Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Quantifying Microorganisms at Low Concentrations Using Digital Holographic Microscopy (DHM)
W tym Artykule
Podsumowanie
Digital holographic microscopy (DHM) is a volumetric technique that allows imaging samples 50-100X thicker than brightfield microscopy at comparable resolution, with focusing performed post-processing. Here DHM is used for identifying, counting, and tracking microorganisms at very low densities and compared with optical density measurements, plate count, and direct count.
Streszczenie
Accurately detecting and counting sparse bacterial samples has many applications in the food, beverage, and pharmaceutical processing industries, in medical diagnostics, and for life detection by robotic missions to other planets and moons of the solar system. Currently, sparse bacterial samples are counted by culture plating or epifluorescence microscopy. Culture plates require long incubation times (days to weeks), and epifluorescence microscopy requires extensive staining and concentration of the sample. Here, we demonstrate how to use off-axis digital holographic microscopy (DHM) to enumerate bacteria in very dilute cultures (100-104 cells/mL). First, the construction of the custom DHM is discussed, along with detailed instructions on building a low-cost instrument. The principles of holography are discussed, and a statistical model is used to estimate how long videos should be to detect cells, based on the optical performance characteristics of the instrument and the concentration of the bacterial solution (Table 2). Video detection of cells at 105, 104, 103, and 100 cells/mL is demonstrated in real time using un-reconstructed holograms. Reconstruction of amplitude and phase images is demonstrated using an open-source software package.
Wprowadzenie
Determination of accurate bacterial counts in very dilute samples is crucial in many applications: a few examples are water and food quality analysis1,2,3; detection of pathogens in blood, cerebrospinal fluid, or sputum4,5; production of pharmaceutical products, including sterile water6; and environmental community analysis in oligotrophic environments such as the open ocean and sediments7,8,9. There is also increasing interest in detection of possible extant microbial life on the icy moons of Jupiter and Saturn, particularly Europa10,11 and Enceladus12,13,14, which are known to have subsurface liquid oceans. Because no mission since Viking in 1978 has attempted to find extant life on another planet, there has been limited development of technologies and instruments for bacterial identification and counting during space missions15.
Traditional methods of plate count find only culturable cells, which can represent a minority of species in environmental strains, sometimes <1%16. Plates require days or weeks of incubation for maximum success, depending upon the strain. Epifluorescence microscopy has largely replaced plate counts as the gold standard for rapid and accurate microbial enumeration. Nucleic-acid-labeling fluorescent dyes such as 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI), SYBR Green, or acridine orange that bind to nucleic acids are the typical dyes used17,18,19, though many studies use fluorescent indicators of Gram sign20,21,22,23,24. Using these methods without pre-concentration steps leads to limits of detection (LoDs) of ~105 cells per mL. Improvements in LoD are possible using filtration. A liquid sample is vacuum-filtered onto a membrane, usually polycarbonate and ideally black to reduce background. Low-background dyes such as the DNA stains mentioned above may be applied directly to the filter25. For accurate counting by eye, ~105 cells are required per filter, which means that for samples more dilute than ~105 cells per mL, significant sample volumes must be collected and filtered. Laser-scanning devices have been developed in order to systematically explore all regions of the filter and thus reduce the number of cells required for counting, pushing limits of detection down to ~102 cells per mL26. However, these are not available in most laboratories, and require sophisticated hardware as well as software that permit expert confirmation that observed particles are bacteria and not debris.
For reference, adults with sepsis usually begin showing symptoms at <100 cells/mL of blood, and infants at <10 cells/mL. A blood draw from an adult takes 10 mL, and from an infant, 1 mL. PCR-based methods are inhibited by the presence of human and non-pathogenic flora DNA and by PCR-inhibiting components in the blood27,28. Despite a variety of emerging techniques, cultures remain the gold standard for the diagnosis of bloodstream infections, especially in more rural areas or developing nations. For detection of life on other planets, thermodynamic calculations can estimate the energy budget for life and thus the expected possible biomass. 1 - 100 cells/mL are expected to be thermodynamically reasonable on Europa29. It can be readily seen from these numbers that detection of very small numbers of cells in large amounts of aqueous solution is an important unsolved problem.
In this paper, we demonstrate detection of Serratia marcescens and Shewanella oneidensis (wild-type and non-motile mutant) at concentrations of 105, 104, 103, and 100 cells/mL using an off-axis digital holographic microscope (DHM). The key advantage of DHM over traditional light microscopy is the simultaneous imaging of a thick sample volume at high resolution—in this implementation, the sample chamber was 0.8 mm thick. These sample chambers were constructed by the soft-lithography of polydimethylsiloxane (PDMS) from a precision-machined aluminum mold with a tolerance of ± 50 µm. This represents an approximately 100-fold improvement in depth of field over high-power light microscopy. DHM also provides quantitative phase information, allowing for measurements of optical path length (product of refractive index and thickness). DHM and similar techniques have been used for monitoring bacterial and yeast cell cycle and calculation of bacterial dry mass30,31,32; scattering differences may even be used to differentiate bacterial strains33.
The instrument we use is custom-built specifically for use with microorganisms, as previously published34,35, and its design and construction are demonstrated and discussed. Aqueous solutions are continuously supplied to a 0.25 µL volume sample chamber via syringe pump; the flow rate is determined by camera frame rate in order to ensure imaging of the entire sample volume. A statistical calculation predicts the number of sample volumes that must be imaged in order to detect a significant number of cells at a given concentration.
For cell-detection applications, reconstruction of the holograms into amplitude and phase images was not required; analysis was performed on the raw hologram. This saves significant computational resources and disk space: a 500 Mb hologram video will be 1 - 2 Tb when reconstructed. However, we do discuss reconstruction through depth of the sample to confirm that the holograms represent the desired species. An important feature of DHM is its ability to monitor both intensity and phase of the images. Organisms that are nearly transparent in intensity (such as most biological cells) appear clearly in phase. As it is a label-free technique, no dyes are used. This is an advantage for possible space flight applications, since dyes may not survive the conditions of a mission and—more importantly—cannot be assumed to work with extraterrestrial organisms, which may not use DNA or RNA for encoding. It is also an advantage for work in extreme environments such as the Arctic and Antarctic, where dyes may be difficult to bring to the remote location and may degrade upon storage. Reconstruction of images into phase and amplitude is performed using an open-source software package that we have made available on GitHub (SHAMPOO) or using ImageJ.
Protokół
1. Growth and Enumeration of Bacteria
NOTE: This is applicable to almost any bacterial strain grown in the appropriate medium36. In our example, we use three strains: Serratia marcescens as a common, easy identifiable lab strain; and a smaller, highly motile environmental strain, Shewanella oneidensis MR-1. To compare detection of motile vs. non-motile cells, a non-motile Shewanella mutant, Δ FlgM, is also used for comparison37. All strains are grown in lysogeny broth (LB).
- Prepare sterile LB medium (per liter of distilled water: 10 g bacto tryptone, 5 g bacto yeast, 10 g NaCl; filter or autoclave). Prepare standard 100 mm diameter LB-agar plates (same recipe as medium plus 15 g agar per liter; autoclave (121 °C, 20 min) to sterilize and pour when cool enough to handle). Store medium and plates in refrigerator.
- The day before the experiment seed 5 - 6 mL of "Master" culture medium from a fresh bacterial colony, using correct sterile and biosafety technique. Incubate on a shaker (120 rpm) at 30 °C for ~12 h. Incubation time will be dependent on the strain and growth rate. In our experiments, the Shewanella strains are incubated for 12 hours; however, Serratia is harvested after 8 hours, to ensure the culture will be exhibiting mid-logarithmic growth.
- The day of the experiment, take a spectrophotometric reading (OD600) of the bacterial Master culture, which is expected to be in the range of 0.6 to 0.7. It should be in the logarithmic growth phase (as determined previously). If not, subculture 100 µL into 5 mL of medium and allow cell growth to the mid-log phase.
- Take a sample of the Master culture and count the cells directly using a Petroff-Hausser counting chamber. This will enumerate both live and dead cells.
- Extract a 10 µL sample of the undiluted culture with a micropipette and transfer into the chamber.
- Image under a high-dry objective microscope (40X or 63X, NA 0.7 - 0.8) using phase contrast.
- Count the bacteria in at least 20 squares and average.
NOTE: Count only those bacteria which are entirely within a square and only those crossing over the top and left boundaries (or bottom and right, if you prefer). If squares are separated by several lines, choose one as a boundary. - Calculate concentration as the average of 20 squares x dilution factor. Note that direct counts do not work well for concentrations <107/mL.
- Make serial dilutions of the culture for counting of colony-forming units (CFU). This will enumerate live cells only.
- Make a serial dilution of each of the selected bacterial samples with sterile 0.9% saline solution. Transfer 20 µL of the bacterial solution and dilute it with 180 µL saline. Repeat until the lowest concentration is ~103 cells/mL.
- Take 100 µL from at least two dilutions-suggested are 103 and 104/mL — and plate on an appropriate solid media plate. Spread with a sterile spreader. Perform at least 3 replicates of each dilution.
- Incubate at an appropriate temperature for your bacteria overnight or until colonies grow.
- Count colonies and calculate colony forming units (CFU) according to (# of colonies x dilution factor)/volume plated = CFU/mL. Average CFU over the replicates.
2. Preparation of Highly Dilute Samples for DHM
- Make serial dilutions of the Master culture for DHM and post-DHM counting of colony-forming units on LB media plates (See 1.4.1 - 1.4.4). Perform this double-blind so that the person doing the recordings is unaware of the concentration in each measured sample.
- Dilute the bacteria into 10 - 15 mL of a minimal medium that will encourage motility (as appropriate) but inhibit cell division, so that the concentration of cells does not change appreciably during the experiment. This may be 0.9% saline or a more specific motility medium. For example, if using Escherichia coli, motility medium must contain EDTA38. For these examples, we use 0.9% saline.
3. Recording DHM Videos
- Using a sterile syringe, pull in about 10 mL of the dilution of interest.
- Connect the syringe to the DHM sample chamber using sterile fittings and tubing.
NOTE: A custom-made microfluidic chamber was constructed in order to allow the consistent flow of sample through the optical path of the instrument. See the Results section for more details. Prior to the experiment, all components of the sample chamber should be sterilized by autoclaving. - Flow the sample from the syringe through the sample chamber continuously using a syringe pump.
NOTE: Appropriate flow rates vary depending on fluidic channel dimensions, desired throughput as well as data acquisition limitations. - As the sample is flowed through the sample chamber, acquire holograms consecutively at an appropriate frame rate. A time-stamp file will be created which logs the time of each image capture to be used later during data analysis (protocol section 4). Obtain all holograms at ambient temperature (23° C). Record holograms by executing a pre-written C++ executable file provided by the camera manufacturer.
NOTE: Appropriate frame rates vary depending on the flow rate chosen. For this experiment, it is recommended to use a frame rate such that a bacterium would be imaged >2 times as it traveled across the instrument's field of view. - Allow sufficient time for the entire 10 mL of sample to be flowed through the DHM.
- To ensure that bacteria are not growing or dying during the experiments, inoculate enriched media plates with 100 µL of the spent media post-DHM image capture. Spread with a sterile spreader and incubate at appropriate temp for 24 hours; count colonies present.
4. Calculation of Cell Density and Limits of Detection
- With the acquired hologram videos, analyze the data for presence of bacteria.
- Calculate the median pixel value for a time series of holograms then subtract this median value from each respective pixel to eliminate stationary artifacts in the hologram. This may be done in ImageJ by performing the following steps:
- Convert to 32-bit (Image-Type-32 bit)
- Calculate the median (Image-Stack-Zproject-Median)
- Subtract the median from the rest of the stack (Process-ImageCalculator-Subtract)
- Count the number of visible Airy rings and in-focus cells manually. In ImageJ, this is done by pointing at the objects with the Point tool.
- Each series of holograms will be accompanied by a time-stamp file (recorded at time of hologram acquisition). Use the time-stamp file to calculate the total amount of sample pumped at the time the image was captured.
- Calculate the median pixel value for a time series of holograms then subtract this median value from each respective pixel to eliminate stationary artifacts in the hologram. This may be done in ImageJ by performing the following steps:
- Calculate the cell density by dividing the total number of cells detected by the total volume of sample imaged. Average 5 - 10 frames for accurate statistics.
5. Image Reconstruction to Amplitude and Phase
- Choose representative videos with 10 - 200 cells per frame. Load raw (not median-subtracted) holograms into the reconstruction software (examples: ImageJ Numerical Propagation39; SHAMPOO [GitHub]).
- Using ImageJ, go to OD-Numerical Propagation-Numerical Diffraction.
- At the prompt, draw a Fourier mask to choose the real or virtual image and eliminate any sinusoidal noise features.
- Reconstruct amplitude and phase at appropriate z-steps by entering the reconstruction distance.
- Median subtract the amplitude data to reduce noise as in 4.1.1.
- Plot data as a 3D cube or projection. Go to Plugins—Volume Viewer.
Wyniki
The results should indicate the ability to detect living and dead bacteria at very low levels by DHM. The number of bacteria counted should be consistent with the results obtained using the Petroff-Hauser counting chamber and plate counts. Standard statistical methods provide information about the accuracy of the different detection methods at various bacterial concentrations.
Figure 1 shows the Pet...
Dyskusje
Numerical reconstruction of holograms: For the numerical reconstruction of holograms, the angular spectrum method (ASM) is used. This involves the convolution of the hologram with the Green's Function for the DHM. The complex wavefront of the image at a particular focal plane can be calculated by employing the Fourier Convolution Theorem as follows:
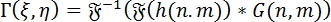 (1)
(1)
Where
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors acknowledge the Gordon and Betty Moore Foundation Grants 4037 and 4038 to the California Institute of Technology for funding this work.
Materiały
| Name | Company | Catalog Number | Comments |
| Bacto Yeast | BD Biosciences | 212750 | |
| Bacto Tryptone | BD Biosciences | 211705 | |
| Sodium chloride | Sigma-Aldrich | 7710 | Many options for purchase |
| Bacto Agar | BD Biosciences | 214010 | |
| 10 cm Petri dishes | VWR | 10053-704 | |
| 15 mL culture tubes | Falcon (Corning Life Sciences) | 352002 | Loose-capped |
| Petroff-Hauser chamber | Electron Microscopy Sciences | 3920 | |
| 10 mL syringes | BD Biosciences | 309604 | Luer-Lok tip not necessary |
| Male Luer to 1/16” barbed fitting | McMaster-Carr | 51525K291 | |
| Autoclavable 1/16” ID PVC tubing for flow | Nalgene | 8000-0004 | Sold by length, purchase accordingly |
| Syringe pump | Harvard Apparatus | PHD 2000 | |
| Sample Chamber | Custom | n/a | See Materials Section |
| Holographic Microscope | Custom | n/a | See Wallace et al. |
| Open-source software | Custom | https://github.com/bmorris3/shampoo |
Odniesienia
- Akineden, O., Weirich, S., Abdulmawjood, A., Failing, K., Buelte, M. Application of a Fluorescence Microscopy Technique for Detecting Viable Mycobacterium avium ssp. paratuberculosis Cells in Milk. Food Anal. Methods. 8, 499-506 (2015).
- Deibl, J., Paulsen, P., Bauer, F. Rapid enumeration of total aerobic microbial counts on meat and in meat products by means of the direct epifluorescence filter technique. Wien Tierarztl Monat. 85, 327-333 (1998).
- Huang, J., Li, Y., Slavik, M. F., Tao, Y., Huff, G. R. Identification and enumeration of Salmonella on sample slides of poultry carcass wash water using image analysis with fluorescent microscopy. Transactions of the Asae. 42, 267-273 (1999).
- Durtschi, J. D., et al. Increased sensitivity of bacterial detection in cerebrospinal fluid by fluorescent staining on low-fluorescence membrane filters. J Med Microbiol. 54, 843-850 (2005).
- Panicker, R. O., Soman, B., Saini, G., Rajan, J. A Review of Automatic Methods Based on Image Processing Techniques for Tuberculosis Detection from Microscopic Sputum Smear Images. J Med Syst. 40, (2016).
- Riepl, M., et al. Applicability of solid-phase cytometry and epifluorescence microscopy for rapid assessment of the microbiological quality of dialysis water. Nephrol Dial Transplant. 26, 3640-3645 (2011).
- Hwang, C. Y., Cho, B. C. Virus-infected bacteria in oligotrophic open waters of the East Sea, Korea. Aquat Microb Ecol. 30, 1-9 (2002).
- Kepner, R. L., Pratt, J. R. Use of Fluorochromes for Direct Enumeration of Total Bacteria in Environmental-Samples - Past and Present. Microbiol Rev. 58, 603-615 (1994).
- Queric, N. V., Soltwedel, T., Arntz, W. E. Application of a rapid direct viable count method to deep-sea sediment bacteria. J Microbiol Meth. 57, 351-367 (2004).
- Prieto-Ballesteros, O., Vorobyova, E., Parro, V., Rodriguez Manfredi, J. A., Gomez, F. Strategies for detection of putative life on Europa. Adv Space Res. 48, 678-688 (2011).
- Gleeson, D. F., et al. Biosignature Detection at an Arctic Analog to Europa. Astrobiology. 12, 135-150 (2012).
- Carr, C. E., Zuber, M. T., Ruvkun, G., Ieee, . 2013 Ieee Aerospace Conference IEEE Aerospace Conference Proceedings. , (2013).
- Konstantinidis, K., et al. A lander mission to probe subglacial water on Saturn's moon Enceladus for life. Acta Astronautica. 106, 63-89 (2015).
- McKay, C. P., Anbar, A. D., Porco, C., Tsou, P. Follow the Plume: The Habitability of Enceladus. Astrobiology. 14, 352-355 (2014).
- Hoover, R. B., Hoover, R. B., Levin, G. V., Rozanov, A. Y., Wickramasinghe, N. C. . Instruments, Methods, and Missions for Astrobiology Xvii. , (2015).
- Handelsman, J. Metagenomics: application of genomics to uncultured microorganisms. Microbiol Mol Biol Rev: MMBR. 68, 669-685 (2004).
- Lebaron, P., Parthuisot, N., Catala, P. Comparison of blue nucleic acid dyes for flow cytometric enumeration of bacteria in aquatic systems. Appl Environ Microbiol. 64, 1725-1730 (1998).
- Marie, D., Partensky, F., Jacquet, S., Vaulot, D. Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I. Appl Environ Microbiol. 63, 186-193 (1997).
- Noble, R. T., Fuhrman, J. A. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat Microb Ecol. 14, 113-118 (1998).
- Forster, S., Snape, J. R., Lappin-Scott, H. M., Porter, J. Simultaneous fluorescent gram staining and activity assessment of activated sludge bacteria. Appl Environ Microbiol. 68, 4772-4779 (2002).
- Lauer, B. A., Reller, L. B., Mirrett, S. Comparison Of Acridine-Orange And Gram Stains For Detection Of Microorganisms In Cerebrospinal-Fluid And Other Clinical Specimens. J Clin Microbiol. 14, 201-205 (1981).
- Mason, D. J., Shanmuganathan, S., Mortimer, F. C., Gant, V. A. A fluorescent gram stain for flow cytometry and epifluorescence microscopy. Appl Environ Microbiol. 64, 2681-2685 (1998).
- Saida, H., Ytow, N., Seki, H. Photometric application of the Gram stain method to characterize natural bacterial populations in aquatic environments. Appl Environ Microbiol. 64, 742-747 (1998).
- Sizemore, R. K., Caldwell, J. J., Kendrick, A. S. Alternate Gram Staining Technique Using A Fluorescent Lectin. Appl Environ Microbiol. 56, 2245-2247 (1990).
- Bitton, G., Dutton, R. J., Foran, J. A. New Rapid Technique For Counting Microorganisms Directly On Membrane Filters. Stain Technology. 58, 343-346 (1983).
- Broadaway, S. C., Barton, S. A., Pyle, B. H. Rapid staining and enumeration of small numbers of total bacteria in water by solid-phase laser cytometry. Appl Environ Microbiol. 69, 4272-4273 (2003).
- Yagupsky, P., Nolte, F. S. Quantitative aspects of septicemia. Clin Microbiol Rev. 3, 269-279 (1990).
- Kang, D. K., et al. Rapid detection of single bacteria in unprocessed blood using Integrated Comprehensive Droplet Digital Detection. Nat Commun. 5, 5427 (2014).
- Hand, K. P. . Report of the Europa Lander Science Definition Team. , (2017).
- Popescu, G., et al. Optical imaging of cell mass and growth dynamics. Am J Physiol-Cell Physiol. 295, C538-C544 (2008).
- Mir, M., et al. Optical measurement of cycle-dependent cell growth. Proc Natl Acad Sci U S A. 108, 13124-13129 (2011).
- Rappaz, B., et al. Noninvasive characterization of the fission yeast cell cycle by monitoring dry mass with digital holographic microscopy. J.Biomed Opt. 14, 034049 (2009).
- Jo, Y., et al. Label-free identification of individual bacteria using Fourier transform light scattering. Opt. Express. 23, 15792-15805 (2015).
- Wallace, J. K., et al. Robust, compact implementation of an off-axis digital holographic microscope. Opt. Express. 23, 17367-17378 (2015).
- Lindensmith, C. A., et al. A Submersible, Off-Axis Holographic Microscope for Detection of Microbial Motility and Morphology in Aqueous and Icy Environments. Plos One. 11, e0147700 (2016).
- Dumas, E. M., Ozenne, V., Mielke, R. E., Nadeau, J. L. Toxicity of CdTe Quantum Dots in Bacterial Strains. IEEE Trans. NanoBiosci. 8, 58-64 (2009).
- Frisk, A., Jyot, J., Arora, S. K., Ramphal, R. Identification and functional characterization of flgM, a gene encoding the anti-sigma 28 factor in Pseudomonas aeruginosa. J Bacteriol. 184, 1514-1521 (2002).
- Adler, J., Templeton, B. The effect of environmental conditions on the motility of Escherichia coli. Journal Gen Microbiol. 46, 175-184 (1967).
- Piedrahita-Quintero, P., Castaneda, R., Garcia-Sucerquia, J. Numerical wave propagation in Image J. Appl Opt. 54, 6410-6415 (2015).
- Schnars, U., Jüptner, W. P. Digital recording and numerical reconstruction of holograms. Meas. Sci. Technol. 13, R85 (2002).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone