A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Constructing a Collagen Hydrogel for the Delivery of Stem Cell-loaded Chitosan Microspheres
In This Article
Summary
A major hurdle in current stem cell therapies is determining the most effective method to deliver these cells to host tissues. Here, we describe a chitosan-based delivery method that is efficient and simple in approach, while allowing adipose-derived stem cells to maintain their multipotency.
Abstract
Multipotent stem cells have been shown to be extremely useful in the field of regenerative medicine1-3. However, in order to use these cells effectively for tissue regeneration, a number of variables must be taken into account. These variables include: the total volume and surface area of the implantation site, the mechanical properties of the tissue and the tissue microenvironment, which includes the amount of vascularization and the components of the extracellular matrix. Therefore, the materials being used to deliver these cells must be biocompatible with a defined chemical composition while maintaining a mechanical strength that mimics the host tissue. These materials must also be permeable to oxygen and nutrients to provide a favorable microenvironment for cells to attach and proliferate. Chitosan, a cationic polysaccharide with excellent biocompatibility, can be easily chemically modified and has a high affinity to bind with in vivo macromolecules4-5. Chitosan mimics the glycosaminoglycan portion of the extracellular matrix, enabling it to function as a substrate for cell adhesion, migration and proliferation. In this study we utilize chitosan in the form of microspheres to deliver adipose-derived stem cells (ASC) into a collagen based three-dimensional scaffold6. An ideal cell-to-microsphere ratio was determined with respect to incubation time and cell density to achieve maximum number of cells that could be loaded. Once ASC are seeded onto the chitosan microspheres (CSM), they are embedded in a collagen scaffold and can be maintained in culture for extended periods. In summary, this study provides a method to precisely deliver stem cells within a three dimensional biomaterial scaffold.
Protocol
1. Isolating Adipose-Derived Stem Cells (ASC)
Note: All procedures were performed at room temperature unless otherwise noted.
- Isolate rat perirenal and epididymal adipose and wash with sterile Hank's buffered salt solution (HBSS) containing 1% fetal bovine serum (FBS) as previously described6.
- Mince the tissue and transfer 1-2 g into 25 mL of HBSS containing 1% FBS into a 50 mL tube and centrifuge at 500 g for 8 min at room temperature.
- Collect the free-floating adipose tissue layer and transfer to 125 mL erlenmeyer flask and treat with 25 mL of collagenase type II (200 U/mL) in HBSS for 45 min at 37 °C on an orbital shaker (125 rpm).
- Carefully remove the liquid fraction (below oil and adipose layer) and filter sequentially through a 100-μm and 70-μm nylon mesh filter. Centrifuge the filtrate at 500 g for 10 min at room temperature, aspirate the supernatant and wash the pellet twice with 25 mL HBSS.
- Resuspend the cell pellet in 50 mL of growth medium (MesenPRO RS Basal Medium) supplemented with MesenPRO RS Growth Supplement, antibiotic-antimycotic (100 U/mL of penicillin G, 100 μg/mL streptomycin sulfate, and 0.25 μg/mL Amphotericin B), and 2 mM L-glutamine and pipet cells into 2 T75 flasks (25 mL/flask).
- Culture the ASC in a 5% CO2 humidified incubator at 37 °C (Passage 2-4 ASC are used for all experiments).
2. Preparing Chitosan Microspheres (CSM)
Note: All procedures were performed at room temperature unless otherwise noted.
- CSM are prepared by a water-in-oil emulsification process along with an ionic coacervation technique using our previous protocol6. Emulsify an aqueous solution of chitosan (6 mL of 3%w/v chitosan in 0.5 M acetic acid) in a 100 mL of an oil phase mixture consisting of soya oil, n-octanol (1:2 v/v) and 5% sorbitan-mono-oleate (span 80) emulsifier, using overhead (1700 rpm) and magnetic stirring (1000 rpm) simultaneously, in opposite directions. This dual method of mixing ensures that micelles formed early on before cross-linking occurs can remain in solution and not settle to the bottom. Furthermore, the magnetic stir bar aids in de-aggregating chitosan during micelle formation and rigidization.
- The mixture is continuously stirred for approximately 1 hour until a stable water-in-oil emulsion is obtained. Ionic crosslinking is initiated with the addition of 1.5 mL of 1% w/v potassium hydroxide in n-octanol every 15 min for 4 h (24 mL total).
- After completion of the crosslinking reaction, slowly decant the oil phase of the mixture containing CSM and immediately add the spheres to 100 mL of acetone. Wash the spheres with acetone until the oil phase is completely removed.
- Dry the recovered spheres in a vacuum desiccator and analyze without further processing. The average CSM particle size, surface area per milligram and the unit cubic volume was determined using particle size analyzer.
- For subsequent experiments, wash CSM three times with sterile water to remove residual salts and sterilize with absolute alcohol.
3. Determining the Number of Free Amino Groups in CSM
Note: All procedures were performed at room temperature unless otherwise noted.
- Determine the number of free amino groups present in CSM after ionic crosslinking by using the trinitro benzenesulfonic (TNBS) acid assay of Bubins and Ofner7. Incubate 5 mg of microspheres with 1 mL of 0.5% TNBS solution in a 50 mL glass tube for 4 h at 40 °C and hydrolyze with the addition of 3 mL of 6N HCl at 60 °C for 2h.
- Cool the samples to room temperature, and extract the free TNBS by adding 5 mL of deionized water and 10 mL of ethyl ether.
- Warm a 5 mL aliquot of the aqueous phase to 40 °C in a water bath for 15 min to evaporate any residual ether, cool to room temperature, and dilute with 15 mL of water.
- Measure the absorbance at 345 nm with a spectrophotometer using TNBS solution without chitosan as blank and the chitosan used for CSM preparation to determine the total number of amino groups. Estimate the number of free amino groups of the CSM relative to chitosan.
4. Loading ASC in CSM
Note: All procedures were performed at room temperature unless otherwise noted.
- Equilibrate 5 mg of sterilized CSM from section 2.5 in sterile HBSS overnight and add to an 8-μm pore size membrane culture plate insert (24-well plate).
- After the CSM have settled onto the membrane, carefully aspirate the HBSS and add 300 μL of growth medium to the inside of the insert and 700 μL of growth media to the outside of the insert.
- Resuspend ASC at the appropriate concentration (1 x 104 to 4 x 104) in 200 μL of growth medium and seed over the CSM inside the culture plate insert. The final volume of medium within the culture insert, after seeding, is 500 μL.
- Incubate the ASC seeded on CSM for 24 h in a 5% CO2 humidified incubator at 37 °C.
5. Determining the Percentage of ASC Loading and Cell Viability in CSM
Note: All procedures were performed at room temperature unless otherwise noted.
- After incubation, collect the ASC-loaded CSM in a sterile 1.5- ml microcentrifuge tube without disturbing the cells that have migrated into the insert membrane.
- Remove the residual medium and add 250 μL of fresh growth medium to the tube.
- To each tube add 25 μL MTT [3-(4,5-dimethylthiozole-2-yl)-2,5-diphenyltetrazolium bromide] solution (5 mg/mL) and incubate for 4 h in a 5% CO2 humidified incubator at 37 °C.
- After incubation, remove the medium, add 250 μL of dimethyl sulfoxide, and vortex the mixture for 2-5 min to solubilize the formazan complex.
- Centrifuge the CSM at 2700 x g for 5 min, and determine the supernatant absorbance measured at 570 nm using 630 nm as reference.
- Determine the cell number associated with the CSM relative to the absorbance value obtained from known numbers of viable ASC.
6. Characterizing ASC-CSM-Embedded Collagen Gel
Note: All procedures were performed at room temperature unless otherwise noted.
- Mix ASC-loaded CSM (5 mg containing ≈ 2 x 104 cells) with type 1 collagen (7.5 mg/mL) extracted from rat tail tendon according the method of Bornstein8 and fibrillate after adjusting the pH to 6.8 using 2 N NaOH.
- Add the fibrillated collagen-ASC-CSM mixture to a 12-well plate and incubate for 30 min at in a 5% CO2 humidified incubator at 37 °C.
- After complete fibrillation, incubate the collagen-ASC-CSM gels for up to 14 days in a 5% CO2 humidified incubator at 37 °C.
- Release and migration of cells from CSM into the gel were observed using standard microscopy techniques.
7. Representative Results
In the present study, we have developed an in vitro strategy to deliver stem cells from chitosan microspheres (CSM) into a collagen gel scaffold. Porous CSM of uniform size (175-225 μm in diameter) and composition were prepared and used as cell carriers (Figure 2). Upon incubating ASC with the CSM, the cells attached at a concentration of 2 x 104 cells/5mg of CSM. The cells were capable of spreading over the microsphere, while extending filopodia into the porous crevices of the microsphere (Figure 3). Once the cell-loaded CSM were mixed with the collagen gel, the cells immediately began migrating into the gels (Figure 4).
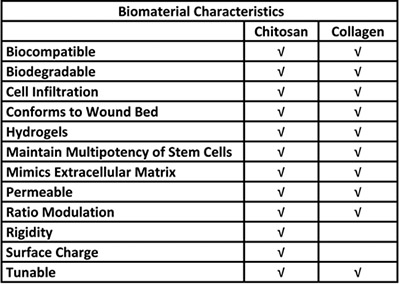
Table 1. Biological advantages for the use of chitosan and collagen in a stem cell delivery system.
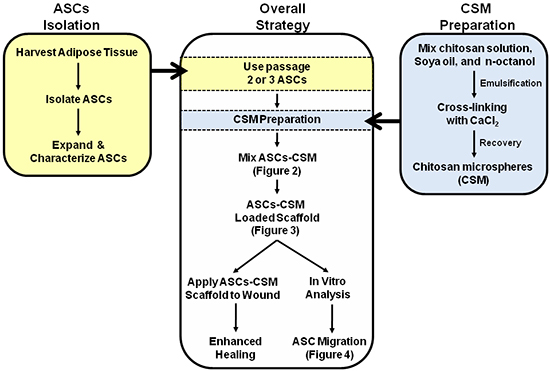
Figure 1. Schematic depicting the overall strategy for the dual use of ASC-CSM loaded collagen scaffolds. Figures 2, 3, and 4 are annotated within the schematic to assist with interpretation of the images.
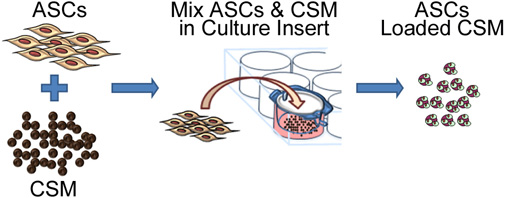
Figure 2. Schematic representation depicting the process of seeding stem cells onto chitosan microspheres. The process involves co-culturing ASC with CSM in an 8- μm pore size membrane culture plate insert. After 24 hours, the microspheres are removed from the insert and are ready for embedding into a biomaterial matrix.
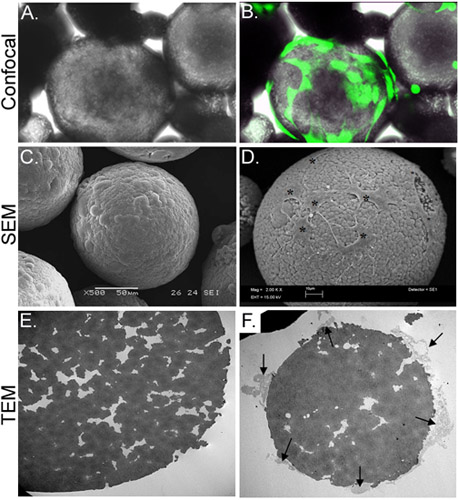
Figure 3. Morphological characterization of CSM-loaded with ASC. Panel A depicts a light micrograph of ASC-loaded CSM, while panel B shows the same field of view superimposed with an image obtained by confocal fluorescence microscopy. ASC were preloaded with calcein AM (green). Panel C depicts an image of an SEM image of an unloaded microsphere, while panel D shows cells loaded onto the microsphere (asterisks). The TEM image in panel E shows a cross-section of an unloaded microsphere. A multitude of pores and crevices are located throughout the microsphere. Panel F shows a cross-sectioned microsphere with cells (arrows) attached and extending filopodia into the crevices. Original magnifications: A & B= 70X; C= 500x , D= 2,000x; E&F= 2,500x.

Figure 4. Migration of ASC from the CSM into the three-dimensional collagen scaffold. Panels A and B depict the CSM with cells migrating away from microsphere and into the collagen matrix on day 3 (A, arrows). Panel B shows a similar culture after 12 days. Transmission electron microscopy (TEM) images are depicted in C, D, and E. Asterisks in C and D show a microsphere that has been cross-sectioned with cells migrating away from the microsphere (arrows). Higher magnification of panel D is depicted in panel E, and shows cell filopodia attached to the collagen fibrils (inset). Original magnification: A&B = 100x; C&D = 6,000x; E = 20,000x, inset = 150,000x.
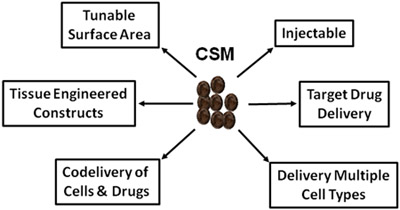
Figure 5. Schematic depicting the vast uses of CSM in regenerative medicine and drug delivery.
Access restricted. Please log in or start a trial to view this content.
Discussion
A major hurdle in stem cell-based therapy is developing efficient methods for delivery of cells to the specified regions for repair. Due to patient to patient variability, the tissue type, injury size and depth; the methodology of delivering stem cells must be determined on a case-by-case basis. Although embedding stem cells within a matrix and delivering them to the wound site appears to be a next logical approach for tissue engineering, some technical hurdles remain. This includes the ability of the embedded cel...
Access restricted. Please log in or start a trial to view this content.
Disclosures
No competing financial interests exist.
Disclaimers
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the U.S. Government. The authors are employees of the U.S. Government, and this work was prepared as part of their official duties. All work was supported by the U.S. Army Medical Research and Materiel Command. This study was conducted under a protocol reviewed and approved by the US Army Medical Research and Materiel Command Institutional Review Board, and in accordance with the approved protocol.
Acknowledgements
D.O.Z. is supported by a grant awarded from The Geneva Foundation. S.N. was supported by a Postdoctoral Fellowship Grant from the Pittsburgh Tissue Engineering Initiative.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Hanks BalancedSalt Solution (HBSS) | GIBCO, by Life Technologies | 14175 | Consumable |
| Fetal Bovine Serum | Hyclone | SH30071.03 | Consumable |
| Collagenase Type II | Sigma-Aldrich | C6685 | Consumable |
| 70-μm nylon mesh filter | BD Biosciences | 352350 | Consumable |
| 100-μm nylon mesh filter | BD Biosciences | 352360 | Consumable |
| MesenPRO Growth Medium System | Invitrogen | 12746-012 | Consumable |
| L-glutamine | GIBCO, by Life Technologies | 25030 | Consumable |
| T75 Tissue Culture Flask | BD Biosciences | 137787 | Consumable |
| Chitosan | Sigma-Aldrich | 448869 | Consumable |
| Acetic Acid | Sigma-Aldrich | 320099 | Consumable |
| N-Octanol | Acros Organics | 150630025 | Consumable |
| Sorbitan-Mono-oleate | Sigma-Aldrich | S6760 | Consumable |
| Potassium Hydroxide | Sigma-Aldrich | P1767 | Consumable |
| Acetone | Fisher Scientific | L-4859 | Consumable |
| Ethanol | Sigma-Aldrich | 270741 | Consumable |
| Trinitro Benzenesulfonic Acid | Sigma-Aldrich | P2297 | Consumable |
| Hydrochloric Acid | Sigma-Aldrich | 320331 | Consumable |
| Ethyl Ether | Sigma-Aldrich | 472-484 | Consumable |
| 8-μm Tissue Culture Plate Inserts | BD Biosciences | 353097 | Consumable |
| 1.5-ml Microcentrifuge Tubes | Fisher Scientific | 05-408-129 | Consumable |
| MTT Reagent | Invitrogen | M6494 | Consumable |
| Dimethyl Sulfoxide | Sigma-Aldrich | D8779 | Consumable |
| Qtracker Cell Labeling Kit (Q tracker 655) | Molecular Probes, Life Technologies | Q2502PMP | Consumable |
| Type 1 Collagen | Travigen | 3447-020-01 | Consumable |
| Sodium Hydroxide | Sigma-Aldrich | S8045 | Consumable |
| 12-Well Tissue Culture Plates | BD Biosciences | 353043 | Consumable |
| Centrifuge | Eppendorf | 5417R | Equipment |
| Orbital Shaker | New Brunswick Scienctific | C24 | Equipment |
| Humidified Incubator with Air-5% CO2 | Thermo Fisher Scientific, Inc. | Model 370 | Equipment |
| Overhead Stirrer | IKA | Visc6000 | Equipment |
| Magnetic Stirrer | Corning | PC-210 | Equipment |
| Vacuum Desiccator | - | - | Equipment |
| Particle Size Analyzer | Malvern Instruments | STP2000 Spraytec | Equipment |
| Water Bath | Fisher Scientific | Isotemp210 | Equipment |
| Spectrophotometer | Beckman Coulter Inc. | Beckman Coulter DU800UV/Visible Spectrophotometer | Equipment |
| Vortex | Diagger | 3030a | Equipment |
| Microplate Reader | Molecular Devices | SpectraMax M2 | Equipment |
| Light/Fluorescence Microscope | Olympus Corporation | IX71 | Equipment |
| Confocal Microscope | Olympus Corporation | FV-500 Laser Scanning Confocal Microscope | Equipment |
| Scanning Electron Microscope | Carl Zeiss, Inc. | Leo 435 VP | Equipment |
| Transmission Electron Microscope | JEOL | JEOL 1230 | Equipment |
References
- Krampera, M. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone. 39, 678-683 (2006).
- Patrick, C. W. Tissue engineering strategies for adipose tissue repair. Anat. Rec. 263, 361-366 (2001).
- Pountos, I., Giannoudis, P. V. Biology of mesenchymal stem cells. Injury. 36, Suppl 3. S8-S12 (2005).
- Kim, I. Y. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 26, 1-21 (2008).
- Shi, C. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J. Surg. Res. 133, 185-192 (2006).
- Natesan, S. Adipose-derived stem cell delivery into collagen gels using chitosan microspheres. Tissue Eng. Part. A. 16, 1369-1384 (2010).
- Bubnis, W. A., Ofner, C. M. 3rd The determination of epsilon-amino groups in soluble and poorly soluble proteinaceous materials by a spectrophotometric method using trinitrobenzenesulfonic acid. Anal. Biochem. 207, 129-133 (1992).
- Bornstein, M. B. Reconstituted rattail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest. 7, 134-137 (1958).
- Benoit, D. S. Integrin-linked kinase production prevents anoikis in human mesenchymal stem cells. J. Biomed. Mater. Res. A. 81, 259-268 (2007).
- Nuttelman, C. R., Tripodi, M. C., Anseth, K. S. Synthetic hydrogel niches that promote hMSC viability. Matrix Biol. 24, 208-218 (2005).
- Shanmuganathan, S. Preparation and characterization of chitosan microspheres for doxycycline delivery. Carbohydr. Polym. 73, 201-211 (2008).
- Haque, T., Chen, H., Ouyang, W., Martoni, C., Lawuyi, B., Urbanska, A., Prakash, S. Investigation of a new microcapsule membrane combining alginate, chitosan, polyethylene glycol and poly-L-lysine for cell transplantation applications. Int. J. Artif. Organs. 28, 631-637 (2005).
- Goren, A., Dahan, N., Goren, E., Baruch, L., Machluf, M. Encapsulated human mesenchymal stem cells: a unique hypoimmunogenic platform for long-term cellular therapy. FASEB J. 24, 22-31 (2010).
- Zielinski, B. A., Aebischer, P. Chitosan as a matrix for mammalian cell encapsulation. Biomaterials. 15, 1049-1056 (1994).
- Girandon, L., Kregar-Velikonja, N., Božikov, K., Barliç, A. In vitro Models for Adipose Tissue Engineering with Adipose-Derived Stem Cells Using Different Scaffolds of Natural Origin. Folia Biol. (Praha). 57, 47-56 (2011).
- Baruch, L., Machluf, M. Alginate-chitosan complex coacervation for cell encapsulation: effect on mechanical properties and on long-term viability. Biopolymers. 82, 570-579 (2006).
- Wei, Y., Gong, K., Zheng, Z., Wang, A., Ao, Q., Gong, Y., Zhang, X. Chitosan/silk fibroin-based tissue-engineered graft seeded with adipose-derived stem cells enhances nerve regeneration in a rat model. J. Mater. Sci. Mater. Med. , (2011).
- Wang, Q., Jamal, S., Detamore, M. S., Berkland, C. PLGA-chitosan/PLGA-alginate nanoparticle blends as biodegradable colloidal gels for seeding human umbilical cord mesenchymal stem cells. J. Biomed. Mater. Res. A. 96, 520-527 (2011).
- Alves da Silva, M. L., Martins, A., Costa-Pinto, A. R., Correlo, V. M., Sol, P., Bhattacharya, M., Faria, S., Reis, R. L., Neves, N. M. Chondrogenic differentiation of human bone marrow mesenchymal stem cells in chitosan-based scaffolds using a flow-perfusion bioreactor. J. Tissue Eng. Regen. Med. , (2010).
- Kang, Y. M., Lee, B. N., Ko, J. H., Kim, G. H., Kang, K. N., Kim da, Y., Kim, J. H., Park, Y. H., Chun, H. J., Kim, C. H., Kim, M. S. In vivo biocompatibility study of electrospun chitosan microfiber for tissue engineering. Int. J. Mol. Sci. 11, 4140-4148 (2010).
- Bozkurt, G., Mothe, A. J., Zahir, T., Kim, H., Shoichet, M. S., Tator, C. H. Chitosan channels containing spinal cord-derived stem/progenitor cells for repair of subacute spinal cord injury in the rat. Neurosurgery. 67, 1733-1744 (2010).
- Leipzig, N. D., Wylie, R. G., Kim, H., Shoichet, M. S. Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds. Biomaterials. 32, 57-64 (2011).
- Altman, A. M., Gupta, V., RÃos, C. N., Alt, E. U., Mathur, A. B. Adhesion, migration and mechanics of human adipose-tissue-derived stem cells on silk fibroin-chitosan matrix. Acta Biomater. 6, 1388-1397 (2010).
- Altman, A. M., Yan, Y., Matthias, N., Bai, X., Rios, C., Mathur, A. B., Song, Y. H., Alt, E. U. IFATS collection: Human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells. 27, 250-258 (2009).
- Machado, C. B., Ventura, J. M., Lemos, A. F., Ferreira, J. M., Leite, M. F., Goes, A. M. 3D chitosan-gelatin-chondroitin porous scaffold improves osteogenic differentiation of mesenchymal stem cells. Biomed. Mater. 2, 124-131 (2007).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved