestive. The number of cell types varies considerably depending upon the physiological phase. Secretory and digestive cells both originate from reserve cells18,19,20.
The construction of cDNA libraries to examine the composition of the tick gut has led to the identification of antigenic proteins, such as Bm86, as potential vaccine candidates2,3,4. The glycoprotein Bm86 is localized at the surface of tick gut cells and induces a protective immune response against the cattle tick (R. microplus) in vaccinated cattle. Anti-Bm86 IgGs produced by the immunized host are ingested by the tick, recognize this antigen on the surface of tick gut cells, and subsequently disturb tick gut tissue function and integrity. Vaccines based upon Bm86 antigens have shown effective control of R. microplus and Rhipicephalus annulatus, by reducing the number, weight and reproductive capacity of engorging females, resulting in a reduced larval infestation in subsequent tick generations4. However, Bm86 based vaccines are not effective against all tick stages and have demonstrated unsatisfactory efficacy against some geographical strains of R. microplus, consequently the beef and dairy industries have poorly adopted these vaccines2,4.
The ability to isolate epithelial cells from the tick gut is a significant innovation which would enable the progression of research to determine protein membrane composition including morphology and physiology under different environmental conditions. The method described here utilizes the chelating agent ethylenediaminetetraacetic acid (EDTA) and the reducing agent tris(2-carboxyethyl)phosphine (TCEP) to release the epithelium from its sub-epithelial support tissues10. The epithelium is recovered following mechanical disruption of the tissues by shaking, followed by discontinuous gradient centrifugation in Percoll. This paper describes a feasible and novel technique for the isolation of tick gut epithelial cells. Biotinylated cell surface proteins, isolated from the surface of these epithelial cells can subsequently analyzed in downstream applications such as FACS and/or LC-MS/MS-analysis.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Dissection of the Gut Epithelium from R. microplus
- Collect semi-engorged ticks from cattle on the day of experiment. Dissect ticks within 24 h after removal from the host.
- Adhere a strip of duct tape to the bottom of the 92 mm x 16 mm Petri dish. Add a drop of super glue to the tape. Place the tick, ventral side down on the super glue, allow to dry for 2 min.
- Pour 100 mL of phosphate buffered saline (PBS) into the petri dish, or until the tick is completely submerged.
- Utilizing a size 11 scalpel, cut from the top of the eyes to the bottom festoons, on both sides of the tick.
- Using sterile forceps, completely remove the scutum and alloscutum, to expose the internal organs.
- Remove the fine white thread-like organs (trachea) and other membranes to prevent contamination.
- Remove the gut using forceps, by pinching the upper region and pulling from the carcass. Remove any remaining gut tissues, ensuring that no other tissues have been dissected.
- Store gut in ice-cold Hank's Balanced Salt Solution (HBSS) without calcium chloride and magnesium sulfate with proteinase-inhibitor cocktail (PIC). Snap freeze the guts in dry ice and store at -20 °C.
Note: To assist with gut dissection and details of the tick internal organs refer to Chapter 3.1 of D. Sonenshine, "Biology of Ticks"19.
2. Epithelial Cell Dissociation
- Pour the dissected gut onto a 70 µm cell strainer inside a 50 mL tube.
- Flush the gut tissue with 50 mL ice cold HBSS with PIC until the solution runs clear, and the guts take on a white/clear appearance.
- Re-suspend guts in 30 mL ice cold HBSS with PIC, mix gently and centrifuge at 500 x g at 4 °C for 10 min to pellet the gut. Remove the supernatant and repeat the wash process three times.
- To dislodge epithelial gut cells, re-suspend the gut in 10 mL of cell culture medium Dulbecco's modified eagle medium (DMEM), 2% fetal calf serum (FCS), 0.5 mM ethylenediaminetetraacetic acid (EDTA), 1 mM Tris(2-carboxyethyl)phosphine (TCEP), PIC and incubate for 60 min at 37 °C under slow rotation using a roller at 6 rpm.
- Filter the suspension through a 250 µm cell strainer, vortex the flow-through and filter through a 70 µm cell strainer collecting the remaining flow-through.
- Centrifuge the suspension at 500 x g at 4 °C for 20 min to pellet the single cells.
3. Isolation of Single Epithelial Cells using a density centrifugation gradient
- Prepare density centrifugation gradient (e.g., Percoll) by filtering through an AP15 pre-filter paper. Prepare 40% and a 20% Percoll in mqH20 (v/v) and cool at 4 °C for 1 h prior to layering the gradient.
- Using a peristaltic pump set at the lowest speed, layer 3 mL of 40% density centrifugation gradient into a 16 mL ultracentrifuge tube, allowing it to settle on ice for 15 min. Speed of the pump should lead to a <1 mL per min flow rate.
- Tilting the tube to a 45° angle, use the peristaltic pump to layer the 20% density centrifugation gradient on top of the 40% layer. Speed of the pump should lead to <1 mL per min flow rate. Allow the layers to settle on ice for 15 min.
- Use the peristaltic pump at <1 mL per min flow rate to layer 3 mL of DMEM medium containing tick gut cells over the 20-40% density centrifugation gradient.
- Program the centrifuge for maximum acceleration and minimum deceleration. Centrifuge at 600 x g for 10 min. Collect interphases between the DMEM:20% density centrifugation gradient, and the 20%: 40% density centrifugation gradients to isolate epithelial single cells. Store collected interphases at 4 °C for subsequent analyses.
4. Assessment of Cell Isolation
- Hemacytometer
- Clean the hemacytometer slide with alcohol.
- .Re-suspend the cells by gently pipetting the cells up and down. Pipette 100 µL of the cell suspension and place in a 1.5 mL microcentrifuge tube.
- Add 400 µL of 0.4% Trypan Blue. Gently mix by flicking the tube.
- Pipette 100 µL of the Trypan Blue-treated cell suspension to slowly fill both chambers of the hemocytometer.
- Place the hemocytometer under a light microscope, focusing upon the grid lines of the hemocytometer with a 10X objective.
- Using a hand-held tally counter, count the live unstained cells within a set of 16 squares. Within the same square, count the blue dead cells. Continue counting until four sets of 16 squares are counted.
- Calculate the total cells per mL by using the formula:

- Calculate the percentage cell viability by using the formula:
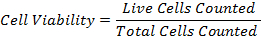
- Cell Isolate Visualization
- Dilute 1 µL of the isolated cells in 9 µL of HBSS in a 1.5 mL microcentrifuge tube. Flick the microcentrifuge tube gently to mix.
- Pipette 5 µL of isolated cells in HBSS into the middle of a glass slide. Apply three drops of mounting medium with 4', 6-diamidino-2-phenylindole (DAPI).
- Incubate the slide at room temperature for 5 min. Carefully place a cover slip over the preparation, avoiding air bubbles.
- Visualize cells stained with DAPI at excitation 360 nm and emission at 460 nm under a fluorescent microscope.
5. Cell Surface Protein Biotinylation
- Biotinylate 100 µL of single cell epithelial cells using Biotin (Type A) Conjugation kit, at a molar ratio of 1:1 surface protein to conjugate, as per manufacturer's instructions
- For cell lysis, add 100 µL of PBS, 1% Triton X-100, 10% glycerol, 100 µM oxidized glutathione and PIC to the biotinylated cells. Incubate on ice for 1 h with gentle mixing every 10 min.
- Centrifuge the biotinylated cells at 20,000 x g at 4 °C for 20 min to pellet insoluble material. Collect the supernatant containing cytoplasmic, and biotinylated membrane proteins.
- Determine the protein concentration using the Bradford assay.
6. Isolation of Biotinylated Surface Proteins
- Add 50 µL of Streptavidin Magnetic Beads into a 1.5 mL microcentrifuge tube.
- Place the tube into a magnetic stand, collecting the beads against the side of the tube. Remove and discard the supernatant.
- Add 1000 µL of TBS, 0.1% Tween-20 to the tube. Mix gently and collect the beads with the magnetic stand. Remove and discard the supernatant.
- Combine 40 µg of biotinylated cell surface proteins, diluted to 300 µL in 1x PBS with washed magnetic beads. Incubate for 2 h at room temperature with agitation.
- Collect the beads with a magnetic stand, remove and discard the supernatant.
- Add 300 µL of TBS, 0.1% Tween-20 to the tube, gently mixing to re-suspend the beads. Collect beads, remove and discard the supernatant. Repeat this wash step twice.
- Add 100 µL of the 0.1 M glycine pH 2.0 to the magnetic beads, and incubate at room temperature for 5 min. Collect beads and remove supernatant containing eluted biotinylated surface proteins.
- Visualize isolated surface proteins on a 4-20% Tris-MOPS SDS-PAGE gel.
7. Assessment of Biotinylated Surface Protein
- Dot Blot
- Cut a 7 cm x 3 cm strip of nitrocellulose membrane.
- Apply 10 µg total tick gut contents (from 1.8 above), and 10 µg of biotinylated surface protein to the membrane. Allow drying for 15 min at room temperature.
- Transfer to a container and submerge in 100 mL of blocking buffer. Incubate at room temperature with agitation for an hour. Discard blocking buffer.
- Wash nitrocellulose membrane in 100 µL PBS, 0.05% Tween-20 for 5 min with agitation. Discard wash buffer and repeat the washes for three times.
- Incubate in 1/5000 Streptavidin- horseradish peroxidase (HRP)in 100 µL PBS, 0.05% Tween-20 for 2 h with agitation at room temperature, and discard any remaining solution.
- Wash nitrocellulose membrane in 100 µL of PBS, 0.05% Tween-20 for 5 min with agitation. Discard wash buffer and repeat the wash three times.
- For detection, dissolve 1 tablet of 4-chloro-1-napthol in 10 mL of ice cold methanol. Add 4 mL of methanol stock to 20 mL of TBS. Add 10 µL of fresh 30% hydrogen peroxide and immediately apply to nitrocellulose membrane.
- Incubate with agitation at room temperature until substrate produces an insoluble blue end product. This may take between 2-15 min.
- Discard detection solution and wash the membrane three times in 100 µL mqH2O.
- ELISA Assay
- Dilute 200 ng of each sample with 400 µL of 100 mM Carbonate coating buffer and coat 2 lanes of the first row (A#) of the ELISA plate (flat bottom wells)
- Add 100 µL of 100 mM carbonate coating buffer to all other corresponding wells in rows (B-H).
- Prepare serial dilutions of each sample by pipetting 100 µL from each well in row A, and transferring into row B. Mix gently by pipetting and avoid producing bubbles. Repeat the dilutions down each row, discarding the final 100 µL from each well in row H.
- Cover the plate with parafilm and incubate at 4 °C overnight.
- Wash the plate with 200 µL PBS, 0.05% Tween-20 per well three times.
- Add 200 µL of blocking buffer to the coated wells, cover with Parafilm and incubate at 4 °C overnight.
- Dilute 1/15,000 Streptavidin-HRP in blocking buffer and add 100 µL to each well of the plate. Cover with parafilm and incubate at 4 °C overnight.
- Wash the plate in 200 µL PBS, 0.05% Tween-20 per well five times.
- To detect, add 100 µL of TMB reagent per well. After sufficient color development, usually between 10-15 min, add 100 µL of 1 M phosphoric acid to stop the reaction.
- Read the absorbance of each well at λ = 450nm.
Access restricted. Please log in or start a trial to view this content.
Wyniki
Epithelial cells were isolated from the gut tissues of R. microplus as per the schematic presented in Figure 1. Representative fluorescence microscopy imagery of tick gut epithelial cells prepared using this protocol are shown in Figure 2A and 2B. As the cell isolation is conducted upon semi-engorged R. microplus, cells appear as singular, spher...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
Cattle tick infestations constitute a major problem for the cattle industry in tropical and subtropical regions of the world, with the most common method of control reliant upon the use of acaricides1,4. Bm86 was previously identified within the tick gut epithelial surface as a protective antigen against R. microplus infestation10, with limited success as a vaccine strategy due to Bm86 geographic sequence variation and the require...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors wish to thank the Biosecurity Tick Colony (Queensland Department of Agriculture & Fisheries, Australia) for the provision of Rhipicephalus microplus ticks utilized for this study, and Lucas Karbanowicz for assistance with video filming.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| 0.4% Trypan Blue | ThermoFisher Scientific | 15250061 | |
| 1.5 mL microcentrifuge tube | Eppendorf | 3322 | |
| 100mM Carbonate Buffer | 3.03 g Na2CO3, 6.0 g NaHCO3 1000 ml distilled water pH 9.6 | ||
| 16 mL centrifuge tubes with sealing cap | Thermo Scientific | 3138-0016 | Cool in ice prior to gradient |
| 250 µM cell strainer | Thermo Fisher | 87791 | |
| 3,3′,5,5′-Tetramethylbenzidine (TMB) Liquid Substrate System for ELISA | Sigma | T0440 | Stored at 4C |
| 30% Hydrogen Peroxide | Labscene | BSPA5.500 | |
| 4-20% Tris-MOPS Gel | Gen Script | M42015 | |
| 4-Chloro-1-naphthol tablet | Sigma-Aldrich | C6788 | |
| 50 mL Falcon Tube | Corning Blue | 30 x 115mm style. Polyproplyene conical tube. | |
| 70 µM cell strainer | BD Falcon | 352350 | |
| AP15 filter paper | Millipore | AO1504200 | |
| Biotin (Type A) Conjugation Kit | Abcam | Ab102865 | |
| Dissection microscope | Olympus | SZX7 | |
| DP Manager | Olympus | 2.2.1.195 | Cell imagery software |
| Duct Tape | Home Handyman | 48mm x 25mm Duct Tape | |
| Dulbecco’s Modified Eagle Medium | Gibco | 11995-065 | DMEM - ice cold for protocol |
| EDTA | Amresco | 0105-500G | |
| F96 Maxisorp Immuno Plate | Nunc | 439454 | |
| Fetal Bovine Serum | Sigma-Aldrich | 12003C | FCS |
| Fluorescence microscope | Olympus | BX51 | |
| Fluoroshield with DAPI | Sigma-Aldrich | F6057-20ML | DAPI |
| Forceps | Dumont | #9 Dumont - Switerzland | |
| Glycerol | Sigma-Aldrich | G5516 | Glycerol for molecular biology >99% |
| Glycine | Sigma-Aldrich | 410225 | |
| Hand-Held Counter | Officeworks | JA0376230 | |
| Hank’s Balanced Salt Solution | Sigma Life Sciences | H9394 | HBSS – ice cold for protocol |
| Hemacytometer | Optik Lakor | - | - |
| L-Glutathione oxidized | Sigma-Aldrich | G4376 | |
| Magnetic Separation Stand | Novagen | - | 4-Tube Magnetic Separation Rack |
| Methanol | Sigma-Aldrich | 179337 | |
| Milli-Q Water | Millipore | ZRXQ003WW | Integral Water Purification System for Ultrapure Water |
| Nitrocellulose Membrane | Life Sciences | 66485 | 30cm x 3M pure nitrocellulose membrane |
| PageRuler Prestained protein Ladder | Thermo-Fisher | SM0671 | |
| PBS | 1.16 g Na2HPO4, 0.1 g KCl, 0.1 g K3PO4, 4.0 g NaCl (500 ml distilled water) pH 7.4 | ||
| Percoll | Sigma-Aldrich | P1644-500ML | |
| Peristaltic Pump | Masterflex | 7518-10 | |
| Phosphoric Acid | Sigma-Aldrich | P6560 | |
| Pierce Protein-Free T20 PBS Blocking Buffer | Thermo-Scientific | 37573 | Stored at 4C. Blocking Buffer |
| Protease Inhibitor Cocktail | Sigma-Aldrich | P8215-5ML | PIC – stored at -20 °C |
| Quick Start Bradford Dye Reagent 1x | Biorad | 500-0205 | For Bradford Assay |
| Quick Start BSA Standards | Biorad | 500-0207 | BSA standards for Bradford Assay |
| Scalpel | Lab. Co | Size 11 Scalpel | |
| SilverQuest TM Staining Kit | Invitrogen | LC6070 | |
| Simply Blue TM Safe Stain | Invitrogen | LC6060 | |
| Sorvall C6+ Ultracentrifuge | Thermo Scientific | 46910 | |
| Streptavidin (HRP) | Abcam | AB7403 | |
| Streptavidin Magnetic Beads | New England Biolabs | S1420S | |
| Super Glue - Ultra Fast Mini | UHU | UHU Super Glue 1mg. Ultra Fast mini | |
| Table-top Centrifuge | Eppendorf | 22331 | |
| TCEP | Thermo Fisher | 20490 | |
| Triton X-100 | Biorad | 161-0407 | |
| Tween-20 | Sigma | P2287-500ML | |
| Vortex Mixer | Ratek | VM1 | |
| Water Bath | Grant | GD100 |
Odniesienia
- Rodriguez-Valle, M., et al. Efficacy of Rhipicephalus (Boophilus) microplus Bm86 against Hyalomma dromedarii and Amblyomma cajennense tick infestations in camels and cattle. Vaccine. 30, 3453-3458 (2012).
- De Rose, R., et al. Bm86 antigen induces a protective immune-response against Boophilus microplus following DNA and protein vaccination in sheep. Vet. Immunol. Immunopathol. 71, 151-160 (1999).
- García-García, J. C., et al. Sequence variations in the Boophilus microplus Bm86 locus and implications for immunoprotection in cattle vaccinated with this antigen. Exp. Appl. Acarol. 23, 883-895 (1999).
- Abbas, R. Z., Zaman, M. A., Colwell, D. D., Gilleard, J., Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet. Parasitol. 203, 6-20 (2014).
- Kearney, S. Acaricide (chemical) resistance in cattle ticks. , AgNote No. K58. (2013).
- Foil, L. D., et al. Factors that influence the prevalence of acaricide resistance and tick-borne diseases. Vet. Parasitol. 125, 163-181 (2004).
- Rodriguez, M., et al. High level expression of the B. microplus Bm86 antigen in the yeast Pichia pastoris forming highly immunogenic particles for cattle. J Biotechnol. 33, 135-146 (1994).
- Rodriguez, M., et al. Effect of vaccination with a recombinant Bm86 antigen preparation on natural infestations of Boophilus microplus in grazing dairy and beef pure and cross-bred cattle in Brazil. Vaccine. 13 (18), 1804-1808 (1995).
- Lew-Tabor, A. E., Rodriguez Valle, M. A review of reverse vaccinology approaches for the development of vaccines against ticks and tick borne diseases. Ticks Tick Borne Dis. 7, 573-585 (2016).
- Capella, A. N., Terra, W. R., Ribeiro, A. F., Ferreira, C. Cytoskeleton removal and characterization of the microvillar membranes isolated from two midgut regions of Spodoptera frugiperda (Lepidoptera). Insect Biochem. Mol. Biol. 27, 793-801 (1997).
- Cioffi, M., Wolfersberger, M. G. Isolation of separate apical, lateral and basal plasma membrane from cells of an insect epithelium. A procedure based on tissue organization and ultrastructure. Tissue Cell. 15, 781-803 (1983).
- Koefoed, B. M. A simple mechanical method to isolate the basal lamina of insect midgut epithelial cells. Tissue Cell. 17, 763-768 (1985).
- Roche, J. K. Isolation of a purified epithelial cell population from human colon. Methods Mol. Med. 50, 15-20 (2001).
- Terra, W. R., Costa, R. H., Ferreira, C. Plasma membranes from insect midgut cells. An. Acad. Bras. Ciênc. 78, 255-269 (2006).
- Vargas, A. E., Markoski, M. M., Cañedo, A. D., Helena, F., Nardi, N. B. Identification, isolation and culture of intestinal epithelial stem cells from murine intestine. Stem Cells. 879, 479-490 (2012).
- Autengruber, A., Gereke, M., Hansen, G., Hennig, C., Bruder, D. Impact of enzymatic tissue disintegration on the level of surface molecule expression and immune cell function. Eur. J. Microbiol. Immunol. 2, 112-120 (2012).
- Karhemo, P. R., et al. An optimized isolation of biotinylated cell surface proteins reveals novel players in cancer metastasis. J. Proteomics. 77, 87-100 (2012).
- Obenchain, F. R., Galun, R. Physiology of Ticks. Current Themes in Tropical Science Volume 1. , Pergamon Press. 201-205 (1982).
- Sonenshine, D., Roe, R. Chapter 3.1. "Biology of Ticks". 1, Two, Oxford University Press. (2014).
- Raikhel, A. S., Balashov, Y. S. "An Atlas of Ixodid Tick Ultrastructure" (English Translation). , Entomology Society of America Entomological Society of America. (1983).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone