Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Reducing Willow Wood Fuel Emission by Low Temperature Microwave Assisted Hydrothermal Carbonization
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
A protocol for the emission precursor depletion from low quality biomass by low temperature microwave assisted hydrothermal carbonization treatment is presented. This protocol includes the microwave parameters and the analysis of the biocoal product and process water.
Streszczenie
Biomass is a sustainable fuel, as its CO2 emissions are reintegrated in biomass growth. However, the inorganic precursors in the biomass cause a negative environmental impact and slag formation. The selected short rotation coppice (SRC) willow wood has a high ash content (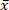 = 1.96%) and, therefore, a high content of emission and slag precursors. Therefore, the reduction of minerals from SRC willow wood by low temperature microwave assisted hydrothermal carbonization (MAHC) at 150 °C, 170 °C, and 185 °C is investigated. An advantage of MAHC over conventional reactors is an even temperature conductance in the reaction medium, as microwaves penetrate the whole reactor volume. This allows a better temperature control and a faster cooldown. Therefore, a succession of depolymerization, transformation and repolymerization reactions can be analyzed effectively. In this study, the analysis of the mass loss, ash content and composition, heating values and molar O/C and H/C ratios of the treated and untreated SCR willow wood showed that the mineral content of the MAHC coal was reduced and the heating value increased. The process water showed a decreasing pH and contained furfural and 5-methylfurfural. A process temperature of 170 °C showed the best combination of energy input and ash component reduction. The MAHC allows a better understanding of the hydrothermal carbonization process, while a large-scale industrial application is unlikely because of the high investment costs.
= 1.96%) and, therefore, a high content of emission and slag precursors. Therefore, the reduction of minerals from SRC willow wood by low temperature microwave assisted hydrothermal carbonization (MAHC) at 150 °C, 170 °C, and 185 °C is investigated. An advantage of MAHC over conventional reactors is an even temperature conductance in the reaction medium, as microwaves penetrate the whole reactor volume. This allows a better temperature control and a faster cooldown. Therefore, a succession of depolymerization, transformation and repolymerization reactions can be analyzed effectively. In this study, the analysis of the mass loss, ash content and composition, heating values and molar O/C and H/C ratios of the treated and untreated SCR willow wood showed that the mineral content of the MAHC coal was reduced and the heating value increased. The process water showed a decreasing pH and contained furfural and 5-methylfurfural. A process temperature of 170 °C showed the best combination of energy input and ash component reduction. The MAHC allows a better understanding of the hydrothermal carbonization process, while a large-scale industrial application is unlikely because of the high investment costs.
Wprowadzenie
The application of microwaves for hydrothermal carbonization (MAHC) was used for the thermochemical transformation of biomass model compounds like fructose, glucose1,2 or cellulose3, and for organic substrates, preferably waste material4,5,6,7,8,9,10. The utilization of microwaves is advantageous as it allows an even heating of the treated biomass2,10 mainly through thermal losses of a dielectric solvent11,12, though the microwaves do not transfer enough energy to directly break chemical bonds and induce reactions13. The microwaves penetrate the whole reaction volume of the HTC reactor vessel and transfer the energy directly to the material, which is not possible with a conventional reactor that shows a slower heating rate due to the high heating capacity of the steel mantle and the sample itself14. The even excitation of the sample’s water molecules by microwaves allows an improved process control, as the temperature in the microwave reactor is evenly distributed11,14,15 and the cooldown after the reaction is much faster. Furthermore, conventional reactors heat up much slower and the chemical reactions occurring during the heating can bias the results that are usually assigned to the final temperature. The improved process control in an MAHC reactor enables a precise elaboration of the temperature dependency of selected HTC reactions (e.g., dehydration or decarboxylation). Another advantage of the even temperature distribution in the HTC-reactor volume is the lower adhesion of immobilized and completely carbonized particles on the inner reactor wall2. However, water is only an average microwave absorbing solvent that even shows decreasing microwave absorbance at higher temperatures, which limits the achievable maximum temperature. This negative effect is compensated when acids are produced during the HTC process or catalyzers (ionic or polar species) are added before the treatment. Microwave induced reactions show higher product yields in general11,15 and specifically of 5-hydroxymethylfurfural (5-HMF) from fructose in comparison to sand-bed catalyzed reactions12. They also have a much better energy balance then conventional heating methods15,16.
The fundamental chemical concept of hydrothermal carbonization is the degradation and successive polymerization of the biomass. In the course of these complex interacting reactions the tissue is depleted of oxygen, which increases the heating value. At first, the polymers hemicellulose and cellulose are hydrolyzed to sugar monomers17, though low temperatures mainly affect the hemicellulose18,19,20,21. In this early stage of the HTC reactions, organic acids are formed from the transformation of the sugar aldehydes and the deacetylation of hemicellulose. These acids can be acetic, lactic, levulinic, acrylic or formic acid20,21,22 and they decrease the pH of the reaction water in the reactor. Due to dissociation, they form free negative ions that increase the ion product in the process water. The increasing ion product allows the solving of cations, which are the major constituents of the ash in the biomass. By this mechanism, the tissue is depleted from emission precursors and slag formers (e.g., potassium, sodium, calcium, chlorine and heavy metals)23,24.
The formed organic acids can support the dehydration of sugar monomers to furans. A common sugar dehydration product is furfural and 5-hydroxymethylfurfural, which are feasible products for the chemical industry, as they serve as platform products (e.g., for the synthesis of biopolymers). 5-Methylfurfural can be formed by catalyzed reactions from cellulose25,26 or 5-hydroxymethylfurfural27. While the biopolymer synthesis is an artificial repolymerization under controlled conditions, the furans can also condense, polymerize and form high molecular weight aromatic structures in the complex chemical environment of the MAHC reactor. The interaction of the solubilized organic and inorganic compounds with the modified wood cell matrix add to the complexity of the reaction system20. The furan polymerization reaction pathways employ aldol condensation or/and intermolecular dehydration18,20 and yield hydrochar particles with a hydrophobic shell and a more hydrophilic core28. It is not yet revealed whether biomass particles are completely decomposed and then repolymerized or if the biomass particles serve as a template for the carbonization. However, the degradation and repolymerization reactions comprise dehydration and decarboxylation reactions, as well29,30, which induces the drop in the van Krevelen diagram towards the O/C and H/C ratios of black carbon.
While other studies proved the mineral reducing effect of conventional reactor based hydrothermal treatment31, of a water washing with combined mechanical leaching32 or water/ammonium acetate/hydrochloric acid washing33, our studies investigate the mineral leaching during low temperature carbonization with microwaves for the first time. As this study focuses on emission precursor leaching for fuel upgrading, it investigates the fate of potassium, sodium, magnesium, calcium, chlorine, sulfur, nitrogen and heavy metals. Fine dust precursors form volatile salts (e.g., KCl or K2SO4) at elevated temperatures in the gaseous phase. When these salts accumulate in the flue gas, heavy metals like zinc can scavenge them as nucleation particles, which leads to a particle growth chain reaction. At lower flue gas temperatures, salt condensation further triggers the particle growth and results in cancerogenous fine dust emission from the chimney. These emissions are at present the main factor that compromises the sustainability of biomass fuels. A sustainable energy supply relies on their reduction by expensive filters or their reduction in the fuels (e.g., by MAHC). As this study follows a practical approach, short rotation coppice (SRC) willow wood was chosen as a potential bioenergy feedstock with high growth rates. It can be grown by farmers on their fields for a self-sustainable power supply by gasification, but also for heat generation by direct combustion. A disadvantage of willow SRC is its high bark content due to a low stem:bark ratio at mature stage. The bark contains a lot of minerals in comparison to wood34,35,36,37 and yields higher quantities of gaseous or particle emissions38. Low temperature HTC can improve the combustion properties of SRC willow wood and, thereby, contribute to a sustainable heat and power supply. Another important parameter of the HTC biocoal investigated in this study is its energy density, its higher initial combustion temperature and its higher final combustion temperature39.
Protokół
1. Preparation of sample material
- Harvest five year old willow, clone type “Tordes” ([Salix schwerinii x S. viminalis] x S. vim.), with a height of 12−14 m and a breast diameter of approximately 15 cm.
- Chip the wood and dry the chips in a kiln dryer for 24 h at 105 °C.
- Cut the wood chips with a cutting mill and grind with a centrifugal mill to a particle size of 0.12 mm.
2. Microwave assisted hydrothermal carbonization
- Use a microwave oven with 850 W and a magnetron frequency of 2,455 MHz.
- Place 500 mg of raw material from step 1.3 in a 50 mL polytetrafluoroethylene (PTFE) reaction vessel with a spatula. Add 10 mL of demineralized water. Screw down the reaction vessel cap so that the pressure valve in the cap is on the same level as the cap brim.
- For each treatment temperature, put twelve reaction vessels with raw materials in the microwave oven and close the oven.
- Set up three temperature programs, with the microwave for the three temperatures: 150 °C (ramp +12.5 °C min-1, hold 60 min, peak power 50%), 170 °C (ramp +9.6 °C min-1, hold 60 min, peak power 80%), and 185 °C (ramp +5.3 °C min-1, hold 30 min, ramp -1.1 °C min-1 to 150 °C, peak power 100%). Start the microwave oven, for each single program.
- After the program is completed, remove the reaction vessels, allow them to cool and reactivate. Then open them under a fume cupboard after releasing the pressure inside.
- Add 35 mL of twice distilled water to each reaction vessel. Pour the solution in each vessel to a centrifuge cylinder and centrifuge at 1,714 x g for 10 min.
- The process water is drained into another tube and stored frozen at -5 °C for pH and gas chromatography-mass spectrometry (GC-MS) analysis.
- Freeze the centrifuge cylinder with the remaining biocoal pellet at -5 °C. Then take out the biocoal pellet and dry it at 105 °C for 24 h. Weigh the biocoal pellet and calculate the weight loss induced by the MAHC treatment.
- Repeat steps 2.2−2.8 four times per temperature (48 reaction vessels per temperature) to produce enough biocoal (approximately 22 g) for the subsequent analysis.
3. Ash content determination
- Weigh 20 empty ceramic dishes individually. Add in each 1 g of sample (5 x 1 g of raw material, and 5 x 1 g of biocoal from each temperature treatment).
NOTE: Because the dishes cannot be labeled, a plan must be drawn for the arrangement of the vessels in the oven. - Place the open ceramic dishes into a muffle furnace and close the furnace.
- Program a temperature program for the muffle furnace (+6 °C min-1 from 25 °C to 250 °C, hold 60 min, +10 °C min-1 to 550 °C, hold 120 min) and start the program.
- After the program is completed, let the muffle furnace cool down to 105 °C. Then open the furnace and take out the ceramic dishes.
- Place the ceramic dishes in an extractor (Table of Materials) filled with a drying agent consisting of silica gel. Close the desiccator and vacuum dry with the help of a vacuum pump.
- Take out the ceramic dishes after 24 h of cooling. Weigh the ceramic dish containing the ash and calculate the ash weight by subtracting the weight of the empty ceramic dish.
- Determine the ash content in percent by dividing the ash weight by the dry mass of the raw material or biocoal.
4. Determination of the higher and lower heating values
- Activate the water pump of the calorimeter and open the oxygen valve to supply 99.5% oxygen to the calorimeter.
- Weigh 1 g of glucose and place it into a plastic sample bag with a defined calorific value of 46,479 J/g. Put the sample bag into the combustion crucible of a calorimeter bomb.
- Add 5 mL of twice deionized water in the bottom of the bomb and screw down the bomb. Put the bomb into the calorimeter and close the calorimeter.
- Enter the weight of the sample and change settings to sample bag method. Start the calorimeter.
- After the measurement is completed, take out the bomb, turn it upside down and shake it slowly for 1 min.
- Unscrew the bomb, remove 5 mL of twice demineralized water and store it in a screw cap container for subsequent ion chromatography analysis.
- Repeat steps 4.2−4.6 three times to obtain the calibration standard.
- Repeat steps 4.2−4.6 five times with each MAHC biocoal (150 °C, 170 °C, 185 °C) and the raw material.
- Calculate the lower heating value using the following equation40:
where LHV is the lower heating value, HHV is the higher heating value obtained from the calorimeter in step 4.4, and ω is the hydrogen content [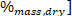 obtained from elemental analysis.
obtained from elemental analysis.
5. Ion chromatography for the quantification of chlorine
NOTE: Check the calibration of ion chromatograph before analysis.
- Take out the 5 mL of solution from step 4.8 and add 45 mL of twice demineralized water in a 50 mL volumetric mask.
- Insert the sample suction tube into a sample container and draw approximately 3 mL of the sample with a syringe into the pre-column. Start the analysis run.
- Carry out the measurements according to manufacturer’s instructions.
- Repeat steps 5.2 and 5.3 for each sample prepared in section 4.
6. Elemental analysis for the determination of the O/C and H/C ratios
- Select an appropriate method from the instrument manual for the samples to be measured.
- Make all the 20 mg sulfonamide standards and blanks required to calibrate the device.
- To prepare a material sample, put 20 mg of sample in tin foil on the micro balance. Weigh the sample on the tin foil, close the foil around the sample and press the package to contain as little air as possible. Afterwards repeat this 5x for each sample.
NOTE: In order to be able to analyze the biochar samples, the same amount of tungsten trioxide as the amount of the sample must be added in a ratio of 1:1. This is needed to compensate the missing oxygen in the biochar to ensure complete combustion in the elemental analyzer. - Insert the prepared samples in the autosampler of the elemental analyzer.
- Open the oxygen and helium valve for the combustion chamber of the elemental analyzer.
- Start the analysis when the device has reached the temperature specified by the device. In this case, wait till the temperature reaches 900 °C.
- Calculate the moles of each element in the sulfonamide standard by the sulfonamide standard weight (step 6.2), and the weight of 1 mole of the respective element.
- Calculate the relationship between moles of C, H, S, and N in sulfonamide, obtained from step 6.7, and the respective peak areas.
- Subtract the sample ash content, obtained from step 3.7, from the total sample weight.
- Compare the respective element peak area in the sulfonamide standard and the sample, and multiply by the mole of each element in sulfonamide to obtain the mole of the element in the sample.
- Calculate the weight of C, H, S, and N in the sample by multiplying the mole of the element, obtained from step 6.10, with the respective molar mass of the element from the periodic table.
- Calculate the weight of oxygen in the sample by using the ash free sample mass, obtained from step 6.9, and subtracting the weight of C, H, N, and S, obtained from step 6.11.
- Calculate the molar H/C and O/C ratios in the raw material and the MAHC biocoal samples.
7. Induced coupled plasma optical emission spectroscopy
- Weigh 400 mg of dried raw material or MAHC biocoal and put it into a 50 mL PTFE reaction vessel with a spatula. Add 3 mL of 69% nitric acid and 9 mL of 35% hydrochloric acid.
- Screw down the reaction vessel cap so that the pressure valve in the cap is on the same level as the cap brim.
- Put the reaction vessels of the samples to be analyzed in the microwave oven and close the oven.
- Program the temperature program for the complete degradation of the organic material: ramp +15.5 °C min-1 to 200 °C, hold 30 min, cool down to 180 °C, hold for 5 min. Start the microwave oven.
- After the program is completed, remove the reaction vessels, allow them to cool and reactivate. Then open the vessels under a fume cupboard after releasing the pressure inside.
- Pour the samples into a 50 mL bulb cylinder. Then rinse the reaction vessel thoroughly with twice deionized water and transfer it to the bulb cylinder. Top up the cylinder to the 50 mL mark with twice deionized water to ensure even dilution of all samples.
- Filter the sample from step 7.6 with 150 µm mesh filter paper. Fill the filtrate in 50 mL conical centrifuge tubes.
- Put the standard samples in the autoinjector of the ICP-OES. The standard samples are of the known concentrations (0.0001 ppm, 0.001 ppm, 0.1 ppm, 1 ppm 10 ppm, 20 ppm, 50 ppm) of the elements to be quantified (Ca, As, B, Be, Fe, Se, Zn, Ag, Al, Ba, Bi, Cd, Co, Cr, Cu, Ga, K, Li, Mg, Mn, Mo, Na, Ni, Pb, Rb, Sr, Te, Tl,V).
- Put the samples in the autoinjector of the ICP-OES and run the ICP-OES analysis with the same parameters.
- After the ICP-OES analysis, obtain the elemental concentration from the software, automatically calculated in mg/kg, based on the calibration curves obtained from standard samples in step 7.8.
- Calculate the elemental concentration reduction in the produced biocoal:
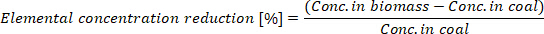
where Conc. in biomass is the elemental concentration in the biomass and Conc. In coal is the elemental concentration in the biocoal.
8. Measuring the pH of the HTC process water
- Fill each liquid fraction from the MAHC treatment (step 2.7) of the raw material and the three biocoals in four respective beakers.
- Calibrate the pH probe with standard solutions.
- Measure the pH of the liquid fraction of the raw material and the three MAHC biocoals.
9. Gas chromatography-mass spectrometry
- Filter the liquid fractions from the MAHC treatment (step 2.7) with 150 µm mesh filter paper. Add 20 mL of methanol to 1 mL of the filtered liquid fractions.
- Transfer 200 µL to a GC-MS autosampler vial and put the vial in the GC-MS autosampler.
- Dilute pure standards of furfural and 5-methylfurfural (analytical grade) down to 10-2, 10-3, 10-4, and 10-5 with methanol.
- Put the standards in the GC-MS autosampler and analyze them with the parameters: 1 µL injection volume at 230 °C injector temperature and 1:40 split; 5MS non-polar column (Table of Materials) with 15 m length and 0.25 mm film thickness; temperature program 30 °C, hold 2 min, ramp of +40 °C/min to 250 °C, hold 2 min; ionization with 70 mV and MS detector at the scan mode with a m/z range of 35−400, each scan in 0.3 s.
- Establish calibration curves by the total ion count (TIC) peak area and the compound concentration.
- Run the prepared HTC biocoal liquid phase samples with the same analytical parameters and identify furfural and 5-methylfurfural by means of retention time of the standard and the spectrum match in a spectra library.
- Determine the concentrations of furfural and 5-methylfurfural by using the calculated calibration curve (step 9.6) and inserting the sample peak areas of furfural and 5-methylfurfural.
10. Statistics
- Analyze the data with the Shapiro Wilks test for normal distribution.
- Use the Mann-Whitney U-test for non-normally distributed data sets and the t-test for normally distributed data sets to find significant differences between data sets.
NOTE: If one data set is normally distributed and the other not, use the Mann-Whitney U test.
Wyniki
The results of the elemental analysis revealed differences between the O/C-H/C ratio of the willow wood and the MAHC biocoals (Figure 1). The raw material shows higher O/C-H/C ratios and a higher variation of the values. The MAHC treatment reduced the value variation due to homogenization in the microwave reactor. The precision of the microwave reactor allowed the differentiation of three stages of degradation. The H/C ratio was reduced at 150 °C and the...
Dyskusje
The MAHC allows the differentiation of the steps of the chemical degradation by applying different intensities of thermal treatment. Therefore, it is possible to assess the interactions between the mass loss, O/C-H/C ratio, heating value, ash component reduction, pH increase of the process water and accumulation of furans in the process water. The advantage of the MAHC method over the conventional HTC reactor method is based on the thermal conduction via microwaves that penetrate the whole reactor volume and conduct the ...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors like to thank Christoph Warth, Michael Russ, Carola Lepski, Julian Tejada and Dr. Rainer Kirchhof for their technical support. The study was funded by the BMBF (Project BiCoLim-Bio-Combustibles Limpios) under the grant number 01DN16036.
Materiały
| Name | Company | Catalog Number | Comments |
| 5MS non-polar cloumn | Thermo Fisher Scientific,Waltham, USA | TraceGOLD SQC | GCMS |
| 9µm polyvinylalcohol particle column | Methrom AG, Filderstadt, Germany | Metrosep A Supp 4 -250/4.0 | Ion chromatography |
| argon | Westfalen AG, Münster, Germany | UN 1006 | ICP-OES |
| calorimeter | IKA-Werke GmbH & Co.KG, Stauffen, Germany | C6000 | higher and lower heating value |
| centrifuge | Andreas Hettich GmbH & Co.KG, Germany | Rotofix 32 A | |
| centrifuge mill | Retsch Technology GmbH, Haan, Germany | ZM 200 | |
| ceramic dishes | Carl Roth GmbH&Co.KG, Karlsruhe, Germany | XX83.1 | Ash content |
| cutting mill | Fritsch GmbH, Markt Einersheim, Germany | pulverisette 19 | |
| D(+) Glucose | Carl Roth GmbH&Co.KG, Karlsruhe, Germany | X997.1 | higher and lower heating value |
| elemental analyzer | elementar Analysesysteme GmbH, Langenselbold, Germany | varioMACRO cube | elemental analysis |
| exicator | DWK Life Sciences GmbH, Wertheim, Germany | DURAN DN300 | Ash content |
| GC-MS system | Thermo Fisher Scientific,Waltham, USA | Trace 1300 | GCMS |
| hydrochloric acid | Carl Roth GmbH&Co.KG, Karlsruhe, Germany | HN53.3 | ICP-OES |
| ICP OES | Spectro Analytical Instruments GmbH, Kleve, Germany | Spectro Blue-EOP- TI | ICP-OES |
| Ion chromatograph | Methrom GmbH&Co.KG, Filderstadt, Germany | 833 Basic IC plus | Ion chromatography |
| kiln dryer | Schellinger KG, Weingarten, Germany | ||
| kiln dryer | Schellinger KG, Weingarten, Germany | Ash content | |
| mesh filter paper | Carl Roth GmbH&Co.KG, Karlsruhe, Germany | L874.1 | ICP-OES |
| microwave oven | Anton Paar GmbH, Graz, Austria | Multiwave Go | |
| muffel furnance | Carbolite Gero GmbH &Co.KG, Neuhausen, Germany | AAF 1100 | Ash content |
| nitric acid | Carl Roth GmbH&Co.KG, Karlsruhe, Germany | 4989.1 | ICP-OES |
| oxygen | Westfalen AG, Münster, Germany | UN 1072 | higher and lower heating value |
| pH-meter | ylem Analytics Germany Sales GmbH & Co. KG, Weilheim,Germany | pH 3310 | pH |
| sample bag | IKA-Werke GmbH & Co.KG, Stauffen, Germany | C12a | higher and lower heating value |
| Standard Laboratory Vessels and Instruments | |||
| standard samples | Bernd Kraft GmbH, Duisburg, Germany | ICP-OES | |
| sulfonamite | elementar Analysesysteme GmbH, Langenselbold, Germany | SLBS4782 | elemental analysis |
| teflon reaction vessels | Anton Paar, Austria | HVT50 | |
| teflon reaction vessels | Anton Paar, Austria | HVT50 | ICP-OES |
| tin foil | elementar Analysesysteme GmbH, Langenselbold, Germany | S12.01-0032 | elemental analysis |
| tungstenVIoxide | elementar Analysesysteme GmbH, Langenselbold, Germany | 11.02-0024 | elemental analysis |
| twice deionized water | Carl Roth GmbH&Co.KG, Karlsruhe, Germany | ||
| twice deionized water | Carl Roth GmbH&Co.KG, Karlsruhe, Germany | higher and lower heating value | |
| twice deionized water | Carl Roth GmbH&Co.KG, Karlsruhe, Germany | ICP-OES |
Odniesienia
- Li, C., Zhao, Z. K., Cai, H., Wang, A., Zhang, T. Microwave-promoted conversion of concentrated fructose into 5-hydroxymethylfurfural in ionic liquids in the absence of catalysts. Biomass and Bioenergy. 35 (5), 2013-2017 (2011).
- Möller, M., Harnisch, F., Schröder, U. Microwave-assisted hydrothermal degradation of fructose and glucose in subcritical water. Biomass and Bioenergy. 39, 389-398 (2012).
- Guiotoku, M., Rambo, C. R., Hansel, F. A., Magalhães, W. L. E., Hotza, D. Microwave-assisted hydrothermal carbonization of lignocellulosic materials. Materials Letters. 63 (30), 2707-2709 (2009).
- Guiotoku, M., Rambo, C. R., hansel, F. A., Magalhães, W. L. E., Hotza, D. Microwave-assisted hydrothermal carbonization of lignocellulosic materials. Materials Letters. (63), 2707-2709 (2009).
- Kannan, S., Gariepy, Y., Raghavan, G. S. V. Optimization and characterization of hydrochar produced from microwave hydrothermal cabonization of fish waste. Waste Management. , 159-168 (2017).
- Elaigwu, S. E., Greenway, G. M. Microwave-assisted and conventional hydrothermal carbonization of lignocellulosic waste material: Comparison of the chemical and structural properties of the hydrochars. Journal of Analytical and Applied Pyrolysis. 118, 1-8 (2016).
- Elaigwu, S. E., Greenway, G. M. Microwave-assisted hydrothermal carbonization of rapeseed husk: A strategy for improving its solid fuel properties. Fuel Processing Technology. 149, 305-312 (2016).
- Chen, W. -. H., Ye, S. -. C., Sheen, H. -. K. Hydrothermal carbonization of sugarcane bagasse via wet torrefaction in association with microwave heating. Bioresource technology. 118, 195-203 (2012).
- Nizamuddin, S., et al. Upgradation of chemical, fuel, thermal, and structural properties of rice husk through microwave-assisted hydrothermal carbonization. Environmental science and pollution research international. 25 (18), 17529-17539 (2018).
- Nizamuddin, S., et al. An overview of microwave hydrothermal carbonization and microwave pyrolysis of biomass. Reviews in Environmental Science and Bio/Technology. 17 (4), 813-837 (2018).
- Dallinger, D., Kappe, C. O. Microwave-assisted synthesis in water as solvent. Chemical reviews. 107 (6), 2563-2591 (2007).
- Qi, X., Watanabe, M., Aida, T. M., Smith, J. R. L. Catalytic dehydration of fructose into 5-hydroxymethylfurfural by ion-exchange resin in mixed-aqueous system by microwave heating. Green Chemistry. 10 (7), 799 (2008).
- Nüchter, M., Ondruschka, B., Bonrath, W., Gum, A. Microwave assisted synthesis - a critical technology overview. Green Chem. 6 (3), 128-141 (2004).
- Schanche, J. -. S. Microwave synthesis solutions from personal chemistry. Molecular Diversity. 7 (2-4), 291-298 (2003).
- Kappe, C. O. Controlled microwave heating in modern organic synthesis. Angewandte Chemie (International ed. in English). 43 (46), 6250-6284 (2004).
- Gronnow, M. J., White, R. J., Clark, J. H., Macquarrie, D. J. Energy Efficiency in Chemical Reactions: A Comparative Study of Different Reaction Techniques. Organic Process Research & Development. 9 (4), 516-518 (2005).
- Kruse, A., Dahmen, N. Hydrothermal biomass conversion: Quo vadis?. The Journal of Supercritical Fluids. 134, 114-123 (2018).
- Reza, M. T., et al. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Applied Bioenergy. 1 (1), (2014).
- Libra, J. A., et al. Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels. 2 (1), 71-106 (2011).
- Reza, M. T., Uddin, M. H., Lynam, J. G., Hoekman, S. K., Coronella, C. J. Hydrothermal carbonization of loblolly pine: reaction chemistry and water balance. Biomass Conversion and Biorefinery. 4 (4), 311-321 (2014).
- Funke, A., Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels, Bioproducts and Biorefining. 4 (2), 160-177 (2010).
- Kruse, A., Funke, A., Titirici, M. -. M. Hydrothermal conversion of biomass to fuels and energetic materials. Current opinion in chemical biology. 17 (3), 515-521 (2013).
- Reza, M. T., Lynam, J. G., Uddin, M. H., Coronella, C. J. Hydrothermal carbonization: Fate of inorganics. Biomass and Bioenergy. 49, 86-94 (2013).
- Zhang, D., et al. Comparison study on fuel properties of hydrochars produced from corn stalk and corn stalk digestate. Energy. 165, 527-536 (2018).
- Huang, Y. -. B., Yang, Z., Dai, J. -. J., Guo, Q. -. X., Fu, Y. Production of high quality fuels from lignocellulose-derived chemicals: a convenient C-C bond formation of furfural, 5-methylfurfural and aromatic aldehyde. RSC Advances. 2 (30), 11211 (2012).
- Van de Vyver, S., Geboers, J., Jacobs, P. A., Sels, B. F. Recent Advances in the Catalytic Conversion of Cellulose. ChemCatChem. 3 (1), 82-94 (2011).
- Delidovich, I., Leonhard, K., Palkovits, R. Cellulose and hemicellulose valorisation: an integrated challenge of catalysis and reaction engineering. Energy & Environmental Science. 7 (9), 2803 (2014).
- Sevilla, M., Fuertes, A. B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon. 47 (9), 2281-2289 (2009).
- Yao, Z., Ma, X. Characteristics of co-hydrothermal carbonization on polyvinyl chloride wastes with bamboo. Bioresource technology. 247, 302-309 (2018).
- Funke, A., Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels, Bioproducts and Biorefining. (4), 160-177 (2010).
- Liu, Z., Balasubramanian, R. Upgrading of waste biomass by hydrothermal carbonization (HTC) and low temperature pyrolysis (LTP): A comparative evaluation. Applied Energy. 114, 857-864 (2014).
- Khalsa, J., Döhling, F., Berger, F. Foliage and Grass as Fuel Pellets-Small Scale Combustion of Washed and Mechanically Leached Biomass. Energies. 9 (5), 361 (2016).
- Saddawi, A., Jones, J. M., Williams, A., Le Coeur, C. Commodity Fuels from Biomass through Pretreatment and Torrefaction: Effects of Mineral Content on Torrefied Fuel Characteristics and Quality. Energy & Fuels. 26 (11), 6466-6474 (2012).
- Kaltschmitt, M., Hartmann, H., Hofbauer, H. . Energie aus Biomasse: Grundlagen, Techniken und Verfahren. , (2016).
- Fengel, D., Wegener, G. . Wood: Chemistry, Ultrastructure, Reactions. , (1989).
- Obernberger, I., Thek, G. Physical characterisation and chemical composition of densified biomass fuels with regard to their combustion behaviour. Biomass and Bioenergy. 27 (6), 653-669 (2004).
- Kenney, W. A., Sennerby-Forsse, L., Layton, P. A review of biomass quality research relevant to the use of poplar and willow for energy conversion. Biomass. 21 (3), 163-188 (1990).
- Tharakan, P. J., Volk, T. A., Abrahamson, L. P., White, E. H. Energy feedstock characteristics of willow and hybrid poplar clones at harvest age. Biomass and Bioenergy. 25 (6), 571-580 (2003).
- Liu, Z., Quek, A., Balasubramanian, R. Preparation and characterization of fuel pellets from woody biomass, agro-residues and their corresponding hydrochars. Applied Energy. , 1315-1322 (2014).
- Technischen Komitee ISO/TC 238. . "Solid biofuels" und Technisches Komitee CEN/TC 335 "Biogene Festbrennstoffe" Solid biofuels - Determination of calorific value (ISO 18125:2017); German version EN ISO 18125:2017. , (2017).
- Kambo, H. S., Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renewable and Sustainable Energy Reviews. 45, 359-378 (2015).
- Knappe, V., et al. Low temperature microwave assisted hydrothermal carbonization (MAHC) reduces combustion emission precursors in short rotation coppice willow wood. Journal of Analytical and Applied Pyrolysis. 134, 162-166 (2018).
- Liu, Z., Quek, A., Kent Hoekman, S., Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel. 103, 943-949 (2013).
- Hoekman, S. K., Broch, A., Robbins, C., Zielinska, B., Felix, L. Hydrothermal carbonization (HTC) of selected woody and herbaceous biomass feedstocks. Biomass Conversion and Biorefinery. 3 (2), 113-126 (2013).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone