Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Surgical Approach and Complications of Stand-alone Lateral Trans-Psoas Interbody Fusion
W tym Artykule
Podsumowanie
Interbody fusion of the lumbar spine can be achieved surgically using different techniques. Minimally invasive techniques, including lateral interbody fusion, have been developed within recent decades to reduce rates of complications and allow for quicker patient recovery. We provide insight into stand-alone lateral trans-psoas interbody fusion, highlighting potential complications and pitfalls.
Streszczenie
Interbody fusion of the lumbar spine is a standard procedure for symptomatic degenerative lumbar spine disease if conservative treatment fails. Surgical decompression and fusion of the segment can be achieved using several different techniques. Over the last decades, minimally invasive techniques, such as lateral interbody fusion (LLIF), have been developed to reduce tissue damage and complications and allow quicker patient recovery. With growing popularity, indications for LLIF have expanded to treat spinal deformities and foraminal/central stenosis. Mechanically, it allows for an unsurpassed fixation through the left-to-right apophyseal ring placement of the cage. LLIF utilizes a minimally disruptive retroperitoneal corridor and includes both trans-psoas and pre-psoas approaches. For the pre-psoas approach, the risk of damage from manipulation of the intramuscular lumbar plexus is reduced compared to a trans-psoas approach. However, increased risks of major vascular, ureteral, bowel injury and sympathetic plexus damage are reported.
This article aims to provide a detailed and comprehensive guide on stand-alone lateral trans-psoas interbody fusion, including its indications, surgical procedure, potential complications, and outcomes based on a decade of experience from a single center.
Wprowadzenie
Lower back pain, an important symptom of degenerative lumbar disease (DLD), is common in patients over 65 years1. Other symptoms of DLD include radiculopathy and claudication. When non-surgical treatment fails, surgical decompression or, if indicated, interbody fusion of the spine may be a viable treatment option2. Several techniques and approaches have been developed to achieve interbody fusion or decompression of the segment. Traditional approaches include posterior lumbar interbody fusion (PLIF), transforaminal lumbar interbody fusion (TLIF), and anterior lumbar interbody fusion (ALIF). Approaches to the lumbar spine are illustrated in Figure 1.
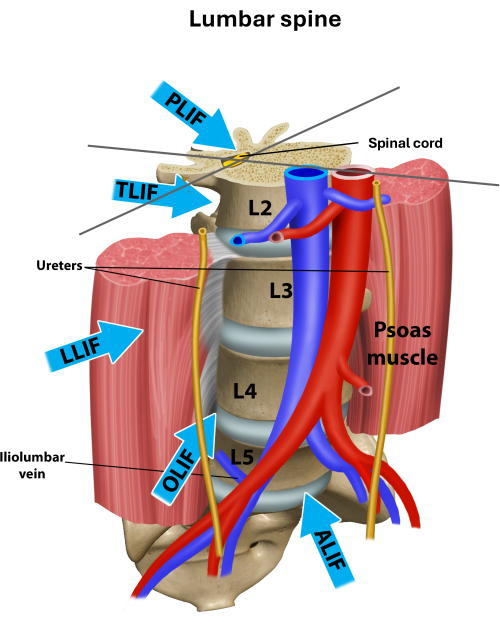
Figure 1: Different approaches to the lumbar spine for interbody fusion12. Overview of the different approaches used for lumbar interbody fusion. LLIF is a trans-psoas approach, and OLIF is a pre-psoas approach. Please click here to view a larger version of this figure.
Over the last decades, minimally invasive techniques for interbody fusion of the lumbar spine have been developed to reduce tissue damage and complications, allowing for quicker patient recovery, reducing complications, and surpassing the technical limitations of traditional approaches to the spine. In 2001, Pimenta et al. introduced a minimally invasive retroperitoneal approach to the lumbar spine by splitting the psoas, providing direct disc exposure by expanding the retroperitoneal space. This has been introduced as the lateral trans-psoas approach3,4. This technique was modified with the use of special retractors and popularized by Ozgur et al.5. In recent years, extreme lateral interbody fusion in a prone patient position has been developed. This technique offers efficiency with combined posterior procedures and improved lumbar lordosis6,7.
Lateral interbody fusion (LLIF) can be achieved utilizing various approaches. These include trans-psoas approaches (extreme lateral interbody fusion (XLIF8), direct lateral interbody fusion (DLIF9) using different instruments), and a pre-psoas approach (oblique lateral interbody fusion (OLIF10). The approach used for the procedure depends on the patient and the surgeon's training, among others. Anatomical studies showed notable benefits for trans-psoas approaches (minimal blood loss, preservation of the posterior musculature and ligamentous chain, the ability to perform an extensive discectomy, and placement of a large intervertebral graft) but also disadvantages (post-operative nerve palsies, visceral abdominal injuries)11. Reported major but rare complications include intestinal perforation, common iliac vein injury, cage subsidence, vertebral body fractures around the interbody device, retroperitoneal hematoma, and pneumoretroperitoneum with an associated pneumoscrotum12. Overall, pre-psoas approaches are associated with a slightly lower complication rate and fewer postoperative neurological deficits13. To allow optimal outcome, the indication for a lateral interbody fusion needs to be done carefully. We advise obtaining a computed tomography (CT) and magnetic resonance (MR)-scan of the lumbar spine. To assess the segment hypermobility or instability, obtain flexion-extension X-ray images besides regular anteroposterior (AP) and lateral. Common indications are patients with segmental instability and concurrent radiculopathy who have failed non-surgical treatment. In the case of segmental instability or deformity surgery, additional internal fixation may be necessary. LLIF may be limited in the lower lumbar spine, depending on the height of the iliac crest. Supplemental posterior fixation may add valuable construct stiffness and deformity reduction for patients with high-grade instability, deformity, or questionable bone stability. Contraindications for this procedure are typically malignancy, high-grade deformities, or bifurcation abnormalities. A history of retroperitoneal infection or disease and previous retroperitoneal surgery or injury are important considerations. Risk factors for poor outcomes include osteoporosis, smoking, long-term steroid use, severe deformities, and segment hypermobility due to facet effusion or previous laminectomy14,15,16. Also, patients with very low body mass index and anterior psoas location are potentially adverse patients for increased complexity of access. In revision surgery, a pre-psoas approach may be favorable to avoid scar tissue.
The aim of this article is to provide step-by-step guidance to surgeons on a stand-alone lateral trans-psoas interbody fusion, including pitfalls and complication rates after 10 years of single-center experience.
Protokół
The retrospective study was approved by our institutional review board (STUDY2021000113), and an informed consent waiver was granted because of the nature of the study. The procedure demonstrated in the video was performed on a human specimen that was obtained and utilized following the ethical guidelines and protocols established by our institution. The specimen was handled respectfully and in compliance with all relevant ethical standards.
1. Patient selection
- Select patients with degenerative lumbar disease and failed non-surgical treatment.
- Assess segment hypermobility or instability with flexion-extension X-ray images in addition to regular anteroposterior (AP) and lateral views (Figure 2). In cases of segment instability, perform an additional posterior fixation.
- Obtain a computed tomography (CT) and magnetic resonance (MR)-scan of the lumbar spine.
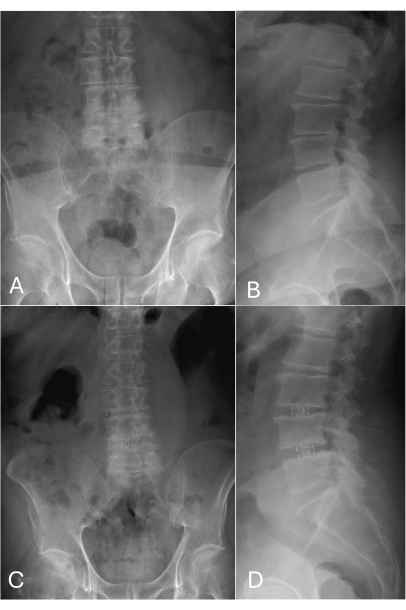
Figure 2: Pre-operative and follow-up X-ray images in A.P and lateral view. (A-B) Multilevel degenerative lumbar spine disease with collapsed disc space L3/4 as well as L4/5 and neuroforaminal stenosis. No signs of instability or hyperflexible were observed. (C-D) One-year post-operative follow up. Correct position of PEEK cages in L3/4 and L4/5 disc space without subsidence. Both disc and neuroforaminal height are restored. Please click here to view a larger version of this figure.
2. Positioning
- Position the patient, under general anesthesia, in a right-sided decubitus position with partially flexed hips and knees on a radiolucent operating table. Pad bony prominences to avoid soft tissue damage due to pressure.
- Secure the patient with surgical tape and belts/pads to prevent movement and ensure safety.
- Flex the operating table to generate more space if needed.
3. Preoperative phase
- Confirm the desired surgical level with AP/lateral fluoroscopy images.
- Use skin markers to visualize the disc space and vertebral bodies of the desired level on the skin surface (Figure 3).
- Place electrodes for electromyography (EMG) on reference muscles for nerves that could be at risk during surgery17.
4. Approach
- After disinfecting the skin and covering the area with the surgical drape, according to institutional standards, make a 3-5 cm long skin incision using a sterile scalpel over the disc space in a single-level surgery.
- Use electrocautery to dissect the fascia abdominalis.
- Open the fascia and use clamps/retractors to bluntly split the three layers of the obliques until the retroperitoneal space is reached.
- Identify the psoas and split it in the longitudinal direction (for example, using retractors).
5. Neuromonitoring
- Look out for any changes in somatosensory evoked potentials (SSEP), motor evoked potentials (MEP), and spontaneous electromyography (EMG) during approach and when dissecting the psoas17.
- Using a handheld EMG probe, identify the exiting nerve root at the desired level.
6. Retractors
- After fluoroscopically confirming the desired level, place a self-retaining retractor system (Figure 4).
7. Preparation of intradiscal space
- Use a scalpel to perform an annulotomy and resect all discal material using a rongeur.
NOTE: In severe degeneration, the disc space may be obscured by osteophytes. These may need resection before evacuation of the discal space. - Place a spreader to open the collapsed intervertebral space.
NOTE: In cases of severe degeneration, only partial restoration of the intervertebral space may be possible. - Carefully remove disc material and cartilage from both endplates using Curettes or Cobb elevators (Figure 4).
8. Placement of trail cage
- Use different trial components to restore normal disc height and to relieve any pressure on nerve roots.
NOTE: The trial position is verified using fluoroscopy from both the AP and lateral views. The endplates should be symmetrically elevated (Figure 4).
9. Cage implantation
- After identifying the correct size, implant the cage under fluoroscopic guidance (Figure 5).
- Obtain final fluoroscopic images in A.P. and lateral view.
10. Closure
- Use irrigation and perform a final hemostasis check.
- Carefully remove all retractors and spreaders.
- Close the wound in a multilayered fashion.

Figure 3: Identification of disc level. (A-B): Marking the desired level in anteroposterior (AP) and lateral view. (C) Identification of the desired level in the lateral view fluoroscopy. Please click here to view a larger version of this figure.
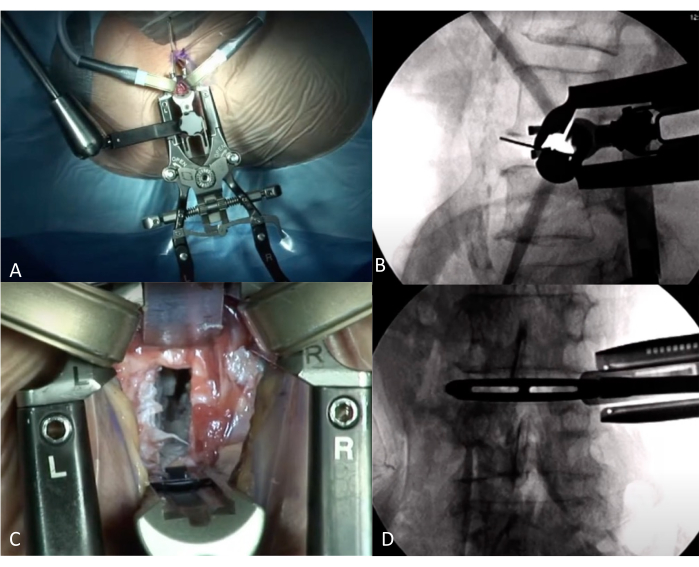
Figure 4: Interoperative view during LLIF. (A) Position of Retractors on AP view. (B) Intraoperative verification of the correct level using fluoroscopy lateral view with retractors in place. (C) View on disc-level through Retractors. The disc was removed, with some cartilage left. (D) Intraoperative fluoroscopy AP view, with a trial cage in place. Please click here to view a larger version of this figure.
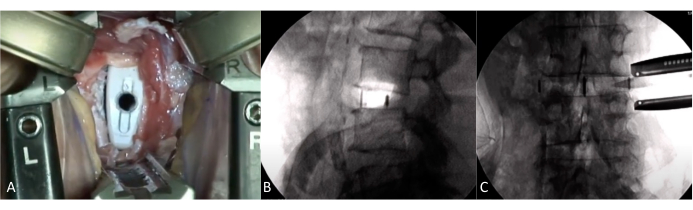
Figure 5: Intraoperative view with implanted cage. (A) Intraoperative view through retractors. Visualization of the implanted cage. (B-C) Fluoroscopy with the cage in place. Correct positioning of the cage with restoration of disc height. Please click here to view a larger version of this figure.
11. In-hospital postoperative phase
- Encourage patients to mobilize without a brace within hours by providing adequate pain medication or other pain management techniques.
- Provide the patient with a clear diet until the patient has passed flatus, then advance to a regular diet.
- Perform a recommended inspection and manual palpation of the flanks and a formal dressing check before discharge.
12. Outpatient postoperative phase
- After two weeks, perform a formal wound check.
- Advise the patients not to perform bending/lifting/twisting movements greater than 15 lbs.
13. Follow up
- At six weeks obtain postoperative X-rays and initiate 6 weeks of formalized physical therapy and education in trunk mechanics (Figure 2).
- At 3 months perform a formal evaluation. If concern for pseudoarthrosis arise obtain a CT with MPR (multiplanar reformatting) or MIP (maximum intensity projection) post-processing tools to assess for continuity of the fusion.
- At six months, perform a neurological evaluation and obtain standard radiographs.
- Release patient from care after a last clinical and radiological follow-up one-year post-surgery.
Wyniki
In our recent study, we investigated the complication rates following a stand-alone lateral interbody fusion12. We retrospectively investigated 158 patients (145 trans-psoas, 13 pre-psoas) who received an LLIF between 2016 and 2020, with a mean follow-up of 14 months (Table 1).
| Patient characteristics | All (n=158) | Trans-psoas (n=145) |
Dyskusje
Lateral Lumbar Interbody Fusion (LLIF) has become a popular minimally invasive technique for achieving arthrodesis. Since its introduction, indications have expanded for the treatment of various lumbar conditions such as degenerative diseases, deformity, and lumbar spinal pathologies. It allows implantation of larger interbody cages than TLIF or PLIF across the apophysis leading to effective correction of coronal deformities and indirect decompression of the neuroforamina by restoring disc height18
Ujawnienia
Conflict of interest Jens Chapman MD receives consulting fees from Globus Medical Inc. Rod Oskouian MD receives royalties from Stryker and consulting fees from Seaspine, Globus Medical Inc., Stryker, DePuy Synthes, Medtronic and ATEC. All other authors have no relevant financial or non-financial interests to disclose.
Podziękowania
None.
Materiały
| Name | Company | Catalog Number | Comments |
| 100mm Aluminum Center Blade | Nuvasive | 3244100 | |
| 100mm Electrode Center Blade | Nuvasive | 3243100 | |
| 100mm Left Blade | Nuvasive | 3241100 | |
| 100mm Right Blade | Nuvasive | 3242100 | |
| 110mm Aluminum Center Blade | Nuvasive | 3244110 | |
| 110mm Electrode Center Blade | Nuvasive | 3243110 | |
| 110mm Left Blade | Nuvasive | 3241110 | |
| 110mm Right Blade | Nuvasive | 3242110 | |
| 120mm Aluminum Center Blade | Nuvasive | 3244120 | |
| 120mm Electrode Center Blade | Nuvasive | 3243120 | |
| 120mm Left Blade | Nuvasive | 3241120 | |
| 120mm Right Blade | Nuvasive | 3242120 | |
| 130mm Aluminum Center Blade | Nuvasive | 3244130 | |
| 130mm Electrode Center Blade | Nuvasive | 3243130 | |
| 130mm Left Blade | Nuvasive | 3241130 | |
| 130mm Right Blade | Nuvasive | 3242130 | |
| 140mm Aluminum Center Blade | Nuvasive | 3244140 | |
| 140mm Electrode Center Blade | Nuvasive | 3243140 | |
| 140mm Left Blade | Nuvasive | 3241140 | |
| 140mm Right Blade | Nuvasive | 3242140 | |
| 150mm Aluminum Center Blade | Nuvasive | 3244150 | |
| 150mm Electrode Center Blade | Nuvasive | 3243150 | |
| 150mm Left Blade | Nuvasive | 3241150 | |
| 150mm Right Blade | Nuvasive | 3242150 | |
| 160mm Aluminum Center Blade | Nuvasive | 3244160 | |
| 160mm Electrode Center Blade | Nuvasive | 3243160 | |
| 160mm Left Blade | Nuvasive | 3241160 | |
| 160mm Right Blade | Nuvasive | 3242160 | |
| 50mm Aluminum Center Blade | Nuvasive | 3244050 | |
| 50mm Left Blade | Nuvasive | 3241050 | |
| 50mm Right Blade | Nuvasive | 3242050 | |
| 60mm Aluminum Center Blade | Nuvasive | 3244060 | |
| 60mm Left Blade | Nuvasive | 3241060 | |
| 60mm Right Blade | Nuvasive | 3242060 | |
| 70mm Aluminum Center Blade | Nuvasive | 3244070 | |
| 70mm Left Blade | Nuvasive | 3241070 | |
| 70mm Right Blade | Nuvasive | 3242070 | |
| 80mm Aluminum Center Blade | Nuvasive | 3244080 | |
| 80mm Left Blade | Nuvasive | 3241080 | |
| 80mm Right Blade | Nuvasive | 3242080 | |
| 90mm Aluminum Center Blade | Nuvasive | 3244090 | |
| 90mm Electrode Center Blade | Nuvasive | 3243090 | |
| 90mm Left Blade | Nuvasive | 3241090 | |
| 90mm Right Blade | Nuvasive | 3242090 | |
| ALGI Penfield, Long Push | Nuvasive | 3300118 | |
| Anterior Crossbar | Nuvasive | 3240003 | |
| Bayonetted Shim Inserter | Nuvasive | 3200215 | |
| Blade Rotation Driver | Nuvasive | 3240005 | |
| Dilator - 12mm | Nuvasive | 1010968 | |
| Dilator - 6mm | Nuvasive | 1010966 | |
| Dilator - 9mm | Nuvasive | 1010967 | |
| Fixation Shim Screw Driver | Nuvasive | 3200052 | |
| Fluoro Modulator | Nuvasive | 3220131 | |
| Hex Driver (3/32”) | Nuvasive | 1011691 | |
| Hex Key (3/32”) | Nuvasive | 1011748 | |
| Initial Dilator Holder | Nuvasive | 3230140 | |
| K-wire (13.5”) | Nuvasive | 3230101 | |
| Light Cable Adapter, Storz | Nuvasive | 1011811 | |
| Lock Shim Inserter/Repositioning Tool | Nuvasive | 3240001 | |
| Lock Shim Inserter/Repositioning Tool, | Nuvasive | ||
| Low-Profile Shim | Nuvasive | 3240002 | |
| MaXcess 4 4th Blade Attachment | Nuvasive | 3240004 | |
| Maxcess 4 Access System | Nuvasive | Tray with XLIF Dilator and Retractors in different seizes | |
| MaXcess 4 Articulating Arm | Nuvasive | 3240121 | |
| MaXcess 4 Blade, 180mm Electrode Ctr | Nuvasive | 3243180 | |
| Needle Electrodes | Nuvasive | 8050029 | NVM5 System |
| Surface Electrodes | Nuvasive | 8020029 | NVM5 System |
| Targeting Instrument | Nuvasive | 3240000 | |
| XL (18mm wide) | Nuvasive | 1000-00-0716 | Implant |
| XL-F (18mm wide) | Nuvasive | 1000-00-0719 | Implant |
| XL-F Wide (22mm wide) | Nuvasive | 1000-00-0720 | Implant |
| XLIF Annulus Cutter, 4x14mm | Nuvasive | 6942414 | |
| XLIF Annulus Cutter, 4x16mm | Nuvasive | 6942416 | |
| XLIF Cobb, 12mm Straight | Nuvasive | 6940012 | |
| XLIF Cobb, 18mm Down | Nuvasive | 6940118 | |
| XLIF Cobb, 18mm Straight | Nuvasive | 6940018 | |
| XLIF Cobb, 22mm Straight | Nuvasive | 6940022 | |
| XLIF Cobb, Serrated | Nuvasive | 6940001 | |
| XLIF Cutter, 8mm Disc | Nuvasive | 6940151 | |
| XLIF Forceps, Bipolar Str Extra Long | Nuvasive | 3200322 | |
| XLIF Handle, Slide | Nuvasive | 6940004 | |
| XLIF Inserter, Angled XL | Nuvasive | D6904485 | |
| XLIF Kerrison, 2mm | Nuvasive | 6940132 | |
| XLIF Kerrison, 5mm | Nuvasive | 6940135 | |
| XLIF Nerve Retractor, Extra Long | Nuvasive | 3300319 | |
| XLIF Paddle Sizer, 4x16mm | Nuvasive | 6940416 | |
| XLIF Paddle Sizer, 6x18mm | Nuvasive | 6940618 | |
| XLIF Paddle Sizer, 6x22mm | Nuvasive | 6940622 | |
| XLIF Penfield, Pull Extra Long | Nuvasive | 3300318 | |
| XLIF Pituitary, Large | Nuvasive | 6940431 | |
| XLIF Pituitary, Medium | Nuvasive | 6940430 | |
| XLIF Pituitary, Small | Nuvasive | 6940429 | |
| XLIF Rasp | Nuvasive | 6940340 | |
| XLIF Scraper, Endplate | Nuvasive | 6940020 | |
| XLIF Slide, Bayonetted | Nuvasive | 6940063 | |
| XLIF Slide, XW Bayonetted | Nuvasive | 6940064 | |
| XLIF Suction, 12FR Extra Long | Nuvasive | 3300320 | |
| XLIF Suction, 15FR Extra Long | Nuvasive | 3300321 | |
| XL-W (22mm wide) | Nuvasive | 1000-00-0717 | Implant |
| XL-XW (26mm wide) | Nuvasive | 1000-00-0718 | Implant |
Odniesienia
- Luoma, K., et al. Low back pain in relation to lumbar disc degeneration. Spine. 25 (4), 487-492 (2000).
- Rabau, O., et al. Lateral lumbar interbody fusion (LLIF): An update. Global Spine J. 10 (2_suppl), 17S-21S (2020).
- Pimenta, L. Less-invasive lateral lumbar interbody fusion (XLIF) surgical technique: video lecture. Eur Spine J. 24 Suppl 3 (S3), 441-442 (2015).
- Pimenta, L. Lateral endoscopic transpsoas retroperitoneal approach for lumbar spine surgery. Presented at VIII Brazilian Spine Society Meeting. , (2001).
- Ozgur, B. M., Aryan, H. E., Pimenta, L., Taylor, W. R. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 6 (4), 435-443 (2006).
- Lamartina, C., Berjano, P. Prone single-position extreme lateral interbody fusion (Pro-XLIF): preliminary results. Eur Spine J. 29, 6-13 (2020).
- Walker, C. T., et al. Single-position prone lateral interbody fusion improves segmental lordosis in lumbar spondylolisthesis. World Neurosurg. 151, e786-e792 (2021).
- . XLIF Nuvasive Available from: https://www.nuvasive.com/procedures/spine/xlif20/ (2025)
- . DLIF Metronic Available from: https://thespinemarketgroup.com/wp-content/uploads/2020/07/Direct-Lateral-Surgical-Technique-Medtronic.pdf (2025)
- . OLIF Medtronic Available from: https://www.medtronic.com/me-en/healthcare-professionals/therapies-procedures/spinal-orthopaedic/olif.html (2025)
- Alonso, F., et al. The subcostal nerve during lateral approaches to the lumbar spine: an anatomical study with relevance for injury avoidance and postoperative complications such as abdominal wall hernia. World Neurosurg. 104, 669-673 (2017).
- Godolias, P., et al. Complication rates following stand-alone lateral interbody fusion: a single institution series after 10 years of experience. Eur J Orthop Surg Traumatol. 33 (5), 2121-2127 (2022).
- Walker, C. T., et al. Complications for minimally invasive lateral interbody arthrodesis: a systematic review and meta-analysis comparing prepsoas and transpsoas approaches. J Neurosurg Spine. 30 (4), 446-460 (2019).
- Amini, D. A., et al. Development of a decision-making pathway for utilizing standalone lateral lumbar interbody fusion. Eur Spine J. 31 (7), 1611-1620 (2022).
- Camino-Willhuber, G., et al. Lumbar lateral interbody fusion: step-by-step surgical technique and clinical experience. J Spine Surg. 9 (3), 294-305 (2023).
- Kwon, B., Kim, D. H. Lateral lumbar interbody fusion. J Am Acad of Orthop Surg. 24 (2), 96-105 (2016).
- Lall, R. R., et al. Intraoperative neurophysiological monitoring in spine surgery: indications, efficacy, and role of the preoperative checklist. Neurosurg Focus. 33 (5), E10 (2012).
- Taba, H. A., Williams, S. K. Lateral lumbar interbody fusion. Neurosurg Clin of N Am. 31 (1), 33-42 (2020).
- Rodgers, W. B., Gerber, E. J., Patterson, J. Intraoperative and early postoperative complications in extreme lateral interbody fusion. Spine. 36 (1), 26-32 (2011).
- Moro, T., Kikuchi, S., Konno, S., Yaginuma, H. An anatomic study of the lumbar plexus with respect to retroperitoneal endoscopic surgery. Spine. 28 (5), 423-427 (2003).
- Ng, J. P. H., Kaliya-Perumal, A. K., Tandon, A. A., Oh, J. Y. L. The oblique corridor at L4-L5: a radiographic-anatomical study into the feasibility for lateral interbody fusion. Spine. 45 (10), E552-E559 (2020).
- Huang, C., Xu, Z., Li, F., Chen, Q. Does the access angle change the risk of approach-related complications in minimally invasive lateral lumbar interbody fusion? An MRI study. J Korean Neurosurg Soc. 61 (6), 707-715 (2018).
- Kramer, D. E., Woodhouse, C., Kerolus, M. G., Yu, A. Lumbar plexus safe working zones with lateral lumbar interbody fusion: a systematic review and meta-analysis. Eur Spine J. 31 (10), 2527-2535 (2022).
- Godolias, P., et al. Lumbosacral plexus 3D printing with dissection validation - a baseline study with regards to lateral spine surgery. Interdiscip Neurosurg. 31, 101666 (2023).
- Agarwal, N., et al. Lateral lumbar interbody fusion in the elderly: a 10-year experience. J Neurosurg Spine. 29 (5), 525-529 (2018).
- Chen, E., et al. Cage subsidence and fusion rate in extreme lateral interbody fusion with and without fixation. World Neurosurg. 122, 969-977 (2019).
- Wang, Y., Beydoun, M. A., Min, J., Xue, H., Kaminsky, L. A., Cheskin, L. J. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 49 (3), 810-823 (2020).
- Barrie, U., et al. Impact of obesity on complications and surgical outcomes after adult degenerative scoliosis spine surgery. Clin Neurol Neurosurg. 226, 107619 (2023).
- Shasby, G. Erratum. Does minimally invasive spine surgery improve outcomes in the obese population? A retrospective review of 1442 degenerative lumbar spine surgeries. J Neurosurg Spine. 36 (6), 1035 (2022).
- Hijji, F. Y., et al. Lateral lumbar interbody fusion: a systematic review of complication rates. Spine J. 17 (10), 1412-1419 (2017).
- Hu, W. -. K., et al. An MRI study of psoas major and abdominal large vessels with respect to the X/DLIF approach. Eur Spine J. 20 (4), 557-562 (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone