Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Obtention of Giant Unilamellar Hybrid Vesicles by Electroformation and Measurement of their Mechanical Properties by Micropipette Aspiration
W tym Artykule
Podsumowanie
The goal of the protocol is to reliably measure membrane mechanical properties of giant vesicles by micropipette aspiration.
Streszczenie
Giant vesicles obtained from phospholipids and copolymers can be exploited in different applications: controlled and targeted drug delivery, biomolecular recognition within biosensors for diagnosis, functional membranes for artificial cells, and development of bioinspired micro/nano-reactors. In all of these applications, the characterization of their membrane properties is of fundamental importance. Among existing characterization techniques, micropipette aspiration, pioneered by E. Evans, allows the measurement of mechanical properties of the membrane such as area compressibility modulus, bending modulus and lysis stress and strain. Here, we present all the methodologies and detailed procedures to obtain giant vesicles from the thin film of a lipid or copolymer (or both), the manufacturing and surface treatment of micropipettes, and the aspiration procedure leading to the measurement of all the parameters previously mentioned.
Wprowadzenie
Giant vesicles obtained from phospholipids (liposomes) have been widely used since the 1970s as the basic cell membrane model1. In the late 1990s, vesicular morphologies obtained from the self-assembly of copolymers, named polymersomes in reference to their lipid analogs2,3, rapidly appeared as an interesting alternative to liposomes that possess weak mechanical stability and poor modular chemical functionality. However, their cell biomimetic character is rather limited compared to liposomes since the latter are composed of phospholipids, the main component of the cell membrane. Furthermore, their low membrane permeability can be an issue in some applications like drug delivery where controlled diffusion of species through the membrane is required. Recently, the association of phospholipids with block copolymers to design hybrid polymer-lipid vesicles and membranes has been the subject of an increasing number of studies4,5. The main idea is to design entities that synergistically combine the benefits of each component (bio-functionality and permeability of lipid bilayers with the mechanical stability and chemical versatility of polymer membranes), which can be exploited in different applications: controlled and targeted drug delivery, biomolecular recognition within biosensors for diagnosis, functional membranes for artificial cells, development of bio-inspired micro-/nano-reactors.
Nowadays, different scientific communities (biochemists, chemists, biophysicists, physico-chemists, biologists) have increasing interest in development of a more advanced cell membrane model. Here, our goal is to present, as detailed as possible, existing methodologies (electroformation, micropipette aspiration) to obtain and characterize the mechanical properties of giant vesicles and the recent "advanced" cell membrane models that are hybrid polymer lipid giant vesicles4,5.
The purpose of these methods is to obtain reliable measurement of the area compressibility and bending moduli of the membrane as well as their lysis stress and strain. One of the most common techniques existing to measure bending rigidity of a giant vesicle is fluctuation analysis6,7, based on direct video microscope observation; but this requires large visible membrane fluctuation, and is not systematically obtained on thick membranes (e.g. polymersomes). Area compressibility modulus can be experimentally determined using the Langmuir Blodgett technique but most often on a monolayer8. The micropipette aspiration technique allows the measurement of both moduli on a bilayer forming giant unilamellar vesicle (GUV) in one experiment.
The following method is appropriate for all amphiphilic molecules or macromolecules able to form bilayers and, consequently, vesicles by electroformation. This requires a fluid character of the bilayer at the temperature of electroformation.
Protokół
1. Fabricating micropipettes
NOTE: Here, micropipettes with an inner diameter ranging from 6 to 12 µm and a taper length around 3-4 mm are necessary. A detailed method of manufacturing micropipette is described in the following.
- Place the borosilicate glass capillary in the drawbar of the puller and fix one of the ends by tightening the knob.
- Carefully slide the glass through the holes at the side of the heater chamber.
- Tighten down the clamping knob at the other end.
- Control the size of the tip and the taper length to achieve the desired specifications. For that, optimize technical parameters such as heating temperature, pull, velocity, delay and pressure. Here is an example of a program used:
Heat: 550 °C
Pull: 10 (Range of the machine: 0-255 in arbitrary units)
Velocity: 30 (Range: 0-255 in arbitrary units)
Delay: 1 (Range: 0-255 in arbitrary units)
Pressure: 500 (Range: 0-999 in arbitrary units) - Click on PULL to execute the events defined by the program. The capillary is then separated into two micropipettes, whose dimensions have to be adjusted using a microforge.
- Insert the micropipette into the metal pipette holder of the microforge (see Figure 1). By using 10x objective, adjust the microscope stage and the pipette manipulator (vertical and horizontal movement) until the pipette tip is close to the glass bead surface.
- Press the foot switch to melt the glass bead. Lower the tip and put it in contact with the molten glass bead. Molten glass will flow into the pipette by capillary action. Wait a few seconds until the level of the molten glass reaches a certain height as shown in the insert of the Figure 1.
- Stop the heating by removing the pressure on the foot switch, and quickly pull the tip away using the vertical pipette manipulator to cause a sharp break.
- Repeat steps 1.7 and 1.8 until the desired diameter is obtained (6 to 12 µm).
NOTE: To improve the accuracy of the diameter measurement, during the last step, use a 32x objective equipped with a reticle.
2. Coating pipette tips with BSA (bovine serum albumin)
- To prepare a 0.1 M solution of glucose containing 1% wt. BSA in pure water.
- Weigh 180 mg of glucose powder, place in a 15 mL polypropylene conical tube and complete with 10 mL of pure water.
- Add 0.1 g of BSA powder and shake gently using a test tube rotatory mixer until complete dissolution (approximately 4 h).
- Take the solution with a 10 mL disposable Luer syringe fitted with a needle. Once filled, remove the needle and install a 0.22 μm acetate cellulose filter. Fill several polypropylene micro-centrifuge tubes (1.5 mL) that will be used to immerse the tip.
- Place the capillaries vertically into holders. Lower the holder and immerse the tip into the glucose/BSA solution overnight. The solution should rise about 1 cm high by capillary action.
- Remove the pipette tip from the glucose/BSA solution. Prepare 5 mL of 0.1 M glucose solution (dilute 90 mg of glucose powder in 5 mL of pure water) and filter through a 0.22 μm acetate cellulose filter.
- Fill the pipette with the glucose solution by using a 500 µL glass syringe equipped with a flexible fused silica capillary. Then, remove all the glucose solution by sucking it back and discard it (Figure 2). Repeat this step several times to remove the unbounded BSA.
3. Formation of GUVs and GHUVs by electroformation
NOTE: Electroformation is a commonly used technic developed by Angelova9. The procedures to obtain an electroformation chamber, deposit a lipid or polymer film (or both for GHUVs (Giant Hybrid Unilamellar Vesicles)) and hydrate the film under an alternative electric field are described in the following. The procedure to collect the GUV obtained is also described.
- Amphiphile, sucrose and glucose solutions preparation
- Prepare an amphiphile solution at a concentration of 1 mg/mL. Weigh 10 mg of amphiphile and dissolve in 10 mL of chloroform. Keep the solution in sealed vials to avoid solvent evaporation.
- Prepare a stock solution of 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (DOPE-Rhod) at 1 mg/mL in chloroform.
- Add 10 µL of fluorescent lipid solution to the amphiphile solution. Keep the solutions in sealed vials to avoid solvent evaporation.
- Prepare sucrose and glucose solutions at a concentration of 0.1 M. Weigh 342 mg and 180 mg of sucrose and glucose, respectively, and dissolve them in 10 mL of pure water.
- Preparation of the electroformation chamber
NOTE: Different conductive materials can be used to make an electroformation device (e.g., platinum wires10, stainless needles11). The electroformation chamber is composed of two ITO slides separated by an O-ring rubber spacer that has been cut on one side to create an aperture. The slides are connected to a voltage generator via two electric wires (Figure 3 and Figure 4A).- Clean the ITO slides with organic solvent (e.g., chloroform). Identify the conductive surface using an ohmmeter.
- Attach the electrical wires on the conductive side using adhesive tape.
- Amphiphile solution deposition
- Dip a capillary in the solution until the level increases by capillary action and collect about 5 μL of the solution.
- Put the capillary in contact with the center of the ITO glass plate and gently spread the solution. Wait 10 seconds to ensure complete solvent evaporation (Figure 4A).
- Repeat this procedure 3 times for each side.
- Add a layer of silicon-free grease on both sides of the opened O-ring spacer. Put it around the area of deposition. Place the conductive face of the second ITO glass plate on the top of the spacer.
- Place the electroformation chamber under vacuum for 3 hours to remove any traces of organic solvent.
- Electroformation procedure
- Plug the electric wires to the generator.
- Use the following settings for the generator:
Alternative sinusoidal tension
Frequency: 10 Hz
Amplitude: 2 Vpeak-to-peak
NOTE: Optimum voltage frequency and duration must be found for each system. - Ensure that the voltage is applied before injection of the solution in the chamber.
- Inject 1 mL of solution using a syringe with 0.8 mm inner diameter needle to fill the chamber. Remove eventual bubbles.
- Let the chamber under the applied voltage/frequency for 75 min (Figure 4B).
- GUVs harvest
- Switch off the generator.
- Using 1 mL syringe with 0.8 mm inner diameter needle, suck a small part of the solution in order to produce an air bubble inside the chamber. Slightly tilt the chamber in order to make this bubble move inside the chamber. This can help the GUVs to detach from the ITO surface (Figure 4C).
- Suck all the solution and transfer it into a 1 mL plastic tube.
- Remove the wires and clean the ITO slides with organic solvents (toluene then chloroform).
4. Micromanipulation set up
NOTE: The principle of micropipette aspiration is to suck a single vesicle through a glass micropipette by applying a depression. The length of the tongue inside the pipette is measured as a function of the suction pressure. The pipette coating with BSA, described previously, is essential to prevent or minimize any adhesion between vesicles membranes and the pipette.
The protocol is illustrated below.
- Pipette and water filled reservoir connection
NOTE: The water filled tank and the micrometer are fixed to a sliding plate. A digital counter with a micrometer head allows a vertical displacement of the device in a range of 0 to 2.5 cm and an accuracy of 1 µm. Displacement along an aluminum optical rail is possible up to 1 meter in length. A silicon tubing connects the reservoir and the capillary holder (Figure 5A).- Fill the tank with pure water. Connect a disposable 5 mL syringe to the capillary metal holder via silicone tubing and aspirate to create a water flow from the tank to the holder.
- Touch the tube slightly to eliminate air bubbles. Simultaneously, raise the water tank to create a positive pressure. The 5 mL syringe is still attached to the holder.
- After coating and cleaning steps described previously (see step 2), fill a capillary with glucose solution until a drop forms at the end. Remove the syringe tubing from the metal holder to create a slight water flow at its end.
- Turn the capillary upside down and connect the glucose drop with the water flow from the holder. Fix the capillary and the holder by screwing them together.
- Position a pipette
NOTE: During this operation, the water tank is still positioned on the aluminum rail to maintain a positive pressure.- Take the homemade aluminum stage equipped with two glass slides (previously cleaned with ethanol) and glue them with vacuum grease on each side. Install it on the microscope stage and form a meniscus between the two slides by using a 1 mL syringe containing 0.1 M of glucose as shown in Figure 5B,C.
- Place the pipette and its holder on the motor unit of the micromanipulator and tighten down clamping knob.
- Use the control panel joystick in coarse mode to lower the micropipette near the glucose meniscus. Adjust the position of the tip to the center of the meniscus using the fine mode.
- Hold the tip immersed in glucose for a few minutes to clean its outer and inner surface (as a positive pressure is maintained, the water flow will rinse the inner surface of the pipette to remove uncoated BSA).
- Store the position of the tip on the micromanipulator keyboard and withdraw it from the meniscus.
- Remove the glucose meniscus and replace it by a fresh one. Suck 2 μL of GUVs in 0.1 M sucrose by using a 20 μL micropipette and put it in the fresh glucose meniscus. Observe in DIC mode (differential interference contrast) the GUVs located at the bottom due to the difference of density between sucrose (mainly inside the GUVs) and glucose (mainly outside the GUVs).
- When the vesicles are slightly deflated, reinsert the suction pipette and focus on the tip. Set the height H0 of the water tank for which pressure is almost 0. For that, take advantage of small vesicles or particles which are naturally present in solution and adjust the water tank height until the motion of these particles is stopped.
- At this point, surround the meniscus with mineral oil to prevent water evaporation, see Figure 5D.
NOTE: The room temperature should be controlled between 20-22 °C by using air conditioning.
- Micropipette aspiration experiment
- Lower the pipette tip (6-12 µm in diameter) and create a small suction (-1 cm) to aspirate a vesicle (15-30 µm in diameter). The membrane of the selected vesicle should slightly fluctuate, and must not present any visible defects (no bud or filament) (Figure 6).
- Raise the pipette to a higher level to isolate the aspirated vesicle from the other vesicles, by using the micromanipulator and keep this position during the whole experiment.
- Pre-stress the vesicle by lowering the water filled tank to approximately -10 cm, then decrease the pressure to return to its initial value (-1 cm). Repeat this step several times to remove membrane excess and small defects from the membrane.
- From a height of -0.5 cm defined by the position of the water tank, slowly decrease the suction pressure with the micrometer to reach a regime in which the membrane fluctuates. Then increase the pressure to clearly visualize a tongue in the tip with a significant projection length (a few microns).
NOTE: The lowest applied pressure (P0) that allows sucking up the smallest membrane projection length (L0) will define the reference point α0 (Figure 7A). All points of the curve will be measured according to this reference (ΔL = L-L0 and ΔP = P-P0). - Increase the suction pressure with the micrometer in a stepwise manner until reaching 0.5 -0.8 mN/m. At each step, wait 5 s and take a snapshot of the tongue. This procedure at low tension enables determination of the bending modulus.
- Keep on increasing the suction pressure from 0.5 mN/m to the rupture tension by adjusting the height of the water filled sliding on the rail (ranging from -2 to -50 cm) (Figure 7B-D). From this experiment at high tension regime, the area compressibility modulus, lysis tension and lysis strain will be measured.
- Stretch about 15-20 vesicles to acquire significant statistics. Each micropipette aspiration experiment takes between 7 and 10 minutes. Perform image analysis using LASAF software for measuring the projection length of the tongue, the diameter of vesicles and the radius of the capillary.
- Measuring bending modulus, area compressibility modulus, lysis tension and lysis strain
- To access these parameters, use the formalism established by E. Evans12. Calculate the suction pressure applied over the membrane from equation 1:
Δ P = ρwg (h−h0) (1)
where g is the gravitational acceleration (9.8 m∙s−2), ρw is the density of the water (ρ = 1 g∙cm−3), h is the position of the water tank and h0 is the initial position where pressure is equal to zero. - Calculate the membrane tension from the Laplace equation:
 (2)
(2)
where ΔP is the suction pressure on the micropipette, Rp and Rv are the micropipette and vesicle radii (outside the micropipette) respectively. The surface area strain (α) of the membrane is defined as:
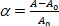 (3)
(3)
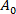 being the membrane area of the vesicle at the lower suction pressure.
being the membrane area of the vesicle at the lower suction pressure. - Calculate α from the increase in projection length ΔL of vesicle inside the capillary tip according to Equation 412:
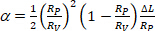 (4)
(4) - Under a very low tension regime, smoothing of thermal bending undulations dominates the apparent expansion. Plot ln(σ) vs α. At low-σ values (typically 0.001–0.5 mN.m−1 13), this gives a straight line whose slope is linked to the bending modulus, Kb (first term of the equation 5)14:
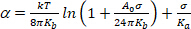 (5)
(5)
NOTE: Under high tensions (> 0.5 mN.m−1), membrane undulations are completely suppressed and membrane area increases as the result of increased spacing between molecules. In this regime, the second term of equation 5 dominates and give access to area compressibility modulus Ka (Figure 8 and Figure 9).
- To access these parameters, use the formalism established by E. Evans12. Calculate the suction pressure applied over the membrane from equation 1:
Wyniki
With the protocol aforementioned, we have studied different synthetic giant unilamellar vesicle (GUV), obtained from a phospholipid: 2-oleoyl-1-palmitoyl-sn-glycero-3-phosphocholine (POPC), a triblock copolymer: Poly(ethyleneoxide)-b-Poly(dimethylsiloxane)-b-Poly(ethyleneoxide) (PEO12-b-PDMS43-b-PEO12) synthesized in a previous study13, and a diblock copolymer Poly(dimethylsiloxane)-b...
Dyskusje
The coating of the micropipette is one of the key points to obtain reliable measurements. Adhesion of the vesicle to the micropipette must be prevented, and a coating is commonly used in literature17,18,19,20,21, with BSA, β-casein or surfasil. Details of the coating procedure are rarely mentioned.
Dissolution of the BSA should...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors gratefully acknowledge the ANR for financial support (ANR Sysa).
Materiały
| Name | Company | Catalog Number | Comments |
| Required equipment and materials for micropipette design | |||
| Borosilicate Glass Capillaries | World Precision Instruments | 1B100-4 | external and internal diameter of 1mm and 0.58 mm respectively. |
| Filament installed | Sutter Instrument Co. | FB255B | 2.5mm*2.5mm Box Filament |
| Flaming/Brown Micropipette Puller | Sutter Instrument Co. | Model P-97 | |
| Microforge | NARISHGE Co. | MF-900 | fitted with two objectives (10x and 32x) |
| Materials for coating pipette tips with BSA | |||
| Bovine Serum Albumin Fraction V (BSA) | Sigma-Aldrich | 10735078001 | |
| Disposable 1 ml syringe Luer Tip | Codan | 62.1612 | |
| Disposable 10 ml syringe Luer Tip | Codan | 626616 | |
| Disposable 5 ml syringe Luer Tip | Codan | 62.5607 | |
| Disposable acetate cellulose filter | Cluzeau Info Labo | L5003SPA | Pore size: 0.22µm, diameter: 25mm |
| Flexible Fused Silica Capillary Tubing | Polymicro Technologies. | TSP530660 | Inner Diameter 536µm, Outer Diameter 660µm, |
| Glucose | Sigma-Aldrich | G5767 | |
| Syringe 500 µL luer Lock GASTIGHT | Hamilton Syringe Company | 1750 | |
| Test tube rotatory mixer | Labinco | 28210109 | |
| Micromanipulation Set up | |||
| Aluminum Optical Rail, 1000 mm Length, M4 threads, X48 Series | Newport | ||
| Damped Optical Table | Newport | used as support of microscope to prevent external vibrations. | |
| Micromanipulator | Eppendorf | Patchman NP 2 | The module unit (motor unit for X, Y and Z movement) is mounted on the inverted microscope by the way of an adapter. |
| Micrometer | Mitutoyo Corporation | 350-354-10 | Digimatic LCD Micrometer Head 25,4 mm Range 0,001 mm |
| Plexiglass water reservoir (100 ml) | Home made | ||
| TCS SP5 inverted confocal microscope (DMI6000) equipped with a resonant scanner and a water immersion objective (HCX APO L 40x/0.80 WU-V-I). | Leica | ||
| X48 Rail Carrier 80 mm Length,with 1/4-20, 8-32 and 4-40 thread | Newport | ||
| Materials for sucrose and amphiphile solution preparation | |||
| 2-Oleoyl-1-palmitoyl-sn-glycero-3-phosphocholine | Sigma-Aldrich | ||
| Chloroform | VWR | 22711.244 | |
| L-α-Phosphatidylethanolamine-N-(lissamine rhodamine B sulfonyl) | Sigma-Aldrich | 810146C | Rhodamine tagged lipid |
| Sucrose | Sigma-Aldrich | S7903 | |
| Electroformation set up | |||
| 10 µL glass capillary ringcaps | Hirschmann | 9600110 | |
| Disposable 1 ml syringe Luer Tip | Codan | 62.1612 | |
| H Grease | Apiezon | Apiezon H Grease | Silicon-free grease |
| Indium tin oxide coated glass slides | Sigma-Aldrich | 703184 | |
| Needle | Terumo | AN2138R1 | 0.8 x 38 mm |
| Ohmmeter (Multimeter) | Voltcraft | VC140 | |
| Toluene | VWR | 28676.297 | |
| Voltage generator | Keysight | 33210A |
Odniesienia
- Bangham, A. D., Standish, M. M., Watkins, J. C. Diffusion of univalent ions across the lamellae of swollen phospholipids. Journal of Molecular Biology. 13 (1), (1965).
- Discher, D. E., Eisenberg, A. Polymer vesicles. Science. 297 (5583), 967-973 (2002).
- Hammer, D., et al. Polymersomes: vesicles from block copolymers. Annals of Biomedical Engineering. 28 (SUPPL. 1), (2000).
- Le Meins, J. F., Schatz, C., Lecommandoux, S., Sandre, O. Hybrid polymer/lipid vesicles: state of the art and future perspectives. Materials Today. 16 (10), 397-402 (2013).
- Schulz, M., Binder, W. H. Mixed Hybrid Lipid/Polymer Vesicles as a Novel Membrane Platform. Macromolecular Rapid Communications. 36, 2031-2041 (2015).
- Schneider, M. B., Jenkins, J. T., Webb, W. W. Thermal fluctuations of large quasi-spherical bimolecular phospholipid vesicles. Journal De Physique. 45 (9), 1457-1472 (1984).
- Dimova, R. Recent developments in the field of bending rigidity measurements on membranes. Advances in Colloid and Interface Science. 208, 225-234 (2014).
- Rodríguez-García, R., et al. Polymersomes: smart vesicles of tunable rigidity and permeability. Soft Matter. 7 (4), 1532-1542 (2011).
- Angelova, M. I., Dimitrov, D. S. Liposome electroformation. Faraday Discussions of the Chemical Society. 81, 303-311 (1986).
- Dao, T. P. T., et al. Membrane properties of giant polymer and lipid vesicles obtained by electroformation and pva gel-assisted hydration methods. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 533, 347-353 (2017).
- Pereno, V., et al. Electroformation of Giant Unilamellar Vesicles on Stainless Steel Electrodes. ACS omega. 2 (3), 994-1002 (2017).
- Evans, E., Rawicz, W. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Physical Review Letters. 64 (17), 2094-2097 (1990).
- Dao, T. P. T., et al. Modulation of phase separation at the micron scale and nanoscale in giant polymer/lipid hybrid unilamellar vesicles (GHUVs). Soft Matter. 13 (3), 627-637 (2017).
- Helfrich, W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 11 (11), 693-703 (1973).
- Dao, T. P. T., et al. The combination of block copolymers and phospholipids to form giant hybrid unilamellar vesicles (GHUVs) does not systematically lead to "intermediate'' membrane properties. Soft Matter. 14 (31), 6476-6484 (2018).
- Shoemaker, S. D., Kyle Vanderlick, T. Material Studies of Lipid Vesicles in the Lα and Lα-Gel Coexistence Regimes. Biophysical Journal. 84 (2), 998-1009 (2003).
- Longo, M. L., Ly, H. V., Dopico, A. M. . Methods in Membrane Lipids. , 421-437 (2007).
- Chen, D., Santore, M. M. Hybrid copolymer-phospholipid vesicles: phase separation resembling mixed phospholipid lamellae, but with mechanical stability and control. Soft Matter. 11 (13), 2617-2626 (2015).
- Mabrouk, E., et al. Formation and material properties of giant liquid crystal polymersomes. Soft Matter. 5, 1870-1878 (2009).
- Henriksen, J., et al. Universal behavior of membranes with sterols. Biophysical Journal. 90 (5), 1639-1649 (2006).
- Ly, H. V., Block, D. E., Longo, M. L. Interfacial Tension Effect of Ethanol on Lipid Bilayer Rigidity, Stability, and Area/Molecule: A Micropipet Aspiration Approach. Langmuir. 18 (23), 8988-8995 (2002).
- Bermudez, H., Hammer, D. A., Discher, D. E. Effect of Bilayer Thickness on Membrane Bending Rigidity. Langmuir. 20, 540-543 (2004).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone