Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Synthesis of Stimuli-responsive Nanogels using Aqueous One-step Crosslinking and Co-nanopolymerization
W tym Artykule
Podsumowanie
Nanogels are an excellent and versatile nanoparticle platform for the delivery of biologics. Stimuli-responsive poly(ethylene) glycol-based polymeric nano-gels, capable of encapsulating protein-based payloads, were synthesized using a one-step cross-linking co-nanopolymerization strategy in aqueous conditions. The optimal fabrication and characterization of these novel nanoparticles are presented here.
Streszczenie
Nanogels consisting of crosslinked-polymeric nanoparticles have been developed for the delivery of numerous chemical and biological therapeutics, owing to their versatile bottom-up synthesis and biocompatibility. While various methods have been employed for nanogel synthesis to date, very few have achieved it without the use of harsh organic solvents or high temperatures that can damage the integrity of the biological payload. In contrast, the methodology presented here accomplishes the synthesis of sub-100 nm sized, protein-loaded nanogels using mild reaction conditions. Here, we present a method for the non-covalent encapsulation of protein-based payloads within nano-gels that were synthesized using an aqueous-based, single-step, crosslinking copolymerization technique. In this technique, we initially electrostatically bind a protein-based payload to a cationic quaternary ammonium monomer and simultaneously cross-link and co-polymerize it using ammonium persulfate and N,N,N',N'-tetramethylethylenediamine to form nanogels that entrap the protein payload. The size and polydispersity index of the nanogels is determined using dynamic light scattering (DLS), while the surface morphology is assessed by transmission electron microscopy (TEM). The mass of protein entrapped within nanogels is determined by calculating the encapsulation efficiency. Furthermore, the controlled-release ability of the nanogels via the gradual degradation of redox-responsive structural elements is also assessed in bioreduction assays. We provide examples of nanoparticle optimization data to demonstrate all caveats of nanogel synthesis and characterization using this technique. In general, uniformly sized nanogels were obtained with an average size of 57 nm and a polydispersity index value of 0.093. A high encapsulation efficiency of 76% was achieved. Furthermore, the nanogels exhibited controlled release of up to 86% of the encapsulated protein by gradual degradation of novel redox-responsive components in the presence of glutathione over 48 h.
Wprowadzenie
Nanogels are three-dimensional, sub-micron-sized hydrogels with crosslinked polymer network structures that can hold large quantities of fluids within their core shell without affecting their morphological integrity1. In general, nanogels are synthesized by the polymerization of functional monomers via physical or chemical crosslinking in heterogeneous colloidal systems, such as water-in-oil inverse microemulsions2,3. Amphiphilic copolymers can self-assemble into nanoscale structures in aqueous environments. However, they must be stabilized using chemical crosslinking strategies involving disulfides or amide-based coupling, click chemistry, or can be physically induced (hydrophobic, electrostatic, or hydrogen-bonding strategies) or photo-induced4. Among these strategies, the physical self-assembly of polymers followed by chemical crosslinking has been reported as a successful nanogel fabrication technique5. While historically, the first nanogel was introduced in the 1990s by Vingradov et al.6, Akiyoshi et al.7, and Lemieux et al.8, lately, a variety of smart nanogels composed of both natural and synthetic polymers have been developed and explored for diverse biomedical applications9.
Nanogels possess extensive cargo-retaining capacity, large surface area, in vivo stability, as well as customizable chemical and mechanical properties10. The synthesis of nanogels is also scalable and can be aqueous-based. In addition, the enhanced water content of the nanogels makes them effective carriers of sensitive biological payloads11. Furthermore, the high surface area can satisfy multiple bioconjugation needs, thereby permitting the attachment of targeting modalities to enable active targeting. Notably, the versatility of nanogel design allows the use of a wide range of stimuli-responsive monomers that permits precise control of their physicochemical properties9. This unique engineerability enables the rational improvement of nanogel design, which is difficult to achieve with conventionally used liposomes, micelles, or polymerosomes12,13. By incorporating stimuli-responsive moieties within specifically designed monomers, nanogels can be engineered to trigger the controlled release of their payload in response to various physiologically-relevant stimuli, such as pH, redox conditions, enzymes, etc.9,14. Such smart nanogels are more useful than conventional nanogels, as they possess superior stability for extended blood circulation, and they can endure physiological conditions to maintain the integrity of their cargo and mediate its controlled release at desired target sites15. Indeed, due to their versatile nature, nanogels have gained traction in the biomedical arena, with notable advances in the development of stimuli-responsive nanogels for numerous theranostic and diagnostic applications2,16,17.
Biologics can represent a category of pharmaceutical products consisting of proteins, peptides, and/or nucleic acids and have revolutionized the therapeutic landscape due to their remarkable selectivity, thereby becoming the fastest-growing class of therapeutics18. Indeed, the growing market for such therapeutics is evident in the steep increase in their approval by the US Federal Drug Association (FDA), where biologics represented ~40% of total drug approvals, in 202319. In addition to their specificity and potency, rapid discoveries of novel drug targets, more efficient bioengineering processes and greater knowledge of the in vivo fate of these therapeutics has led to their increased use20. Traditional biologics include interfering RNA, replacement proteins, cytokines and hormones that are usually generated using recombinant DNA technology21. Since the approval of human recombinant insulin in 1982, biologics have been developed for many conditions including cancer (e.g., trastuzumab, avelumab), inflammatory bowel disease (e.g., adalimumab, certolizumab) and rare genetic diseases (e.g., mipomersan, myozyme, aldurazyme, fabrazyme)21. While the high specificity of the interactions of biologics with their targets should theoretically offset any off-target effects, several clinical concerns have emerged with their use relating to unwanted side effects22. These side effects can be grouped into two categories, including exaggerated pharmacology (over-stimulation of targets) and immunogenicity. Further to this, their short half-lives, limited bioavailability, protease damage, short shelf-life and costly production processes limit their therapeutic benefits21. Conventional methods of mitigating these issues, involve covalent modification of these biologics that can compromise their function, and therefore, efficacy23. Alternatively, the nanomedicine approach to encapsulating therapeutic payloads can confer numerous advantages to pharmacological properties, most importantly, passive targeting to the inflamed site via the enhanced permeation and retention (EPR) effect24. Other nanoparticle-associated benefits can include enhanced circulation times, reduced clearance rate, greater formulation flexibility, improved vasculature permeation and cellular uptake25. While a huge variety of nanoparticle formulations are currently under investigation for the delivery of biological payloads, few can emulate the multifunctionality of nanogels. Indeed, nanogels exceed the loading capacities achieved by liposomal- and micelle-based nanoparticles, and they exhibit greater colloidal stability than most inorganic nanoparticles. As such, nanogels present a valuable platform for the delivery of various biological therapeutics.
We have previously successfully delivered an anti-oxidative enzyme within novel matrix metalloproteinase-responsive crosslinked polymeric nanogels, where the mild encapsulation strategy used maintained the protein's bioactivity upon release26. In this work, we demonstrate the optimized synthesis of redox-responsive nanogels for the delivery of protein-based payloads. Notably, the synthetic methodology enables nanogel synthesis using mild conditions to encapsulate the desired payload, without the use of harsh organic solvents or high temperatures. We exploited the redox homeostasis within the intracellular environment to regulate the release of the encapsulated payload27,28. Typically, the naturally abundant antioxidant glutathione (GSH) controls the extracellular and intracellular redox potentials, where its concentration ranges between 2-20 µM and 1-10 mM, respectively29,30. To date, numerous redox-sensitive nanoparticles have been reported, making this a proven and reliable strategy to enable controlled released of drugs in vivo27,28. Indeed, disulphide bonds have been installed within polymeric nanomaterials by using disulphide-containing crosslinkers31,32, self-assembly of biodegradable polymers from disulphide-containing monomers33, and redox-responsive polymer prodrugs or drug/polymer conjugates34,35. Therefore, this study investigates the incorporation of a unique, highly GSH-sensitive disulphide crosslinker within the polymeric nanoparticles, thereby enabling the controlled release of an encapsulated protein payload.
In this study, nanogel design was centered on the following criteria to address specificity and payload delivery: small size (~100 nm) and a uniform size distribution (polydispersity index (PI)<0.3) to ensure efficient penetration of the endothelium and in vivo stability27; efficient encapsulation of protein payload, and controlled release of payload in response to GSH. We report the synthesis of GSH-responsive crosslinked nanogels, that demonstrated homogenous sub-100 nm sized nanoparticles, with a 76% encapsulation efficiency of the desired protein payload.
Protokół
1. Synthesis of redox-responsive crosslinker
- Add 2-aminoethylmethacrylate hydrochloride (44.79 mg, 0.270 mmol) and 0.063 mL of triethylamine to 3 mL of anhydrous dichloromethane and allow to mix for 20 min under an inert N2 gas in a 25 mL round bottomed flask.
- Dissolve 4,7,10,13,16,19,22,25,32,35,38,41,44,47,50,53-Hexadecaoxa-28,29-dithiahexapentacontanedioic acid di-N-succinimidyl ester (100 mg, 0.045 mmol) in 1 mL of anhydrous dichloromethane and add to the flask.
- Add 4 mL of anhydrous dichloromethane to the flask. Wrap the reaction flask in aluminum foil and leave to stir under N2 at room temperature.
- Confirm product formation by thin-layer chromatography. Ensure correct references for the starting material while confirming product formation by this method.
- Thin-layer chromatography is an affinity-based method used to separate desired compounds from the reaction mixture. Here, use the highly polar and adsorbent silica plate as a stationary phase. Spot the starting material sample and the reaction mixture sample onto one end of the silica plate and place vertically into a closed glass beaker with the eluent (mobile phase: 5% methanol: dichloromethane).
- By capillary action, the mobile phase moves up the silica plate and the sample compounds migrate varying distances depending on their affinities for the stationary and mobile phases. When the solvent front has crossed 3/4th of the plate, remove the plate and dry it. The separated compounds appear as spots on the plate. Quantify the retention factor (Rf) of the desired compound as the distance travelled by compound/distance travelled by solvent. Here, Rf of the product was 0.35. UV light or staining can be used to visualize the spots (potassium permanganate).
- Concentrate the mixture in vacuo and purify via flash column chromatography (isocratic mixture of 5% methanol: dichloromethane)36. Introduce the reaction mixture onto the top of a glass column packed with silica (stationary phase) and then elute with the solvent system (mobile phase)36. Collect all fractions and assay using TLC to distinguish the product fractions using the previously determined Rf value.
- Concentrate the purified fractions by evaporating the solvent using a rotary evaporator (temperature: 40 °C), set vacuum pressure to atmospheric pressure to remove lower boiling dichloromethane first. When the level of the solvent stops decreasing, set the vacuum pressure to 337 mbar to remove methanol and attain a pale-yellow oil, which will then be subjected to further characterization to confirm product formation37.
- Conduct Fourier-Transform Infrared (FT-IR) analysis38, 1H NMR (in deuterated dimethylsulfoxide (DMSO-d6))39, 13C NMR39 and matrix-assisted laser desorption/ionization time-of-flight (MALDI-ToF)40 analysis to confirm product formation before performing next steps.
- For FT-IR analysis, load 1 mg of the oil onto the instrument to obtain the spectra.
- For NMR analyses, re-dissolve 10 mg of the product in 0.75 mL of the relevant NMR solvent, transfer to an NMR tube and seal tightly to prevent leakage. Load the sample onto the NMR instrument, after which the spectra are obtained39.
- For the mass spectrometry analysis, seal 1 mg of the product in a vial, dissolve in DMSO and load onto the instrument.
NOTE: Infrared analysis provides information regarding the key functional groups in the desired product, namely the carbonyl stretches (C=O) identified by the sharp peak at 1650 cm-1 indicates the formation of the amide bond, thereby highlighting product formation. The 1H NMR and the 13C provide information on the chemical environments and thereby can be used to determine the chemical composition of the desired product via the identification of chemical environments present in the compound. Finally, the use of mass spectrometry can verify the exact mass of the desired product.
2. Test cleavage of disulphide crosslinker with glutathione
- Incubate the disulphide crosslinker (to a final concentration of 4.55 mg/mL) with the reducing agent glutathione (final [GSH]: 10 mM), to a final volume of 1 mL, for 24 h in a 1.5 mL glass vial.
- To confirm cleavage of the disulphide linker, submit the vial for MALDI-ToF analysis26,40.
3. Redox-responsive nanogel synthesis
- Ensure all glassware to be used for the reaction is free of dust by washing with filtered (0.20 µm) deionized water and subsequently drying the glassware using compressed air that can be accessed via the compressed air utility on the side of the fumehood. Attach a secure nozzle to the compressed air outlet to ease accessibility. Hold the vial securely and position the nozzle into it. Activate the compressed air, releasing a controlled stream into the vial. Observe the process to ensure effective drying, checking for any remaining contaminants or moisture.
- Prepare a 10 mL glass vial by filling it with deionized water and seal the flask with a rubber septum. Deoxygenate the water by bubbling N2 through it for at least 30 min prior to starting the reaction. To do this, fill a balloon with N2 gas and attach a needle to the balloon; place it on the septum and use the needle to pierce the rubber septum and allow N2 flow. Attach an exit needle to the flask to ensure constant flow of N2.
- Dissolve bovine serum albumin (BSA; 2 mg, 30 nmol) or Cy7-tagged BSA (2 mg, 30 nmol) in 1 mL of deoxygenated deionized water and add to another clean 10 mL glass vial.
- Add [2-(acryloyloxy)ethyl]trimethylammonium chloride (AETC) solution (12.00 mg/10.86 µL, 0.060 mmol) to the flask and seal it using another rubber septum. Ensure the reaction mixture is kept under a constant flow of N2 gas and stir for 30 min.
- Dissolve acrylamide (9.21 mg, 0.13 mmol) in 1 mL of deoxygenated deionized water and add to the mixture in the vial.
- Dissolve disulphide crosslinker (4.44 mg, 0.004 mmol) in 1 mL of deionized water and add to the mixture in the vial. Flush the mixture with N2 gas for 20 min.
- Dissolve sodium dodecyl sulfate (2 mg, 0.007 mmol) in 1 mL of deoxygenated deionized water and add to the mixture via a N2 gas flushed syringe and stir the mixture for 30 min.
NOTE: By flushing the syringe with N2 we minimize the exposure of the reaction mixture to oxygen in the atmosphere which can inhibit the radical polymerization reaction. - Add N,N,N',N'-tetramethylethylenediamine (1.5 µL, 0.010 mmol) and let the reaction mixture deoxygenate for 3 min under N2 gas.
- Dissolve ammonium persulfate (1 mg, 0.004 mmol) in 1 mL of deoxygenated deionized water and add to the reaction mixture. Ensure that N2 gas is bubbling through the reaction mixture for 10 min.
NOTE: If the N2 addition step is omitted, it is likely that white particulates will form in the reaction flask. These are larger-sized particles and/or aggregates and can confound any further characterization. - Seal the flask under a N2 atmosphere and leave to stir for 3 h and 30 min at room temperature.
- To end the reaction, remove the seal from the flask and let the reaction mixture stir for at least 10 min. This would expose the reaction mixture to atmospheric levels of O2 gas, which would then quench any remaining radicals in the reaction mixture. The reaction ends when the mixture assumes a clear blue opalescent appearance.
- In order to purify the nanogels, add the reaction mixture to a 15 mL centrifugal filter unit (100 kDa MWCO) and add deionized water to fill the centrifugal unit. Centrifuge the reaction mixture at 1000 x g for 10 min. This will remove any unreacted chemicals (or unencapsulated payload) that are smaller than 100 kDa and concentrate the nanogel sample.
- Once the level of water in the centrifugal unit decreases, add 2 mL deionized water to dilute the nanogel sample and centrifuge to concentrate the product. Repeat this step 3x.
- Store the sample at 2-8 °C to prevent the degradation of the encapsulated protein.
- Conduct FT-IR and 1H NMR (in deuterated dimethyl sulfoxide (DMSO-d6)) analysis to confirm polymerization39,41. To prepare the sample, freeze-dry 1 mL of the nanogel solution in a 1.5 mL vial. The freeze-drying process involves freezing the sample and then applying a vacuum to remove the frozen solvent through sublimation, leaving behind a powdered residue. After freeze-drying, re-dissolve the sample in the relevant NMR solvent, transfer to an NMR tube and tightly seal it. Load the sample onto the NMR machine, as described previously39. For FT-IR analysis, 1 mg of the dry powder should be loaded onto the instrument to obtain the spectra.
4. Nanogel morphological characterization
- Dynamic light scattering (DLS)
NOTE: Hydrodynamic diameter (nm), polydispersity index (PI), and zeta potential were measured at 25 °C, 632.8 nm laser wavelength and 173° signal detector using a DLS machine. Each run measured 25 sub-scanning cycles.- Hydrodynamic size (nm) measurement
- Prepare a cuvette or a disposable folded capillary cell by cleaning it with filtered deionized water. Blow compressed air into the cuvette (suitable for both DLS and zeta potential measurements) to ensure it is free of large dust particles.
- Fill the cuvette with at least 50-100 µL of the sample and dilute in 750-1000 µL of filtered deionized water. While using a zeta potential cuvette, ensure that the cuvette has been filled with sample without bubble formation, which can perturb measurements.
- Put the cuvette inside the DLS instrument, ensuring that it has been correctly inserted. To do so, open the sample compartment cover of the DLS instrument. Hold the cuvette by its top edges to avoid fingerprints on the optical windows. Ensure the cuvette is properly seated in the cuvette holder making sure it is level and aligned with the instrument's optical path. Close the sample compartment to prevent ambient light interference.
NOTE: The dilution media is filtered using 0.45 µm pore size syringe filter. - Click to start the particle size measurement on the relevant DLS machine software.
- Select the results corresponding to the sample to obtain the z-average (average particle size), average PI, kilo-counts per second (kcps), zeta potential and relevant graphics. Ensure that the correlation function exhibits a smooth sigmoidal curve, as this suggests a uniform sample.
- Once analysis is complete, remove the cuvette from the DLS.
- Hydrodynamic size (nm) measurement
- Transmission electron microscopy (TEM)
- Prepare samples by placing 10 µL of the nanoparticle solution (0.077 mg/mL) onto carbon-coated copper grids. Air dry at room temperature for 1 h.
- Wash the grids with filtered deionized water and stain with 5 µL of filtered 1% uranyl acetate in water for 2 min.
- After soaking the excess staining agent with filter paper, dry the grids overnight at room temperature. Capture TEM images the next day at 45,000x magnification using a bright field camera.
- Use the provided software for image processing (resolution: 2048 x 2048 pixels).
5. Quantification of protein (BSA) encapsulated within the nanogels using a micro-bicinchoninic acid (BCA) assay
- According to the microBCA assay protocol (given on the manufacturer's website), generate a standard curve based on a pre-defined protein concentration42.
- Following their synthesis, purify the protein-encapsulating nanogels using 15 mL centrifugal filter units with 100 kDa MWCO (1000 x g, 10 min) as described in steps 3.12-3.14.
- Collect the filtrate and analyze using the microBCA assay (protocol available on the manufacturer's website). Measure the absorbance at 562 nm using a plate reader. As this measurement correlates to the unencapsulated protein concentration, determine the mass of the encapsulated protein. To calculate the encapsulation efficiency (EE) %, use the following equation:
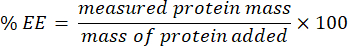
6. Quantification of protein release from nanogels in the presence of glutathione
- Incubate 200 µL of 5 mg/mL of Cy7BSA-loaded nanogels with 200 µL of 10 mM glutathione (GSH) in the 0.5 mL centrifugal filter units (100 kDa MWCO). Place the filter units in a water bath to maintain the temperature at 37 °C.
- Centrifuge at 1000 x g for 3 min every 10 min for at least 1 h - then every 2-8 h.
- Collect the filtrate and use for further analysis. Maintain the volume of the filtrate within the centrifugal filter units at 500 µL by adding more deionized water or PBS.
- Measure the amount of protein in the filtrates by referring to a standard curve for Cy7BSA-loaded nanogels, where the absorbance of the fluorescent protein was measured using a nanodrop instrument at 750 nm.
NOTE: This standard curve can be calculated by preparing serial dilutions of Cy7BSA within the range of 0 mg/mL to 2 mg/mL. Values obtained from the nanodrop can be interpolated against this calibration curve to determine the encapsulation efficiency.
Wyniki
Synthesis and characterization of poly(ethylene glycol) (PEG) disulphide diacrylate crosslinker The redox-responsive crosslinker was synthesized by the nucleophilic substitution of an N-hydroxysuccinimide (NHS) ester by the 2-aminoethyl methacrylate via the formation of an amide linkage (Figure 1). The synthesis of the required product was validated primarily by 1H NMR (Supplementary Figure 1), that was carried out by dissolving the produ...
Dyskusje
The growing demand for target-specific biologics in the biopharmaceutical industry has driven a need for technologies that can improve their in vivo pharmacological profiles, while preventing their rapid physiological degradation and offsetting any unwanted side effects. With this in mind, a straightforward procedure for the synthesis of protein-loaded nanogels is described. As indicated in the protocol, the redox-responsive crosslinker needs to be synthesized prior to nanogel synthesis. Then, the key remaining ...
Ujawnienia
Authors have no conflicts of interest to disclose. There are no financial details to declare.
Podziękowania
We thank the Imperial College London Department of Chemistry and the Medical Research Council Institute of Life Sciences for their support.
Materiały
| Name | Company | Catalog Number | Comments |
| Chemicals | |||
| 2-( acryloyloxy)ethyl]trimethylammonium chloride solution | Sigma Aldrich | 496146 | |
| 2-aminoethyl methacrylatehydrochloride | Sigma Aldrich | 516155 | |
| 4,7,10,13,16,19,22,25,32,35,38,41, 44,47,50,53-Hexadecaoxa-28,29-dithiahexapentacontanedioic acid di-N-succinimidyl ester | Sigma Aldrich | 671630 | |
| Acrylamide | Sigma Aldrich | 23701 | |
| Ammonium persulfate | Sigma Aldrich | 248614 | |
| Bovine serum albumin | Sigma Aldrich | B6917 | |
| Cy7- labelled bovine serum albumin | Nanocs | BS1-S7-1 | |
| Deuterated dimethyl sulfoxide | Sigma Aldrich | 547239 | |
| Dichloromethane | Sigma Aldrich | 270997 (anhydrous) and D65100 | |
| Glutathione | Sigma Aldrich | G4251 | |
| Methanol | Sigma Aldrich | 34860 | |
| N,N,N’,N’-tetramethylethylenediamine | Sigma Aldrich | 411019 | |
| Phosphate buffered saline | ThermoFisher | 10010023 | |
| Sodium dodecyl sulfate | Sigma Aldrich | 436143 | |
| Triethylamine | Sigma Aldrich | 471283 | |
| Uranyl Acetate | Agar Scientific | AGR1260A | |
| Equipment necessary for nanogel synthesis and characterisation | |||
| Amicon Ultra-15 Centrifugal filter units (100kDa MWCO) | Merck Millipore | C7715 | |
| Camera | Olympus | Veleta | |
| Carbon-coated copper grids | Agar Scientific | AGS160 | |
| Dialysis tubing (100kDa MWCO) | Spectrum labs | 11405949 | |
| Dynamic Light Scattering | Malvern | Zetasizer Nano Ultra | |
| Freeze dryer | Labconco | WZ-03336-01 | |
| Infrared spectroscopy | Agilent | Cary 630 FTIR | |
| iTEM software | Olympus | ||
| Mass spectrometry | Waters | Micromass MALDI microMX MALDI Q-ToF | |
| MF-MilliporeTM membrane filter (0.45/0.2μm pore size) | Merck Millipore, UK | HAWP04700, GSWP04700 | |
| Micro BCA Protein Assay Kit | ThermoFisher | 23235 | |
| Plate reader | Beckman | Coulter-PARADIGM | |
| Proton and Carbon-13 nuclear magnetic resonance data | Bruker | 400MHz AV-400 NMR spectrometer | |
| Rotary evaporator | Buchi | R-114 Rotary Vap System | |
| Single-use needles | Sterican | 4665643 | |
| Suba-Seal septa | Sigma Aldrich | Z124575 | |
| Transmission electron microscopy | Phillips | CM 100 TEM | |
| UV-vis spectrophotometer | Nanodrop | Nanodrop One/One C microvolume |
Odniesienia
- Zhang, X., Malhotra, S., Molina, M., Haag, R. Micro- and nanogels with labile crosslinks - from synthesis to biomedical applications. Chem Soc Rev. 44 (7), 1948-1973 (2015).
- Chacko, R. T., Ventura, J., Zhuang, J., Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv Drug Del Rev. 64 (9), 836-851 (2012).
- Hajebi, S., et al. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomaterialia. 92, 1-18 (2019).
- Lee, V. Y., et al. Nanogel star polymer architectures: A nanoparticle platform for modular programmable macromolecular self-assembly, intercellular transport, and dual-mode cargo delivery. Adv Mat. 23 (39), 4509-4515 (2015).
- Ekkelenkamp, A., Rachèl Elzes, M., Engbersen, J. F., Paulusse, J. M. Responsive crosslinked polymer nanogels for imaging and therapeutics delivery. J Mat Chem B. 6 (2), 210-235 (2018).
- Vinogradov, S. V., Zeman, A. D., Batrakova, E. V., Kabanov, A. V. Polyplex nanogel formulations for drug delivery of cytotoxic nucleoside analogs. J Control Rel. 107 (1), 143-157 (2015).
- Akiyoshi, K., Deguchi, S., Moriguchi, N., Yamaguchi, S., Sunamoto, J. Self-aggregates of hydrophobized polysaccharides in water. Formation and characteristics of nanoparticles. Macromol. 26 (12), 3062-3068 (1993).
- Lemieux, P., et al. Block and graft copolymers and NanoGel copolymer networks for DNA delivery into cell. J Drug Target. 8 (2), 91-105 (2000).
- Preman, N. K., Barki, R. R., Vijayan, A., Sanjeeva, S. G., Johnson, R. P. Recent developments in stimuli-responsive polymer nanogels for drug delivery and diagnostics: A review. Euro J Pharma Biopharmac. 157, 121-153 (2020).
- Oh, J. K., Drumright, R., Siegwart, D. J., Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog Poly Sci. 33 (4), 448-477 (2008).
- Jiang, Y., Chen, J., Deng, C., Suuronen, E. J., Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomat. 35 (18), 4969-4985 (2014).
- Napier, M. E., DeSimone, J. M. Nanoparticle drug delivery platform. Poly Rev. 47 (3), 321-327 (2007).
- Malmsten, M. Soft drug delivery systems. Soft Matt. 2 (9), 760-769 (2006).
- Kamaly, N., Yameen, B., Wu, J., Farokhzad, O. C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Che Rev. 116 (4), 2602-2663 (2016).
- Molina, M., et al. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem Soc Rev. 44 (17), 6161-6186 (2015).
- Ma, Y., Ge, Y., Li, L. Advancement of multifunctional hybrid nanogel systems: Construction and application in drug co-delivery and imaging technique. Mat Sci Eng: C. 71, 1281-1292 (2017).
- Wang, H., Qian, J., Ding, F. Recent advances in engineered chitosan-based nanogels for biomedical applications. J Mat Chem B. 5 (34), 6986-7007 (2017).
- Durán-Lobato, M., Niu, Z., Alonso, M. J. Oral delivery of biologics for precision medicine. Adv Mat. 32 (13), 1901935 (2020).
- de la Torre, B. G., Albericio, F. The pharmaceutical industry in 2022: An analysis of FDA drug approvals from the perspective of molecules. Molecules. 28 (3), 1038 (2023).
- Škalko-Basnet, N. Biologics: The role of delivery systems in improved therapy. Biologics. 8, 107-114 (2014).
- Andrews, L., Ralston, S., Blomme, E., Barnhart, K. A snapshot of biologic drug development: Challenges and opportunities. Human Exp Toxicol. 34 (12), 1279-1285 (2015).
- Sathish, J. G., et al. Challenges and approaches for the development of safer immunomodulatory biologics. Nat Rev Drug Disc. 12 (4), 306-324 (2013).
- Pfister, D., Morbidelli, M. Process for protein PEGylation. J Control Rel. 180, 134-149 (2014).
- Blanco, E., Shen, H., Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 33 (9), 941-951 (2015).
- Golombek, S. K., et al. Tumor targeting via EPR: Strategies to enhance patient responses. Adv Drug Del Rev. 130, 17-38 (2018).
- Basak, S., et al. Simultaneous cross-linking and cross-polymerization of enzyme responsive polyethylene glycol nanogels in confined aqueous droplets for reduction of low-density lipoprotein oxidation. Biomacromol. 22 (2), 386-398 (2021).
- Morgulchik, N., Kamaly, N. Meta-analysis of in vitro drug-release parameters reveals predictable and robust kinetics for redox-responsive drug-conjugated therapeutic nanogels. ACS Appl Nano Mat. 4 (5), 4256-4268 (2021).
- Ghorbani, M., Hamishehkar, H. Redox-responsive smart nanogels for intracellular targeting of therapeutic agents: Applications and recent advances. J Drug Target. 27 (4), 408-422 (2019).
- Bajic, V. P., et al. Glutathione "redox homeostasis" and its relation to cardiovascular disease. Oxi Med Cell Long. 2019, e5028181 (2019).
- Forman, H. J., Zhang, H., Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol Asp Med. 30 (1-2), 1-12 (2009).
- Wang, Y. C., et al. Core-shell-corona micelle stabilized by reversible cross-linkage for intracellular drug delivery. Macromol Rapid Comm. 31 (13), 1201-1206 (2010).
- Elkassih, S. A., Kos, P., Xiong, H., Siegwart, D. J. Degradable redox-responsive disulfide-based nanogel drug carriers via dithiol oxidation polymerization. Biomat Sci. 7 (2), 607-617 (2019).
- Hu, X., et al. Stimuli-responsive polymersomes for biomedical applications. Biomacromol. 18 (3), 649-673 (2017).
- Navath, R. S., Wang, B., Kannan, S., Romero, R., Kannan, R. M. Stimuli-responsive star poly(ethylene glycol) drug conjugates for improved intracellular delivery of the drug in neuroinflammation. J Control Release. 142 (3), 447-456 (2010).
- Ling, X., et al. Glutathione-responsive prodrug nanoparticles for effective drug delivery and cancer therapy. ACS Nano. 13 (1), 357-370 (2019).
- Still, W. C., Kahn, M., Mitra, A. Rapid chromatographic technique for preparative separations with moderate resolution. J Org Chem. 43 (14), 2923-2925 (1978).
- JoVE. JoVE science education database - Organic chemistry. Rotary evaporation to remove solvent. JoVE. , (2023).
- JoVE. JoVE science education database - Organic chemistry II. Infrared spectroscopy. JoVE. , (2024).
- JoVE. science education database - Organic chemistry. Nuclear magnetic resonance (NMR) spectroscopy. JoVE. , (2023).
- Kim, J. Sample preparation for matrix-assisted laser desorption/ionization mass spectrometry. Mass Spec Lett. 6, 27-30 (2015).
- Liu, P., Pearce, C. M., Anastasiadi, R. M., Resmini, M., Castilla, A. M. Covalently crosslinked nanogels: An NMR study of the effect of monomer reactivity on composition and structure. Polymers. 11 (2), 353 (2019).
- Smith, P. K., et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 150 (1), 76-85 (1985).
- Yu, M., Wu, J., Shi, J., Farokhzad, O. C. Nanotechnology for protein delivery: Overview and perspectives. J Control Release. 240, 24-37 (2016).
- Zhang, Y., Zhang, D., Wang, J. T., Zhang, X., Yang, Y. Fabrication of stimuli-responsive nanogels for protein encapsulation and traceless release without introducing organic solvents, surfactants, or small-molecule cross-linkers. Poly Chem. 12 (4), 554-563 (2021).
- Kamaly, N., et al. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano. 10 (5), 5280-5292 (2016).
- Lu, R., et al. Probing the secondary structure of bovine serum albumin during heat-induced denaturation using mid-infrared fiberoptic sensors. Analyst. 140 (3), 765-770 (2015).
- Abrosimova, K. V., Shulenina, O. V., Paston, S. V. FTIR study of secondary structure of bovine serum albumin and ovalbumin. J Phys: Conf Ser. 769, 012016 (2016).
- Kabanov, A. V., Vinogradov, S. V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Ang Chem Int Ed Eng. 48 (30), 5418-5429 (2009).
- Li, C., Obireddy, S. R., Lai, W. F. Preparation and use of nanogels as carriers of drugs. Drug Del. 28 (1), 1594-1602 (2021).
- Kulkarni, J. A., et al. The current landscape of nucleic acid therapeutics. Nat Nanotechnol. 16 (6), 630-643 (2021).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone