Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A General Method for Detecting Nitrosamide Formation in the In Vitro Metabolism of Nitrosamines by Cytochrome P450s
W tym Artykule
Podsumowanie
α-hydroxylation of carcinogenic nitrosamines by cytochrome P450s is the accepted metabolic pathway that produces DNA-damaging intermediates, which cause mutations. However, new data indicates further oxidation to nitrosamides can occur. We describe a general method for detecting nitrosamides produced from in vitro cytochrome P450-catalyzed metabolism of nitrosamines.
Streszczenie
N-nitrosamines are a well-established group of environmental carcinogens, which require cytochrome P450 oxidation to exhibit activity. The accepted mechanism of metabolic activation involves formation of α-hydroxynitrosamines that spontaneously decompose to DNA alkylating agents. Accumulation of DNA damage and the resulting mutations can ultimately lead to cancer. New evidence indicates that α-hydroxynitrosamines can be further oxidized to nitrosamides processively by cytochrome P450s. Because nitrosamides are generally more stable than α-hydroxynitrosamines and can also alkylate DNA, nitrosamides may play a role in carcinogenesis. In this report, we describe a general protocol for evaluating nitrosamide production from in vitro cytochrome P450-catalyzed metabolism of nitrosamines. This protocol utilizes a general approach to the synthesis of the relevant nitrosamides and an in vitro cytochrome P450 metabolism assay using liquid chromatography-nanospray ionization-high resolution tandem mass spectrometry for detection. This method detected N′-nitrosonorcotinine as a minor metabolite of N′-nitrosonornicotine in the example study. The method has high sensitivity and selectively due to accurate mass detection. Application of this method to a wide variety of nitrosamine-cytochrome P450 systems will help determine the generality of this transformation. Because cytochrome P450s are polymorphic and vary in activity, a better understanding of nitrosamide formation could aid in individual cancer risk assessment.
Wprowadzenie
N-nitrosamines are a large class of carcinogens found in the diet, tobacco products, and the general environment; they can also be formed endogenously in the human body1. More than 300 N-nitroso compounds have been tested and >90% were evaluated as carcinogenic in animal models2,3. To exhibit their carcinogenicity, these compounds must first be activated by cytochrome P450s1,2,3. Research shows that cytochrome P450s readily oxidize nitrosamines to α-hydroxynitrosamines (Figure 1), which are highly reactive compounds with half-lives of ~5 s before spontaneously decomposing to alkyldiazohydroxides. The latter can alkylate DNA after the loss of H2O and N2. The resulting DNA adducts, if unrepaired, can cause mutations that, if in critical onco- or tumor suppressor genes, lead to cancer development1. For this reason, much effort has been expended to acquire a full understanding of the metabolic pathways, DNA adducts, and downstream metabolites of cytochrome P450 oxidation of carcinogenic nitrosamines. This knowledge has potential application in individual cancer risk assessment4.
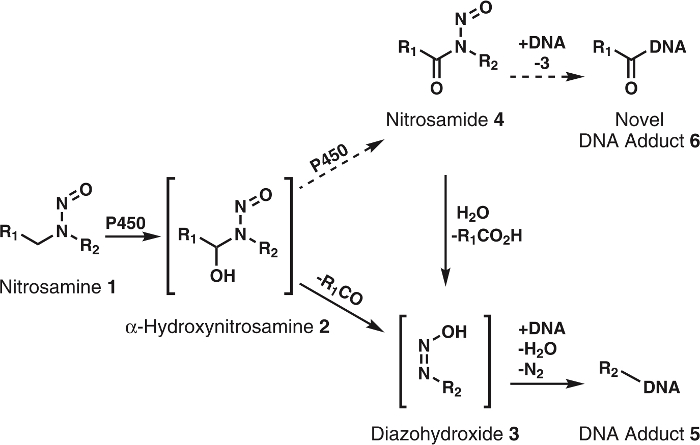
Figure 1: General and proposed metabolism of nitrosamines.
Nitrosamines (1) are oxidized by P450s to α-hydroxynitrosamines (2) that spontaneously decompose to alkyldiazohydroxides (3). These compounds can bind to DNA to form DNA adducts. It is hypothesized that 2 are further oxidized by P450s to nitrosamides 4. These can directly bind to DNA to form novel DNA adducts or be hydrolyzed to 3 to form known DNA adducts. R1 and R2 represent any alkyl group. Please click here to view a larger version of this figure.
Although the α-hydroxynitrosamine hypothesis is solidly supported by extensive data, there are a few inconsistencies; a major one is the short half-life of α-hydroxynitrosamines5,6. It is known that these compounds are produced at the endoplasmic reticulum membrane and later alkylate nuclear DNA. Given their lifetime of a few seconds, it is puzzling how these intermediates survive the required travel though the cytosol. One hypothesis is that a portion of the α-hydroxynitrosamines are processively oxidized to nitrosamides7,8, which are quite stable in comparison9. This would presumably occur via retention of α-hydroxynitrosamines in the cytochrome P450 active site. Precedent for this type of oxidation has been seen with nicotine10, alcohols11, and simple alkylnitrosamines12,13. Additionally, nitrosamides are direct-acting carcinogens2,3. Based on their reactivity9, these compounds are believed to produce DNA adducts identical to those resulting from α-hydroxynitrosamines along with new, unexplored DNA adducts (Figure 1). Thus, this hypothesis not only explains the transport through the cytosol, but also the formation of DNA damage products.
In this paper, a general protocol for assessing the in vitro cytochrome P450-mediated conversion of nitrosamines to nitrosamides is described. The previously reported conversion of N′-nitrosonornicotine (NNN) to N′-nitrosonorcotinine (NNC) by cytochrome P450 2A6 is showcased as an example14. Application of this protocol to a wide range of substrate-enzyme systems will help determine the importance of nitrosamides in overall nitrosamine metabolism.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Materials and general procedures
- Synthesize NNN as previously described15. Obtain norcotinine, P450 2A6 Baculosomes, NADPH regeneration system, 0.5x reaction buffer, and all other chemicals or solvents from commercial sources in reagent grade.
- Record NMR spectra on a 500 MHz spectrometer. Report chemical shifts as parts per million (ppm). Use residual solvent peaks as internal references for 1H-NMR (7.26 ppm CDCl3) and 13C-NMR (77.2 ppm CDCl3).
NOTE: Peak splitting used the following abbreviations: s = singlet, d = doublet, dd = doublet of doublets, dt = doublet of triplets, dq = doublet of quartets, ddd = doublet of doublet of doublets, and m = multiplet. - Perform high resolution mass spectrometry (HRMS) for selected compounds on an LTQ Orbitrap Velos and report data as m/z.
- Use polygram pre-coated silica gel TLC plates (40 mm x 80 mm, 0.2 mm thick) with 254 nm fluorescent indicator for thin-layer chromatography (TLC). Visualize TLC plates by UV lamp irradiation.
- Perform flash chromatography on a 60 Å, 70-150 mesh silica gel using a 1.5 cm x 15 cm glass column.
2. Preparation of nitrosamide positive control (N′-nitrosonorcotinine, NNC)
- Dry, in an oven overnight (16 h, ~140 °C), a 25 mL, round-bottom flask containing a magnetic stir bar. In the morning, cool the flask under a stream of N2 while using an oil bubbler to keep the N2 flow rate under one bubble per second.
NOTE: After cooling, no precautions to keep the flask under N2 are required. - In a fume hood, add norcotinine (31.8 mg, 0.196 mmol), acetic anhydride (5 mL), and acetic acid (1 mL) to the cooled flask. Stir this mixture continuously while cooling to 0 °C with a water-ice bath.
Caution: Acetic anhydride and acetic acid are corrosive. - Add NaNO2 (33.3 mg, 0.483 mmol) in one portion and continuously stir the mixture for 2.5 h. Monitor reaction progress by TLC (100% EtOAc, Rf = 0.19, see step 1.4).
NOTE: Over this time, the mixture will become increasingly yellow with occasional bubbling. - Quench the reaction by pouring the mixture into ice-cold H2O (18 mL). Immediately extract the aqueous solution with 18 mL of CH2Cl2 in a 100 mL separatory funnel. Extract the aqueous layer at least 2 additional times with CH2Cl2 (9 mL each).
- Dry the pooled organics over ~100 mg of MgSO4 for 2 min. Filter and concentrate the solution by rotary evaporation to yield a crude, yellow oil. Heat the water bath to 30 °C during evaporation to remove the residual acetic acid.
NOTE: The protocol can be paused here if the compound is dissolved in CH2Cl2 and the solution stored in the dark at 2 - 8 °C. - Purify the crude compound by column chromatography (see step 1.5) using silica gel as the stationary phase and 100% EtOAc as the eluent16.
Caution: NNC and related nitrosamides should be handled with care as they are assumed to be human carcinogens. - Dissolve the pure compound in CDCl3 and use NMR (see step 1.2) to confirm the structure and determine the molarity of this solution17. Store this solution in the dark at 2 - 8 °C until needed.
NOTE: This solution will be used as the positive control in the in vitro assay (step 3.5). NMR spectra and chemical shifts are available in the Supporting Information. - With a pure NNC solution, confirm the accurate parent mass and determine product ion masses by direct infusion on a high-resolution mass spectrometer (HRMS) under parameters described in steps 1.3 and 4.3.
NOTE: Product masses will be used for detecting this compound in the in vitro metabolism assay (step 4.3).
3. An example nitrosamine-P450 in vitro incubation
- Remove P450 2A6 Baculosomes, Vivid-NADPH-Regeneration System, and 10 mM NADP+ Stock Solution from a -80 °C freezer and let them thaw on ice. Once thawed, dilute the Baculosomes and NADPH-Regeneration System 1:10 and 1:50, respectively, into a single 1 mL tube with 0.5X reaction Buffer. Similarly, add 3 μL of the NADP+ Stock Solution to 97 μL of 0.5X Reaction Buffer.
NOTE: NADP+ is light sensitive. Keep this solution protected by wrapping its container in aluminum foil. Enzyme solutions are sensitive to freeze-thaw cycles; limit this to no more than 2 cycles. Prepare aliquots as necessary for future experiments. - For each incubation, add 50 μL of enzyme solution (containing 5 pmol P450) to a 1 mL tube containing 40 μL of 4 μM NNN solution made with Reaction Buffer. Pre-incubate this new mixture for 2 min at 37 °C using a water bath and then add 10 μL of diluted NADP+ solution. Incubate the full system for 1 - 30 min at 37 °C.
NOTE: Using 5-min intervals for incubation times (e.g. 5, 10, 15 min, etc.) will provide a sufficient nitrosamide formation time course. - After the desired incubation time, quench by first adding 10 μl of 3.0 N ZnSO4 and then 10 μL of 3.0 N Ba(OH)2. Vortex this solution and a white precipitate will form. Centrifuge the sample at 8000 x g for 4 min to pellet the precipitate.
NOTE: The procedure must not pause at this step as the formed nitrosamides have limited stability in aqueous solutions. Long pauses may result in false negatives. - Immediately pipette the supernatant and analyze 2 μL by liquid chromatography positive nanoelectrospray-ionization high-resolution tandem mass spectrometry (LC-NSI+-HRMS/MS, Steps 4.1 - 4.3).
- For positive control incubations, evaporate an aliquot containing 100 fmol of synthesized NNC solution into a 1 mL tube under a stream of N2. To this vial, add the full enzyme system from step 3.2 and proceed as described for steps 3.3 - 3.4.
- For negative control incubations, perform the experiment as described (steps 3.2 - 3.4) except replace the 50 μL enzyme solution with 0.5X reaction buffer.
4. Example parameters for nitrosamide detection by LC-NSI+-HRMS/MS
- For the LC component, apply the following multi-step gradient in step 4.2 to a UPLC system using a commercial, hand-packed18 C18 (5 μm), 100 mm x 75 μm, 15 μm orifice capillary column.
- Using 5 mM NH4OAc as solvent A and MeCN as solvent B, load the sample onto the column by running at 5% B at 1 μL/min from 0 - 5 min, and then slowing the flow rate to 0.3 μL/min afterwards. Next, run a gradient from 5 to 20% B over 4 min, followed by a ramp to 55% B over 10 min, and then re-equilibration to 5% B.
- Perform the LC-NSI+-HRMS/MS on a LTQ Orbitrap. Monitor for NNC by both full scan and MS2 fragmentation. Perform full scan at a resolution of 60,000 and extract the accurate parent mass (192.07670) at a mass tolerance of 5 ppm. Use MS2 fragmentation to isolate parent ions (2.0 amu) and fragment by collision-induced dissociation (CID) with a collision energy of 25 eV, resolution of 15,000, and scan time of 30 ms. Extract the accurate masses for product ions of NNC (m/z 192 m/z 134.04739 and 162.07874) at a mass tolerance of 5 ppm.
Access restricted. Please log in or start a trial to view this content.
Wyniki
Based on the work of White et al.19, norcotinine was nitrosated to NNC cleanly and in high yield (80 - 92%) to produce a standard for the in vitro experiment. Structural evidence for a successful reaction was obtained from spectroscopic analyses including 1H-NMR, 13C-NMR, COSY, and HSQC (Supporting Information) along with HRMS which confirmed the parent mass [M + H]+ within 5 ppm of the theoretical value (
Access restricted. Please log in or start a trial to view this content.
Dyskusje
Elucidating the metabolism of nitrosamines is a critical component to understanding their carcinogenicity. Since the involved cytochrome P450s and other metabolic enzymes are polymorphic, further application of this knowledge could potentially identify high risk individuals1,4. New data indicates that further oxidation of α-hydroxynitrosamines, the presumed major metabolites of nitrosamines involved in DNA binding, to nitrosamides is possible; however, this ...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This study was supported by grant no. CA-81301 from the National Cancer Institute. We thank Bob Carlson for editorial assistance, Dr. Peter Villalta and Xun Ming for mass spectrometry assistance in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, and Dr. Adam T. Zarth and Dr. Anna K. Michel for their valuable discussions and input. The Analytical Biochemistry Shared Resource is partially supported by National Cancer Institute Cancer Center Support Grant CA-77598
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Norcotinine | AKoS GmbH (Steinen, Germany) | CAS 17708-87-1, AKoS AK0S006278969 | |
| Acetic acid | Sigma-Aldrich | 695092 | |
| Acetic Anhydride | Sigma-Aldrich | 242845 | |
| Ammonium Acetate | Sigma-Aldrich | 431311 | |
| Barium Hydroxide | Sigma-Aldrich | 433373 | |
| D-Chloroform | Sigma-Aldrich | 151823 | |
| HPLC Acetonitrile | Sigma-Aldrich | 34998 | |
| Magnesium Sulfate | Sigma-Aldrich | M7506 | |
| Methylene Chloride | Sigma-Aldrich | 34856 | |
| Sodium Nitrite | Sigma-Aldrich | 237213 | |
| ViVid CYP2A6 Blue Screening Kit | Life Technologies | PV6140 | |
| Zinc Sulfate | Sigma-Aldrich | 221376 | |
| 0.5 mL tubes | Fisher | AB0533 | |
| 100 mL round bottom flask | Sigma-Aldrich | Z510424 | |
| 125 mL Erlenmeyer flask | Sigma-Aldrich | CLS4980125 | |
| 125 mL Separatory Funnel | Sigma-Aldrich | Z261017 | |
| 25 mL round bottom flask | Sigma-Aldrich | Z278262 | |
| 500 MHz NMR Spectrometer | Bruker | ||
| Allegra X-22R Centrifuge | Beckman-Coulter | ||
| LC vials | ChromTech | CTC–0957–BOND | |
| LTQ Orbitrap Velos | Thermo Scientific | ||
| Magnetic Stir bar | Sigma-Aldrich | Z127035 | |
| NMR tube | Sigma-Aldrich | Z274682 | |
| P1000, P200, and P10 pipettes | Eppendorf | ||
| Rotary evaporator | Sigma-Aldrich | Z691410 | |
| RSLCnano UPLC system | Thermo Scientific | ||
| Shaking Water Bath | Fisher | FSSWB15 | |
| Stir plate | Sigma-Aldrich | CLS6795420 | |
| PicoFrit Column | New Objective | PF3607515N5 | |
| Luna C18, 5 um | Phenomenex | 535913-1 |
Odniesienia
- Rom, W. N., Markowitz, S. Environmental and Occupational Medicine. , 4th ed, Wolters Kluwer/Lippincott Williams & Wilkins. 1226-1239 (2007).
- Preussmann, R., Stewart, B. W. Chemical Carcinogens, ACS Monograph 182. Searle, C. E. 2, 2nd ed, American Chemical Society. 643-828 (1984).
- Magee, P. N., Montesano, R., Preussmann, R. Chemical Carcinogens. ACS monograph 173. Searle, C. E. , American Chemical Society. 491-625 (1976).
- Zhu, A. Z., et al. Alaska Native smokers and smokeless tobacco users with slower CYP2A6 activity have lower tobacco consumption, lower tobacco-specific nitrosamine exposure and lower tobacco-specific nitrosamine bioactivation. Carcinogenesis. 34 (1), 93-101 (2013).
- Mesić, M., Revis, C., Fishbein, J. C. Effects of structure on the reactivity of alpha-hydroxydialkynitrosamines in aqueous solutions. J. Am. Chem. Soc. 118, 7412-7413 (1996).
- Mochizuki, M., Anjo, T., Okada, M. Isolation and characterization of N-alkyl-N- (hydroxymethyl)nitrosamines from N-alkyl-N- (hydroperoxymethyl)nitrosamines by deoxygenation. Tetrahedron Lett. 21, 3693-3696 (1980).
- Guttenplan, J. B. Effects of cytosol on mutagenesis induced by N-nitrosodimethylamine, N-nitrosomethylurea and à-acetoxy-N-nitrosodimethylamine in different strains of Salmonella:evidence for different ultimate mutagens from N-nitrosodimethylmine. Carcinogenesis. 14, 1013-1019 (1993).
- Elespuru, R. K., Saavedra, J. E., Kovatch, R. M., Lijinsky, W. Examination of a-carbonyl derivatives of nitrosodimethylamine in ethylnitrosomethyamine as putative proximate carcinogens. Carcinogenesis. 14, 1189-1193 (1993).
- Chow, Y. L. ACS Symposium Series. 101, American Chemical Society. 13-37 (1979).
- von Weymarn, L. B., Retzlaff, C., Murphy, S. E. CYP2A6- and CYP2A13-catalyzed metabolism of the nicotine delta5'(1')iminium ion. J. Pharmacol. Exp. Ther. 343 (2), 307-315 (2012).
- Bell-Parikh, L. C., Guengerich, F. P. Kinetics of cytochrome P450 2E1-catalyzed oxidation of ethanol to acetic acid via acetaldehyde. J Biol Chem. 274 (34), 23833-23840 (1999).
- Chowdhury, G., Calcutt, M. W., Nagy, L. D., Guengerich, F. P. Oxidation of methyl and ethyl nitrosamines by cytochrome P450 2E1 and 2B1. Biochemistry. 51 (50), 9995-10007 (2012).
- Chowdhury, G., Calcutt, M. W., Guengerich, F. P. Oxidation of N-nitrosoalkylamines by human cytochrome P450 2A6: sequential oxidation to aldehydes and carboxylic acids and analysis of reaction steps. J Biol Chem. 285 (11), 8031-8044 (2010).
- Carlson, E. S., Upadhyaya, P., Hecht, S. S. Evaluation of nitrosamide formation in the cytochrome P450-mediated metabolism of tobacco-specific nitrosamines. Chem Res Toxicol. 29 (12), 2194-2205 (2016).
- Amin, S., Desai, D., Hecht, S. S., Hoffmann, D. Synthesis of tobacco-specific N-nitrosamines and their metabolites and results of related bioassays. Crit. Rev. Toxicol. 26, 139-147 (1996).
- Clark, A. G., Wong, S. T. A rapid chromatographic technique for the detection of dye-binding. Anal Biochem. 89 (2), 317-323 (1978).
- Pauli, G. F., et al. Importance of purity evaluation and the potential of quantitative (1)H NMR as a purity assay. J Med Chem. 57 (22), 9220-9231 (2014).
- van der Heeft, E., et al. A microcapillary column switching HPLC-electrospray ionization MS system for the direct identification of peptides presented by major histocompatibility complex class I molecules. Anal Chem. 70 (18), 3742-3751 (1998).
- White, E. H. The Chemistry of the N-Alkyl-N-nitrosoamides. I. Methods of Preparation. J. Am. Chem. Soc. 77, 6008-6010 (1955).
- Patten, C., et al. Evidence for cytochrome P450 2A6 and 3A4 as major catalysts for N'-nitrosonornicotine alpha-hydroxylation by human liver microsomes. Carcinogenesis. 18, 1623-1630 (1997).
- Wong, H. L., Murphy, S. E., Hecht, S. S. Cytochrome P450 2A-catalyzed metabolic activation of structurally similar carcinogenic nitrosamines: N'-nitrosonornicotine enantiomers, N-nitrosopiperidine, and N-nitrosopyrrolidine. Chem. Res. Toxicol. 18, 61-69 (2004).
- Hecht, S. S. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 11, 559-603 (1998).
- von Weymarn, L. B., Zhang, Q. Y., Ding, X., Hollenberg, P. F. Effects of 8-methoxypsoralen on cytochrome P450 2A13. Carcinogenesis. 26 (3), 621-629 (2005).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone