A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Using Eggs from Schistosoma mansoni as an In vivo Model of Helminth-induced Lung Inflammation
In This Article
Summary
Schistosoma mansoni eggs are potent stimulators of the T helper type 2 (Th2) immune response, characteristic of parasite infection, asthma and allergic inflammation. This protocol utilizes S. mansoni egg injection to generate a CD4 Th2 cytokine-induced inflammatory response in the lung, characterized by lung granuloma formation around the egg, eosinophilia and macrophage alternative activation.
Abstract
Schistosoma parasites are blood flukes that infect an estimated 200 million people worldwide 1. In chronic infection with Schistosoma, the severe pathology, including liver fibrosis and splenomegaly, is caused by the immune response to the parasite eggs rather than the parasite itself 2. Parasite eggs induce a Th2 response characterized by the production of IL-4, IL-5 and IL-13, the alternative activation of macrophages and the recruitment of eosinophils. Here, we describe injection of Schistosoma mansoni eggs as a model to examine parasite-specific Th2 cytokine responses in the lung and draining lymph nodes, the formation of pulmonary granulomas surrounding the egg, and airway inflammation.
Following intraperitoneal sensitization and intravenous challenge, S. mansoni eggs are transported to the lung via the pulmonary arteries where they are trapped within the lung parenchyma by granulomas composed of lymphocytes, eosinophils and alternatively activated macrophages 3-6. Associated with granuloma formation, inflammation in the broncho-alveolar spaces, expansion of the draining lymph nodes and CD4 T cell activation can be observed. Here we detail the protocol for isolating Schistosoma mansoni eggs from infected livers (modified from 7), sensitizing and challenging mice, and recovering the organs (broncho-alveolar lavage (BAL), lung and draining lymph nodes) for analysis. We also include representative histologic and immunologic data and suggestions for additional immunologic analysis.
Overall, this method provides an in vivo model to investigate helminth-induced immunologic responses in the lung, which is broadly applicable to the study of Th2 inflammatory diseases including helminth infection, fibrotic diseases, allergic inflammation and asthma. Advantages of this model for the study of type 2 inflammation in the lung include the reproducibility of a potent Th2 inflammatory response in the lung and draining lymph nodes, the ease of assessment of inflammation by histologic examination of the granulomas surrounding the egg, and the potential for long-term storage of the parasite eggs.
Protocol
1. Purifying Schistosoma mansoni Eggs
- Infect Swiss-Webster mice with schistosome cercariae, which is the infectious stage in the Schistosoma life cycle 8. Alternatively, obtain schistosome-infected mice from the NIAID Schistosomiasis Resource Center (http://www.schisto-resource.org/). Leave mice for 6-7 weeks following infection, by which time-point, eggs are present in the liver and can be recovered as described below (See Fig. 1).
- Euthanize mice with CO2, and wet with 70% ethanol. Place mouse on back. Use blunt forceps and scissors to cut away skin and underlying peritoneum. Excise the liver, located below the ribcage and diaphragm.
- Finely mince livers and add 20 mL per liver of digestion mixture, composed of collagenase/dispase (0.5 mg/mL), penicillin (100 U/mL) and streptomycin (100 μg/mL) in PBS. Put mixture in a 50 mL Falcon tube and incubate overnight in a 37 °C shaker.
- Centrifuge mixture at 400 x g / 3 min / 20 °C. Gently pour off excess liquid and fill tube with PBS. Centrifuge again and repeat PBS wash. Following last centrifugation, resuspend pellet in 25 mL PBS.
- Strain the resuspended pellet through a standard kitchen metallic strainer (use a 50 mL syringe plunger to mash the liver pieces through into a glass beaker), followed by passing the mixture through a fine sieve (use 10 mL syringe to push mixture through the fine sieve into a second glass beaker).
- Put strained mixture in a 50 mL Falcon tube. Centrifuge at 400 x g / 5 min / 20 °C. Carefully pour out supernatant without losing pellet. Resuspend pellet in 3 mL PBS.
- Overlay mix gently onto a 20% Percoll, 40 mL gradient of 8 mL Percoll and 32 mL 0.25M sucrose (FW=342.3 so 8.56 g sucrose/100 mL H20).
- Centrifuge 10 min / 800 x g / 20 °C. Pipette off and discard gelatinous top layer of liver cells. Remove egg pellet at bottom with a transfer pipette and put into a 15 mL Falcon tube.
- Wash pellet 3X in 10 mL PBS/1 mM EDTA/1 mM EGTA using centrifuge settings: 3 min / 30 x g / 20 °C. Resuspend in 500 μL PBS.
- Overlay mix on a new 25% Percoll, 10 mL gradient (2.5 mL Percoll and 7.5 mL 0.25M sucrose) using centrifuge settings: 10 min / 800 x g / 20 °C.
- Wash 3X in 10 mL PBS using centrifuge settings: 3 min / 30 x g / 20 °C.
- In the last 10 mL wash, remove 100 μL to count eggs. Eggs settle quickly, so the Falcon tube must be mixed well before removing sample. Dilute the 100 μL sample with 900 μL PBS. 100 μL of the mix should be counted as droplets on a microscope slide using a dissecting microscope (1:10 dilution). Wide-bore pipette tips should be used.
- Resuspend eggs at 50,000 eggs/mL in PBS. Eggs can be stored for several months at -80 °C and thawed once or twice for use. Before use, inspect under a microscope to ensure that eggs are still intact (see Fig. 1).
2. Intraperitoneal Sensitization with S. mansoni Eggs: Day 0
- Prepare eggs at 5,000 eggs/100 μL PBS in a 5 mL snapcap tube. For injection, estimate 50% more eggs than necessary.
- Load a 23 gauge3/4inch needle and a 1 mL syringe with 100 μL of egg suspension per mouse. Eggs settle, so load needle with eggs immediately prior to use.
- Rock syringe back and forth to mix eggs prior to each injection, and inject intraperitoneally 100 μL of suspension per mouse.
3. Retro-orbital Intravenous Challenge with S. mansoni Eggs: Day 14
- Prepare eggs as above at 5,000 eggs/100 μL.
- Anesthetize mice in specialized chamber with isoflurane (4%), or intraperitoneally with xylazine (10 mg/kg) and ketamine (120 mg/kg). Depth of anesthesia is determined by pinching of the footpad and ensuring no physical response.
- Following loading of the 1 mL syringe and 23 gauge3/4inch needle with eggs, use forceps to bend the needle at a 90° angle with the bevel facing down into the angle. Ensure that no bubbles are present.
- Place mouse on one side. Retract skin so eye will protrude, and inject 100 μL into the vessels behind the eyeball at a 45° angle to the nose.
- Withdraw needle and apply light pressure to control bleeding.
All of these procedures must be carried out with care, and mice must be monitored until they recover from anesthesia. These protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC).
4. Experimental Harvest: Day 22
- Euthanize mice with CO2.
- Wet mouse with 70% ethanol, and place on back.
- Using blunt forceps, grasp the mouse abdomen and make a small incision in the skin. Carefully remove skin with scissors and expose the rib cage and peritoneum.
- Cut open the peritoneum. Cut the abdominal aorta to recover the blood with a Pasteur pipette. Store on ice.
- Move the liver down to expose the diaphragm. Use fine scissors to cut the diaphragm. Carefully cut open the rib cage to expose the lung tissue.
- Recover the parathymic lymph nodes that drain the lung with fine forceps. These are tucked underneath the rib cage, on either side of the thymus 9. Store on ice in 1mL sterile media (DMEM supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, all available from Invitrogen).
- To recover the BAL, and inflate the lungs for histologic analysis, intratracheal intubation must be performed. Expose the trachea by moving the salivary glands to the side, and placing fine forceps under the trachea. Using fine scissors, cut a hole in the middle of the trachea, and insert tubing into the lungs. Secure tubing by tying with surgical thread.
- Attach PBS-filled 1 mL syringe to the tubing and inflate lungs with 1 mL PBS. Carefully retrieve BAL wash with syringe. Store on ice.
- Attach 4% paraformaldehyde (PFA) in PBS-filled 1 mL syringe to the tubing and inflate the lungs.
- Remove the tubing, and tighten the surgical thread to prevent leaking of the PFA.
- Carefully dissect out lung tissue and place in a 50 mL Falcon tube with 5 mL 4%PFA.
5. Preparation of Recovered Tissues
- Serum: Following storage on ice for 30 min to 2 h for blood to clot, serum is recovered from the blood by centrifugation (13000 x g / 10 min / 4 °C). Serum is stored at -20 °C and can be used for cytokine ELISAs or S. mansoni egg antigen-specific IgG isotype ELISAs 6, 10.
- Lymph Nodes: Single cell suspensions are prepared and cell counts used to evaluate the magnitude of the immune response. To examine Th2 cell polarization, cells are re-stimulated with S. mansoni egg antigen for 72 hours. Intracellar cytokine staining can be examined by flow cytometry, and supernatants can be recovered and stored at -20 °C for ELISA of Th2 cytokines (IL-4, IL-5, IL-13) 6.
- BAL: Cells are pelleted (400 x g / 5 min / 4 °C). BAL fluid is recovered and stored at -20 °C for analysis of Th2 cytokines by ELISA. BAL cells counts are used as a readout for airway inflammation. Cytocentrifuge preparations of 10,000-100,000 cells (500 rpm / 5 min / 4 °C), followed by Diffquik staining will allow enumeration of inflammatory infiltrate 4, 6. Alternatively, BAL cells can be examined by flow cytometry 6.
- Lung tissue: Lung tissue is stored overnight at 4 °C. Fixed tissue is paraffin-embedded, sectioned and mounted on slides for histological and immunofluorescence analysis.
6. Representative Results
This protocol details all the steps necessary to (i) prepare purified Schistosoma mansoni eggs for in vivo use (Fig. 1), (ii) sensitize and challenge mice with these eggs and (iii) recover organs for examination of the lung inflammatory response. This in vivo model reproducibly drives a potent Th2 lung inflammatory response. This is shown by airway inflammation and eosinophilia as visualized by BAL cell counts and cytocentrifuge preparations (Fig. 2). H&E-stained lung sections can be examined for egg-induced granulomas (Fig. 3a), and immunofluorescent staining of lung sections allows visualization of alternatively activated macrophages and Th2 cytokine-induced genes such as Resistin-like molecule (RELM) α (Fig. 3b). The antigen-specific CD4 Th2 cytokine response is examined by ELISAs for IL-5, IL-4 and IL-13 of antigen-stimulated parathymic lymph node cells (Fig. 4).
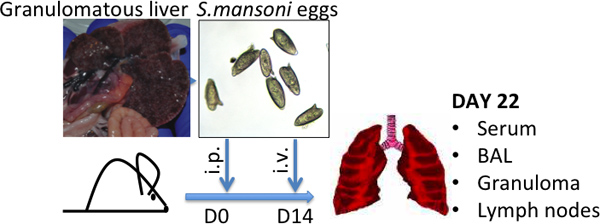
Figure 1. Schistosoma mansoni egg injection model. Representative pictures of granulomatous livers, characteristic of high S.mansoni-egg burdens, and of S.mansoni eggs are shown - average size (L) 120 μm by (W) 50 μm.
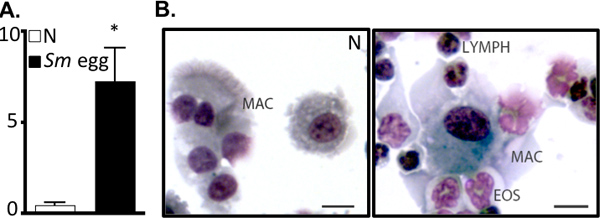
Figure 2. S. mansoni egg injection drives airway inflammation. A. BAL cell numbers (x105) from naïve (N) or S.mansoni (Sm) egg-injected mice. *P<0.05. B. Cytocentrifuge preparation of BAL cells. Mac, MACROPHAGE; Eos, EOSINOPHIL; LYMPH, lymphocyte. Bar, 10 μm.
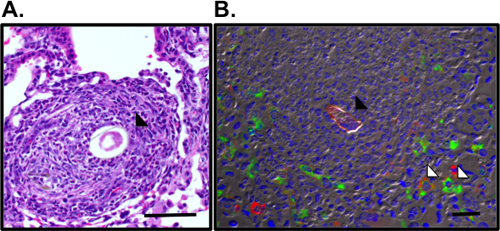
Figure 3. Visualization of pulmonary granulomas and alternatively activated macrophages surrounding the S.mansoni egg. A. Pulmonary granuloma surrounding the Sm egg (black arrow) was visualized in H&E-stained lung sections. B. Immunofluorescence staining for RELMα (green), mannose receptor (red) and DAPI (blue) reveals alternatively activated macrophages (white arrow) in the granuloma surrounding the autofluorescent Sm egg (black arrow). Bar, 50 μm.
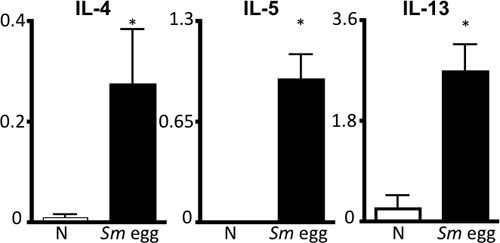
Figure 4. S.mansoni egg antigen-specific Th2 cytokine response. Draining parathymic lymph node cells from naïve (N) or Sm egg-injected mice were stimulated with Sm egg antigen for 72 hours, followed by ELISA of supernatants for IL-4, IL-5 and IL-13. Scale, ng/mL.*P<0.05.
Discussion
Here, a model employing Schistosoma mansoni eggs to induce type 2 lung inflammation is described. Characteristic features of this model include the potent Th2 immune response, airway inflammation and pulmonary granuloma formation. Since these parameters are dependent on CD4 Th2 cell responses 11, this is a useful model to investigate the effects of protein- specific or lineage-specific deletions on Th2 cell responses. Here the readouts would include quantification of antigen-specific Th2 cytokines, an...
Disclosures
No conflicts of interest declared.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) AI091759 and the Crohn's and Colitis Foundation of America's William and Shelby Modell Family Foundation Research Award (to MN). Schistosome-infected mice were supplied by the NIAID Schistosome Resource Center (NIAID Contract N01 A130026).
Materials
| Name | Company | Catalog Number | Comments |
| Collagenase/Dispase | Roche Applied Science | 11 097 113 001 (500mg) | |
| Diff Quik Hema 3 Stain | Pack | Fisher Scientific Co. LLC | 122-911 |
| DMEM | Liquid | Gibco | 11965 |
| Falcon 50mL | Pack | Falcon | 352070 |
| Intramedic PE Tubing | Becton Dickinson and Co. | 427410 (0.58mm) | |
| Isoflurane | Abbot Animal Health | 52600405 (250mL) | |
| Ketamine | Fort Dodge, Animal Health | 100mg/mL | |
| Mannose receptor antibody | Biotin | AbD Serotec | MCA2235B |
| Metallic Strainer | Standard kitchen strainer | Target | Laurentiis |
| Paraformaldehyde | Electron Microsc. Sciences | 15710 (16%) | |
| Penicillin/ Streptomycin | Gibco | 15070 (100x) | |
| Percoll | GE Healthcare Biosciences | 17 0891 01 (1 Liter) | |
| RELMα antibody | Peprotech | 500-P214 (50μg) | |
| Surgical Thread | Surgical Specialties Corp. | SP118 (2.0 USP 100yds) | |
| Syringe Needle | BD Vacutainer Lab. Med. | 305143 (23g, 3/4 inch) | |
| USA Standard Testing Sieve | W.S. Tyler, Inc. | 150μm, 0.0059" ASTME-11, Spec. No. 100 | |
| Xylazine | Butler | 20mg/mL |
References
- World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. WHO Technical Report Series 912. , (2002).
- Pearce, E. J., MacDonald, A. S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2, 499-511 (2002).
- Sandler, N. G. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and th2 responses in tissue repair. J. Immunol. 171, 3655-3667 (2003).
- Perrigoue, J. G. IL-31-IL-31R interactions negatively regulate type 2 inflammation in the lung. J. Exp. Med. 204, 481-487 (2007).
- Sandor, M., Weinstock, J. V., Wynn, T. A. Granulomas in schistosome and mycobacterial infections: a model of local immune responses. Trends Immunol. 24, 44-52 (2003).
- Nair, M. G. Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J. Exp. Med. 206, 937-952 (2009).
- Dalton, J. P. A method for the isolation of schistosome eggs and miracidia free of contaminating host tissues. Parasitology. 115, 29-32 (1997).
- Lewis, F. A. Large-scale laboratory maintenance of Schistosoma mansoni, with observations on three schistosome/snail host combinations. J. Parasitol. 72, 813-829 (1986).
- Tilney, N. L. Patterns of lymphatic drainage in the adult laboratory rat. J. Anat. 109 (Pt. 3), 369-383 (1971).
- Lewis, F., Coligan, J. E. Schistosomiasis. Current protocols in immunology. Chapter 19, Unit 19 (2001).
- Kaplan, M. H. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J. Immunol. 160, 1850-1856 (1998).
- Wynn, T. A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 4, 583-594 (2004).
- Cheever, A. W. Variation of hepatic fibrosis and granuloma size among mouse strains infected with Schistosoma mansoni. Am. J. Trop. Med. Hyg. 37, 85-97 (1987).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved