A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Models of Bone Metastasis
In This Article
Summary
Animal models are frequently utilized to study cancer metastasis to bone. In this protocol we will describe two common methods of tumor inoculation for bone metastasis studies and briefly describe some of the analyses utilized to monitor and quantify these models.
Abstract
Bone metastases are a common occurrence in several malignancies, including breast, prostate, and lung. Once established in bone, tumors are responsible for significant morbidity and mortality1. Thus, there is a significant need to understand the molecular mechanisms controlling the establishment, growth and activity of tumors in bone. Several in vivo models have been established to study these events and each has specific benefits and limitations. The most commonly used model utilizes intracardiac inoculation of tumor cells directly into the arterial blood supply of athymic (nude) BalbC mice. This procedure can be applied to many different tumor types (including PC-3 prostate cancer, lung carcinoma, and mouse mammary fat pad tumors); however, in this manuscript we will focus on the breast cancer model, MDA-MB-231. In this model we utilize a highly bone-selective clone, originally derived in Dr. Mundy's group in San Antonio2, that has since been transfected for GFP expression and re-cloned by our group3. This clone is a bone metastatic variant with a high rate of osteotropism and very little metastasis to lung, liver, or adrenal glands. While intracardiac injections are most commonly used for studies of bone metastasis2, in certain instances intratibial4 or mammary fat pad injections are more appropriate. Intracardiac injections are typically performed when using human tumor cells with the goal of monitoring later stages of metastasis, specifically the ability of cancer cells to arrest in bone, survive, proliferate, and establish tumors that develop into cancer-induced bone disease. Intratibial injections are performed if focusing on the relationship of cancer cells and bone after a tumor has metastasized to bone, which correlates roughly to established metastatic bone disease. Neither of these models recapitulates early steps in the metastatic process prior to embolism and entry of tumor cells into the circulation. If monitoring primary tumor growth or metastasis from the primary site to bone, then mammary fat pad inoculations are usually preferred; however, very few tumor cell lines will consistently metastasize to bone from the primary site, with 4T1 bone-preferential clones, a mouse mammary carcinoma, being the exception 5,6.
This manuscript details inoculation procedures and highlights key steps in post inoculation analyses. Specifically, it includes cell culture, tumor cell inoculation procedures for intracardiac and intratibial inoculations, as well as brief information regarding weekly monitoring by x-ray, fluorescence and histomorphometric analyses.
Protocol
1. Cell Maintenance
- Many bone metastatic subclones of MDA-MB-231 exist that have variable propensity for bone colonization and growth. For our experiments we use a clone derived from the GFP-tagged MDA-MB-231 developed at UTHSCA 2. This particular sub-clone will form visible osteolytic bone lesions at approximately 3 weeks post inoculation, with sacrifice at 4 weeks.
- Maintain cells in DMEM containing 10% FBS and 0.1 mg/ml penicillin-streptomycin at 37 °C in 5-8% CO27.
- Passage cells at 80-90% confluence and re-plate at a 1:10 dilution (this varies depending on cell type and the size plate. For MDA-MB-231 cells in a T-75 this is approximately 6x105), which is approximately every 72 hr.
- MDA-MB-231 cells maintain a generally fibroblastic appearance with numerous pseudopodia. Any rounding is indicative of overcrowding or other undesirable stress, and should be avoided since this may affect metastatic potential in vivo.
- Ideally, cells should be maintained in culture only briefly before inoculation (we recommend less than 1 wk or until you have enough cells for the experiment), and a new vial should be thawed if rounding or changes in cell growth rate occur.
2. Cell Preparation
- Trypsinize cells at 80-90% confluence with 0.15% Trypsin/EDTA (dilute 0.5% Gibco Trypsin EDTA 1:2 in PBS). This concentration allows for fast detachment (<2 min) and reduces clumping of the cells.
- Immediately remove cells from plate with 10 ml ice-cold DMEM containing 10% FBS, pipette into 15 ml conical tube and centrifuge at 200 x g for 5 min.
- Resuspend pellet in a 50 ml conical tube in 25 ml ice-cold PBS (without Ca and Mg) and count.
- Centrifuge to re-pellet, and resuspend at 106 cells per ml (intracardiac) or at 250,000 cells/10 μl (intratibial) in ice-cold PBS. The temperature of PBS is important to prevent clumping of cells and subsequent embolism after injection. The cell suspension should remain on ice while performing injections and will be viable and remain un-clumped for 30 min. While the exact number of plates required will vary from investigator to investigator, we generally use 1 T-75 for between 8-10 mice.
3. Intracardiac Inoculation
- 4-6 week old female (Foxn nu-/-: Harlan) mice are used for these experiments, though other immune-compromised mice (RAG1-/-, RAG2-/-, SCID, XID, and etc.) can also be used with human cancer cell experiments.
- The nude mice should be fed Teklad 2920x diet 5-10 days before injection to decrease mortality from the injection procedure, and reduce background fluorescence in imaging procedures (since the food is alfalfa free).
- If using mice other than nu/nu, remove hair by shaving or using Nair (in our experience Nair works better) on ventral abdominal area before beginning procedure.
- Anesthetize mouse in an isolation chamber using an isoflurane vaporizer (2.5% Iso: 2-3 L/min O2) or other preferred anesthesia (in our experience isoflurane is the best tolerated for intracardiac inoculations).
- Move one mouse at a time to the nose cone of the anesthesia machine inside a stainless steel ventilated hood.
- Position each mouse on its back with chest facing up.
- Wash the chest with a 10% povidone/iodine swab/solution followed by 70% ethanol, repeating 2 times.
- Mark mouse's chest for injection (using a sterile marker or marking slightly to the side to keep the injection site sterile). We use a mark location midway between the sternal notch and top of xyphoid process, and slightly left (anatomical) of the sternum. Draw a small bubble of air into syringe to create space between the plunger and meniscus (important for seeing cardiac pulse) of a 300 μl 28 g ½ insulin syringe, and draw up 100 μl of cells.
- Keep needle upright. While holding skin of mouse taut with other hand, insert needle.
- Successful insertion into left cardiac ventricle should result in a distinct bright red pulse of blood in the syringe. At this depth, carefully depress plunger of syringe (100 μl volume), without significant movement of the needle to avoid puncturing the heart or spilling cells into the chest cavity.
- After cells are injected, pull up slightly on plunger to create a small amount of negative pressure to reduce dripping of cells into the chest cavity.
- Pull needle directly out of chest while being sure to avoid tilting the needle during removal, which can tear the lining of the heart and cause bleeding.
- Apply gentle pressure to the chest over the injection site to reduce bleeding.
- Remove mouse from the nose cone and continue to apply light pressure for about 1 min.
- Move mouse to heating pad until fully conscious.
- If mouse begins to spin or twitch after recovery, the likely cause is an embolism. Inject mouse intraperitonally with 80-120 mg/kg of ketamine to increase blood flow and cardiac output while decreasing vascular resistance. This should help the mouse pass the clot. After this occurs, it is best to change cell preparations, as it indicates that the cells are clumping. We change cell preparations after approximately 8 mice, or 30 min.
4. Intratibial
- Inject mouse with Buprenex (0.1 mg/kg) or similar approved analgesic immediately before procedure.
- Anesthetize mouse using isoflurane, then move to nose cone and maintain anesthesia.
- Clean both legs with 10% povidone/iodine swab/solution, followed by ethanol, repeating 2 times. Depilate legs (with Nair or similar product) prior to cleaning if fur present.
- Gently grasp lateral malleolus, medial malleolus, and lower half of tibia with forefinger and thumb, then bend leg (combination of flexion and lateral rotation, such that the knee is visible and accessible.
- Wet the skin with 70% EtOH to increase visibility of underlying patellar ligament, which should be visible as a distinct, thick, white line. While firmly grasping ankle/leg of mouse insert 28g ½ needle under patella, through the middle of patellar ligament, and into the anterior intercondylar area in top of tibia.
- When inserting needle into tibia, guide carefully through growth plate using steady, firm pressure with slight drilling action.
- Upon penetration of tibial growth plate, the needle will encounter markedly less resistance.
- Use a gentle, lateral movement of needle to ensure needle is in tibia and through the growth plate. Movement will be limited if needle is in proper place within tibia.
- Slowly depress plunger to inject 10 μl of cell solution. Little to no resistance should be felt at this point.
- Slowly extract needle.
- Following the same procedure, proceed to next leg to inject 10 μl of PBS.
- Remove mouse from anesthesia and keep on heating pad until recovered.
- Monitor the mice over the next 24 hr and inject with additional Buprenex every 12 hr if animals continue to show signs of distress.
5. Imaging Time Course
- Luciferase Imaging: Where applicable, luciferase imaging should be utilized beginning at 7 days post-tumor cell inoculation (though in most bone metastasis models, detectable closer to day 14). Mouse is injected intraperitoneally with 150 mg/kg luciferin, anesthetized, and imaged 8 min after injection using IVIS equipment 8.
- GFP imaging: Typically GFP-expressing tumors begin to be detectable around day 10-14 for intratibial injections and 16-21 for intracardiac experiments using Maestro imaging equipment (CRi) 9. Briefly, mice are anesthetized with isoflurane (3%) and maintained under anesthesia through a nose cone in the Maestro imaging chamber. For our eGFP-expressing cells we use the blue filter set (498 excitation/515 emission for eGFP) at 500 ms exposure in 10 nm steps.
- Faxitron (X-ray): X-rays are taken weekly starting between day 7 to 14. Lesions will be visible in the intracardiac model at day 21 and a week earlier in the intratibial injections3. Our radiographic data for ex vivo and in vivo was acquired using a Faxitron LX-60 at 35 kV for 8 seconds of exposure.
- Other Imaging: Imaging modalities will be entirely based upon the specific research. In addition to the above methods, live animal imaging based on 3D micro-computed tomography (μCT), micro-positron emission tomography (μPET), and other modalities 10,11 can provide valuable information.
6. Mouse Sacrifice
- Each model has a slightly different time course. With MDA-MB-231 cells mice usually become cachectic or paraplegic between 21 and 35 days and usually around day 28 (IT injected mice between 21-28 days).
- Once several mice develop lesions that begin to break through the cortical bone, become paraplegic, or lose between 10-20% of body weight, sacrifice all mice in study using ketamine/xylazine overdose with cervical dislocation (or other IACUC approved method).
- Remove bones needed for further study and store in buffered 4% formalin.
- Mice with chest tumors are a sign of a missed injection and should be excluded from the study.
7. Ex Vivo Analysis
- μCT: scanning in 70% EtOH allows for structural and histological parameters to be measured on the same bone specimen.
- Contour and analyze according to individual experimental design and available equipment10. Our data was obtained with a Scanco μCT 40 using 12 μM resolution.
- Histomorphometry: Process specimens using preferred histological methods and stain with Hematoxylin/Eosin7
8. Representative Results
Following intracardiac or intratibial inoculation of tumor cells (Figure 1) mice are monitored weekly by radiography and fluorescence (or luminescence). Lesions normally become apparent by x-ray between week 2 and 3, while they may be visible by fluorescence and luminescence as early as week 2, depending on the model (Figure 2). Lesions are visible as dark holes in the bone, and become more pronounced with time (Figure 3). Lesions were quantified using Metamorph analysis of radiographs and fluorescence was quantified using Maestro (CRi) imaging acquisition and analysis software. As lesions begin to grow, mice frequently become cachectic. Sacrifice is required when mice have lost between 10-20% of their initial body weight. The condition of the mice can change rapidly and must be monitored multiple times during the day. Between 3-4 weeks mice began to drag one or both hind limbs and the entire study was sacrificed. Images were taken prior to sacrifice. After anesthesia overdose, blood was collected to measure markers of bone turnover or other factors. After cervical dislocation, the skin was stripped from the mouse and bones excised and cleaned for ex vivo imaging (Figure 3) which will allow visualization of tumor foci at least 7 days earlier than in vivo imaging techniques. Once imaging was completed bones were placed in labeled cassettes and stored in formalin for fixation and further histological processing or μCT analysis. Histomorphometry was performed on the samples to indicate the tumor burden and BV/TV after μCT was performed on a tibia to quantify the amount of bone destruction (Figure 4).
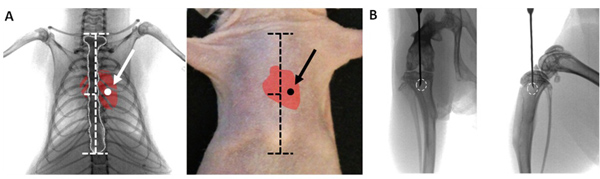
Figure 1. Proper placement of injection. Ventral radiographical view (A) of adult mouse thorax showing proper placement of needle in the 4th intercostal space and into the left ventricle for intracardiac inoculation. Anatomical landmarks are shown with horizontal dashed lines on the sternum (outlined in white). On the mouse's skin the sternal notch and xyphoid process serve as landmarks, and the needle is inserted slightly left of the sternum. Ventral radiographical view (B)of adult mouse leg showing proper placement of needle in the proximal tibia, aligned between the condyles. From a lateral perspective the tip of needle is placed behind the tibial tuberosity and deep to the epiphyseal plate. Ideal location for injection is shown by white circle.

Figure 2. Time course of bone metastasis models. Progression of cancer-induced bone disease shown with x-rays and GFP+ fluorescent images of the tumors in vivo from intratibial injections (A) and intracardiac injections (B).
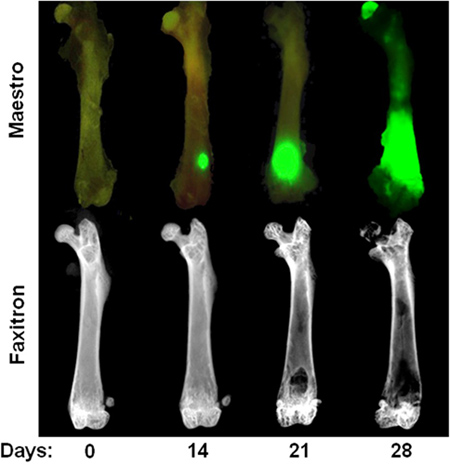
Figure 3. Time course of intracardiac MDA-MB-231 model. Top Row: Ex vivo image showing GFP fluorescence at day 0, 14, 21, and 28 after intracardiac injection of MDA-MB-231. Note that tumor foci at day 14 are only visible in ex vivo after soft tissue removal. Bottom row: Ex vivo radiographic images of the same femora illustrating osteolytic bone lesions. Note that bone destruction is not usually visible until 3 weeks after cardiac injection.
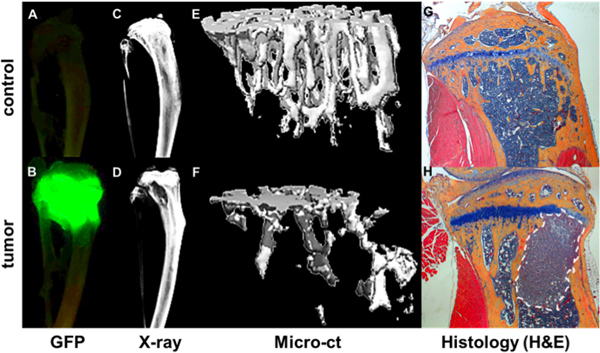
Figure 4. Quantification of bone metastatic progression. GFP+ tumor as visualized by Maestro imaging ex vivo of tibiae of a non-tumor bearing mouse (A) and a mouse 4 weeks after intracardiac injection of a GFP expressing MDA-MB-231 subclone (B). Tumor foci correlate with areas of bone destruction as shown by Faxitron (D) compared a bone without tumor (C). Bone structural and mineral data from microCT measurements is rendered as 3d images of a healthy bone (E) and a tumor bearing bone (F) where areas of bone destruction are shown as void area. Further bone and tumor detail is seen in corresponding H&E stained histological sections (G & H).
Discussion
Studies of tumor-induced bone disease rely on several animal models of which the two most commonly used are intracardiac and intratibial inoculations. In many cases intracardiac is the best option for studying metastasis to bone; however, it does not represent the full metastatic process, and therefore mammary fat pad or other orthotopic injections should ideally be used toward that end. Tumor cells do not metastasize readily from the primary site in most studies using human cells, and if so, they primarily metastasize t...
Disclosures
No conflicts of interest declared.
Acknowledgements
The authors acknowledge the following funding sources: P01CA040035 (FE/JAS) and VA Career Development Award (JAS).
Materials
| Name | Company | Catalog Number | Comments |
| Name of the reagent | Company | Catalogue number | |
| MDA-MB-231 human breast cancer cells | Originally ATCC-Internally cloned and selected | ||
| DMEM | GIBCO | 11885 | |
| PBS | Mediatech Inc | 21-040-CV | |
| FBS | Atlas Biological | F-0050a | |
| Trypsin | GIBCO | 15400 | |
| Faxitron | Faxitron | ||
| Maestro | CRi | ||
| μCT scanner | Scanco | ||
| Athymic Nude Mice | Harlan | Fox nu -/- | |
| Insulin Syringes (300 μl, 28.5 g) | Becton Dickinson | 309300 | |
| IVIS | Caliper | ||
| Irradiated Rodent Diet | Teklad | 2920x |
References
- Sterling, J. A., Edwards, J. R., Martin, T. J., Mundy, G. R. Advances in the biology of bone metastasis: how the skeleton affects tumor behavior. Bone. 48, 6-15 (2011).
- Yoneda, T., Sasaki, A., Mundy, G. R. Osteolytic bone metastasis in breast cancer. Breast Cancer Res. Treat. 32, 73-84 (1994).
- Johnson, R. W. TGF-beta promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical Hedgehog signaling. Cancer Res. 71, 822-831 (2011).
- Li, X. Loss of TGF-beta Responsiveness in Prostate Stromal Cells Alters Chemokine Levels and Facilitates the Development of Mixed Osteoblastic/Osteolytic Bone Lesions. Mol. Cancer Res. , (2012).
- Yoneda, T. Actions of bisphosphonate on bone metastasis in animal models of breast carcinoma. Cancer. 88, 2979-2988 (2000).
- Rose, A. A. Osteoactivin promotes breast cancer metastasis to bone. Mol. Cancer Res. 5, 1001-1014 (2007).
- Sterling, J. A. The hedgehog signaling molecule Gli2 induces parathyroid hormone-related peptide expression and osteolysis in metastatic human breast cancer cells. Cancer Res. 66, 7548-7553 (2006).
- Lu, X. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell. 20, 701-714 (2011).
- Oyajobi, B. O. Detection of myeloma in skeleton of mice by whole-body optical fluorescence imaging. Mol. Cancer Ther. 6, 1701-1708 (2007).
- Johnson, L. C. Longitudinal live animal micro-CT allows for quantitative analysis of tumor-induced bone destruction. Bone. 48, 141-151 (2011).
- Sterling, J., Johnson, R. . Imaging Techniques for the Detection and Examination of Breast Cancer Metastasis to Bone. 46, (2011).
- Steinbauer, M. GFP-transfected tumor cells are useful in examining early metastasis in vivo, but immune reaction precludes long-term tumor development studies in immunocompetent mice. Clin. Exp. Metastasis. 20, 135-141 (2003).
- Oyajobi, B. O. Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood. 102, 311-319 (2003).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved