A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Human Internal Mammary Artery (IMA) Transplantation and Stenting: A Human Model to Study the Development of In-Stent Restenosis
In This Article
Summary
This video shows a model to study the development of intimal hyperplasia after stent deployment using a human vessel (IMA) in an immunodeficient rat model.
Abstract
Preclinical in vivo research models to investigate pathobiological and pathophysiological processes in the development of intimal hyperplasia after vessel stenting are crucial for translational approaches1,2.
The commonly used animal models include mice, rats, rabbits, and pigs3-5. However, the translation of these models into clinical settings remains difficult, since those biological processes are already studied in animal vessels but never performed before in human research models6,7. In this video we demonstrate a new humanized model to overcome this translational gap. The shown procedure is reproducible, easy, and fast to perform and is suitable to study the development of intimal hyperplasia and the applicability of diverse stents.
This video shows how to perform the stent technique in human vessels followed by transplantation into immunodeficient rats, and identifies the origin of proliferating cells as human.
Protocol
1. Internal Mammary Artery (IMA) Preparation
- The arterial endothelium is denuded by the passage of a 2-french Fogarty arterial embolectomy catheter (Baxter Healthcare, Deerfield, IL, USA). The catheter is pulled through the whole vessel length twice to ensure endothelial damage.
- Use any human stent of 8mm length and 2.5 mm-3 mm in diameter (e.g. Translumina, Yukon stent). CAUTION: The diameter of the stent should not exceed the vessel diameter by more than 10% to avoid pre- and post-stent stenosis. CAUTION: Don't switch the length of the stent within the same study.
- Deploy the stent using the appropriate balloon pressure (which is noted on the stent package) to achieve the desired diameter.
- Store stented IMA in 4 °C RPMI + heparin (500 IE/10 ml) on ice until transplantation.
2. Animal Preparation
RNU Nude (Crl:NIH-Foxn1rnu) rats (300-350 g) are housed under conventional conditions in scantainer ventilated cabinets, fed standard rat chow and autoclaved water ad libidum.
- Anesthetize rat with isoflurane (2.5-3%) using an induction chamber.
- Shave the abdominal hair and place the rat on its back and place a facemask over its nose and mouth to keep up the anesthesia.
- Disinfect the abdominal area widely using Provo-Iodine, next use 80% ethanol - repeat this step twice. Check reflexes pinching the hind feet to be sure that the rat is sufficient anesthetized.
- Under microscopic view, perform an upper median laparotomy to expose the infrarenal abdominal aorta.
- Place the intestines in a saline moisturized glove. Fold the glove around the intestines to prevent loss of moisture.
- Dissect the aorta from the infrarenal region to the bifurcation, carefully not to cause damage on the branches of the vessels.
- Use micro clamps to stop the aortic blood flow. Place the proximal clamp first, followed by the distal clamp.
- Remove an app. 0.5-0.7 mm aortic segment and flush the remaining aorta with heparin (200 units).
- Take the stented IMA and shorten it to the adequate length and position it into the gap.
- Connect the IMA to the recipient aorta, by running sutures using 8-0 prolene suture (Ethicon, Norderstedt, Germany).
- Carefully open the first cranial clamp and then the caudal clamp.
- There should be a visible pulse in the transplanted IMA and at the distal end of the aorta.
- Place the intestines back into the abdomen.
- Flush the abdomen with pre-warmed sterile saline.
- Close the muscle layer of the abdominal wall using 6-0 prolene running sutures (Ethicon, Norderstedt, Germany).
- While the rat is still in anesthesia, inject 4-5 mg/kg Carprofen subcutaneously.
- Administer post-surgical analgesia as appropriate (e.g. Carprofen or Meloxicam) for 3 days post surgery.
3. Representative Results
For histology, the specimens were fixed in 4% formalin, dehydrated in a graded series of alcohol, and infiltrated in a mixture (MMA I) of 80% methylmethacrylate and 20% dibutylphthalate for 1 day, MMA I with 1% dry benzoyl peroxide for 1 day, and MMA I with 3% dry benzoyl peroxide (MMA III) for 1-2 days at 4 °C. Thereafter, the specimens were polymerized in fresh MMA III in glass vials in a water bath on a pre-polymerized base. Slow polymerization was achieved by keeping the vials at 26 °C overnight, increasing the temperature to 28 °C the next morning, and then increasing the temperature gradually by 0.5 °C over 12 h until polymerization occurred. The polymerized blocks were sectioned at 5 μm thickness using a MICROM HM 360 microtome equipped with a tungsten carbide knife.
To identify the origin of proliferating cells (Figure 1), slides were stained with antibodies identifying either the green fluorescent protein (GFP) or rat MHC-I and human smooth muscle cells. For these studies, human IMA was incubated with the reporter gene GFP overnight using lentiviral particles for stable transduction of IMA cells. Dividing daughter cells from human origin could be identified by expressing GFP. After deparaffinization, heat-induced epitope retrieval is performed by heating the slides in antigen retrieval solution using a steamer. The Image-iT FX signal enhancer can be used for the blocking step. Cells of human origin are identified using the mouse monoclonal antibodies against GFP (1:100 diluted in primary antibody diluent (Dako)), and further labeled with goat-anti-mouse IgG, Alexa Fluor 488 (1:1000 diluted in secondary antibody diluent). The smooth muscle cells were marked with the rabbit polycolonal anti-smooth muscle α-actin (1:100 diluted in primary antibody diluent), followed by goat-anti-rabbit IgG, Alexa Fluor 555 (1:1000 diluted in secondary antibody diluents). Each antibody incubation step is performed at 37 °C for 1 hour with three times PBS washing in between. Nuclei are stained with DAPI for 10 minutes. After mounting of the slides using Prolong Gold antifade reagent, samples were analyzed using confocal microscopy.
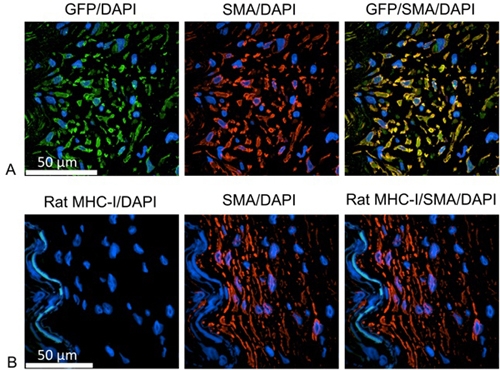
Figure 1. The neointimal cells as human smooth muscle cells. A: Green= anti GFP, labeling cells of human origin; red=anti human smooth muscle cell actin; blue=DAPI, identifying cell nuclei. B: Green= anti rat MHC-I, labeling cells of rat origin; red=anti human smooth muscle cell actin; blue=DAPI, identifying cell nuclei. Proliferating cells are identified as smooth muscle cells and positive for GFP, but negative for the rat MHC-I molecule. Therefore, proliferating cells are human origin.
Discussion
Although different in vivo research models are existing to investigate the development of intimal hyperplasia after stent placement, these models still facing translational hurdles to overcome. Furthermore, large animal models are expensive and special housing conditions as well as surgical equipment is not available for all laboratories.
Using a human IMA to study the development of human intimal proliferation and in-stent restenosis was studied before ex situ in organ ...
Disclosures
No conflicts of interest declared.
Acknowledgements
The authors thank Christiane Pahrmann for her contribution. Special thanks to Ethicon, Norderstedt, Hamburg (Germany) for providing the suture material.
Funding
Sonja Schrepfer has received a research grant from the Deutsche Forschungsgemeinschaft (DFG) (SCHR992/3 1 and SCHR992/4-1).] The work was supported by the ISHLT Shumway Career Development Grant 2010 and the Falk Research Funding (Stanford University).
Materials
| Name | Company | Catalog Number | Comments |
| 2 French Fogarty catheter | Baxter Internationl Inc. | 120602F | |
| Yukon Stent | Translumina GmbH, Hechingen, Germany | Use the stent of your choice according to your study protocol | |
| RPMI media | Biochrom AG | Nr.F1275 | |
| heparin | Baxter Internationl Inc. | 2B0953 | |
| isoflurane | Abbott Laboratories | B506 | |
| Provo-Iodine | Betadine Puredue Pharma | EAN:5995327165830 | |
| 80% ethanol | Geyer | ETV 80/0500 | |
| Micro clamp | Harvard Apparatus | PY2-61-0186 | |
| Sutures 8-0 | Johnson & Johnson | 2808G | |
| Sutures 6-0 | Johnson & Johnson | 1698 H | |
| Carprofen | Feizer Vet | PZN:0110208 | |
| Metamizol | Ratiopharm | ||
| Target retrieval solution, pH9 | Dako | S2368 | |
| Image-iT FX signal enhancer | Invitrogen | I36933 | |
| mouse monoclonal anti-GFP antibody | BD Biosciences | 632381 | |
| primary antibody diluent | Dako | S3022 | |
| goat-anti-mouse IgG, Alexa Fluor 488 | Invitrogen | A11017 | |
| secondary antibody diluent | Dako | S0809 | |
| rabbit polycolonal anti-smooth muscle α-actin | Abcam | ab5694 | |
| goat-anti-rabbit IgG, Alexa Fluor 555 | Invitrogen | A21430 | |
| Prolong Gold antifade reagent | Invitrogen | P36930 |
References
- Deuse, T., Ikeno, F., Robbins, R. C., Schrepfer, S. Imaging In-Stent Restenosis: An Inexpensive, Reliable, and Rapid Preclinical Model. J. Vis. Exp. (31), e1346 (2009).
- Oyamada, S. Trans-iliac rat aorta stenting: a novel high throughput preclinical stent model for restenosis and thrombosis. J. Surg. Res. 166, e91-e95 (2011).
- Chamberlain, J. A novel mouse model of in situ stenting. Cardiovascular research. 85, 38-44 (2010).
- Deuse, T. Introducing the first polymer-free leflunomide eluting stent. Atherosclerosis. 200, 126-134 (2008).
- Finn, A. V. Differential healing after sirolimus, paclitaxel, and bare metal stent placement in combination with peroxisome proliferator-activator receptor gamma agonists: requirement for mTOR/Akt2 in PPARgamma activation. Circulation research. 105, 1003-1012 (2009).
- Tellez, A. Coronary bare metal stent implantation in homozygous LDL receptor deficient swine induces a neointimal formation pattern similar to humans. Atherosclerosis. 213, 518-524 (2010).
- Suzuki, Y., Yeung, A. C., Ikeno, F. The pre-clinical animal model in the translational research of interventional cardiology. JACC Cardiovasc. Interv. 2, 373-383 (2009).
- Holt, C. M. Intimal proliferation in an organ culture of human internal mammary artery. Cardiovascular research. 26, 1189-1194 (1992).
- Swanson, N., Javed, Q., Hogrefe, K., Gershlick, A. Human internal mammary artery organ culture model of coronary stenting: a novel investigation of smooth muscle cell response to drug-eluting stents. Clin. Sci. (Lond). 103, 347-353 (2002).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved