Method Article
Engineering 3D Cellularized Collagen Gels for Vascular Tissue Regeneration
In This Article
Summary
In this work, we present a technique for the rapid fabrication of living vascular tissues by direct culturing of collagen, smooth muscle cells and endothelial cells. In addition, a new protocol for the mechanical characterization of engineered vascular tissues is described.
Abstract
Synthetic materials are known to initiate clinical complications such as inflammation, stenosis, and infections when implanted as vascular substitutes. Collagen has been extensively used for a wide range of biomedical applications and is considered a valid alternative to synthetic materials due to its inherent biocompatibility (i.e., low antigenicity, inflammation, and cytotoxic responses). However, the limited mechanical properties and the related low hand-ability of collagen gels have hampered their use as scaffold materials for vascular tissue engineering. Therefore, the rationale behind this work was first to engineer cellularized collagen gels into a tubular-shaped geometry and second to enhance smooth muscle cells driven reorganization of collagen matrix to obtain tissues stiff enough to be handled.
The strategy described here is based on the direct assembling of collagen and smooth muscle cells (construct) in a 3D cylindrical geometry with the use of a molding technique. This process requires a maturation period, during which the constructs are cultured in a bioreactor under static conditions (without applied external dynamic mechanical constraints) for 1 or 2 weeks. The “static bioreactor” provides a monitored and controlled sterile environment (pH, temperature, gas exchange, nutrient supply and waste removal) to the constructs. During culture period, thickness measurements were performed to evaluate the cells-driven remodeling of the collagen matrix, and glucose consumption and lactate production rates were measured to monitor the cells metabolic activity. Finally, mechanical and viscoelastic properties were assessed for the resulting tubular constructs. To this end, specific protocols and a focused know-how (manipulation, gripping, working in hydrated environment, and so on) were developed to characterize the engineered tissues.
Introduction
Vascular tissue engineering envisions different strategies aimed at the fabrication of engineered vessels, including grafts based on synthetic scaffolds, cell sheet-based tissue-engineered blood vessels (TEBVs), and extracellular matrix (ECM) components-based TEBVs. Among these approaches, synthetic polymers exhibit good mechanical properties, but share a common drawback as they lack bioactivity1. The cell sheet-based method allows the production of engineered vascular substitutes with high mechanical properties, but the time required to produce such grafts is approximately 28 weeks2. Natural biopolymers of the ECM, such as collagen, elastin, fibrin3or a combination thereof, remain the gold standard materials for tissue engineering scaffolds. This is primarily for the reason that these materials possess a generally good biocompatibility while being able to induce functional cellular responses4-5. Among these biopolymers, type I collagen is one of the most abundant and predominant load-bearing protein of the ECM in many tissues such as skin, blood vessels and tendons. Extensive work has been conducted on the mechanical properties of collagen6–8, but there have been only a few studies on cellular remodeling of collagen gels during static maturation. Cellular remodeling refers to the structural modifications of the collagen matrix induced by cells that could affect the stability of the collagen fibrils network9. As a natural scaffold, relatively large quantities of type I collagen can be isolated, sterilized and stored from different sources such as rat-tail tendons10. Understanding cellular interactions with collagen and the related overall mechanical behaviors of the cellularized collagen scaffolds (constructs) is an essential step for the construction of tissues. Collagen-based TEBVs can be processed by directly mixing cells with collagen during gel preparation and further molded into specific shapes such as tubular and planar11. Vascular cells inside the gels proliferate and remodel type I collagen12. Thus, this method bypasses the need for specific macroporosity that represents one of the significant issues in the development of scaffolds for tissue engineering applications. However, the major drawbacks of collagen gels are their low mechanical properties compared to synthetic materials13.
In this study, a viable tissue with homogenous distribution of cells was engineered by direct mixing of collagen with cells in a one-step process. “Static bioreactors” were used for the 1 or 2 weeks of static maturation of the cellularized collagen gels (without applied external dynamic mechanical constraints). During the culture, collagen matrix remodeling occurred, thus providing structural reinforcement to the constructs. Furthermore, these constructs were ready to be transferred to a rotating-wall bioreactor and a homogenous endothelium was achieved. In addition, in this work a specific mechanical testing protocol is also proposed to provide an appropriate novel approach in characterizing the mechanical properties of tubular soft tissues.
In summary, this work presents a method for the in vitro rapid fabrication and maturation of vascular tissues that are strong enough to be handled not only for biological and mechanical characterizations, but also for further mechanical conditioning in a dynamic bioreactor, which is considered a crucial step in the regeneration of tissues.
Protocol
1. Fabrication and Assembly of the Static Bioreactor
- Fabrication of the Reservoir
- Prepare 50 ml centrifuge tubes as a culture medium reservoir for the bioreactor.
- Make two ports by drilling two 5 mm diameters holes at 20 mm from the bottom and the top of the reservoir, respectively. Then insert two luer fittings in 5 mm length silicone tubes. Press-fit these luer fittings through the holes, and seal all the connections with medical grade silicone glue.
- Insert a 0.22 µm filter into the upper port of the reservoir (Figure 1A).
- Insert a luer septum into the lower port of the reservoir (Figure 1A).
- Mandrel-cap Assembly
- Drill a 4.5 mm diameter hole at the center of the ventilated cap of the reservoir tube without damaging the filter membrane that covers the aerating holes.
- Prepare a stir bar (diameter = 4.5 mm, length = 100 mm) as a mandrel for the construct.
- Prepare two silicone conical stoppers (length = 10 mm, middle hole diameter = 4.5 mm).
- Assemble the mandrel and the cap (mandrel-cap complex) as described in Figure 1B.
- Press-fit the mandrel into the hole. Insert the 2 stoppers over the mandrel so that the cap is fitted between them. Adjust the position of the mandrel so that its useful length is 78 mm.
- Apply a primer and then medical grade silicone glue to the surfaces that will be in contact before joining the cap and the silicone conical stoppers together. Remove the excess glue on the cap.
- Let it dry at room temperature for 1-3 days.
- Fabrication of the Gauze-grips
- Prepare 3 silicone tubes (tube 1: inner diameter = 6.4 mm, length = 5 mm; tube 2: diameter = 6.4 mm, length = 10 mm, and tube 3: diameter = 3.1 mm, length = 12 mm).
- Assemble the gauze-grips as described in Figure 1C.
- Cut tube 1 longitudinally, and open it over tube 2. Stick them together with the silicone glue.
- Cut sterile surgical gauze to 5 cm x 7 cm sheet, and then roll tightly the gauze over the tube 3 along the longest side of the gauze. Insert the tube 1-tube 2 complex over the gauze.
- Add silicone glue to stick together the gauze, the tube 1-tube 2 complex and the tube 3. Cut the gauze at a length of 8 mm.
- Assembly and Sterilization
- Assemble the mandrel-cap complex and the gauze-grips as described in Figure 2.
- Coat the mandrel with medical grade grease (Figure 2A). Place the gauze-grips over the mandrel (Figure 2B). Distance the grips at fixed value of 35 mm from each other.
- Prepare a tubular mold by removing the bottom part of a 10 ml syringe using a table saw (final length = 8 mm) (Figure 2B).
- Insert the mold over the gauze grips-equipped mandrel-cap assembly (housing-mold complex), snap-fitting the mold on the silicone stopper (Figure 2C).
- Autoclave the reservoir and the housing-mold complex.
Note: Be careful to hold on the silicone stopper tightly when inserting the mold to avoid its detachment.
- Assemble the mandrel-cap complex and the gauze-grips as described in Figure 2.
2. Engineering Smooth Muscle Cell Collagen Gel-based Constructs and Static Maturation
- Engineering Constructs
- Expand porcine aortic smooth muscle cells (pSMCs) in 175 cm2 culture flasks filled with 20 ml of complete culture medium consisting of Dulbecco’s Modified Eagle Medium supplemented with 10% (v/v) porcine serum (PS), 10% (v/v) fetal bovine serum (FBS), 1% (v/v) penicillin-streptomycin (pen-strep).
- At ≈90% confluence, detach pSMCs (passage 2-4) by removing the culture medium from the flask of pSMCs, adding 5 ml of trypsin solution (1x in phosphate-buffered saline solution, PBS), and incubating for 10 min (T = 37 °C, 5% CO2, 100% humidity).
- Resuspend the pSMCs at a concentration of 4 x 106 cells/ml in complete culture medium.
- Prepare collagen solution as previously described10.
- Extract and collect collagen bundles from rat-tail tendons in a PBS solution.
- Transfer the collagen fibers subsequently into acetone (5 min), isopropanol 70% (v/v) (5 min) and acetic acid (0.02 N, 48 hr, 4 °C) solutions.
- Blend the viscous solution and freeze at -20 °C for 3 days.
- Lyophilize the frozen solution to obtain collagen sponges.
- Solubilize the collagen sponges into acetic acid solution (0.02 N) at a concentration of 4 g/L and centrifuge at 29,581 g force for 45 min.
- Sterilize the collagen solution through dialysis process against subsequent solutions of acetic acid (0.02 N, 1 hr), chloroform 1% (v/v, 1 hr) and acetic acid (0.02 N, sterile solution changed every 2 days for 1 week).
- Collect the sterile collagen solution (4 g/L) in a sterile cell culture hood.
- Prepare cellularized collagen gels as shown in Figure 3.
- Prepare 50 ml of sterile buffer solution by mixing 35 ml of DMEM (5x), 4 ml of HEPES (1 N), 3 ml of NaOH (1 N) in 8 ml of sterile deionized water.
- Prepare cells and collagen gel mixture in a container placed in ice by mixing 50% (v/v) of sterile collagen solution (4 g/L of acetic acid 0.02 N) with 25% (v/v) of buffer solution and 25% (v/v) of the suspension of pSMCs in complete culture medium.
- Measure the pH of the mixture and ensure that it is between 7.0 and 7.4.
- Pour gently 9 ml of cells-and-collagen mixture into the above mentioned housing/mold complex (step 1.4.3, Figure 3A-B).
- Let it gel at room temperature for 1 hr under the cell culture hood (Figure 3B).
- Maturation in Static Bioreactor
- Remove the mold (Figure 3C) and transfer carefully the construct into the reservoir, containing 35 ml of culture medium (Figure 3D).
- Incubate the construct (T = 37 °C, 5% CO2, 100% humidity) in vertical position for 1 or 2 weeks of static maturation.
- Install a web camera (sealed in order to ensure insulation) inside the incubator in front of the construct.
- Change the culture medium every 2 days by aspirating the old medium from the luer septum port and re-filling the reservoir with an equivalent amount of fresh culture medium.
- Measurement of Thickness and Metabolic Activity of SMCs-collagen Gel-based Constructs During Static Culture
- Place the scanning laser interferometer into the cell culture hood and flip it from the vertical to the horizontal position using a spirit level.
- Transfer the bioreactor into the cell culture hood and remove the construct from the reservoir.
- Transfer the construct (still mounted on the mandrel) into the pathway of the laser beam, and place it strictly orthogonally with respect to the beam axis (as shown in Figure 4).
- Read the value displayed on the screen of the scanning laser interferometer, corresponding to the external diameter of the construct.
- Calculate the wall thickness of the construct based on its external and internal diameter (i.e. the mandrel diameter).
Note: Repeat the steps 2.3.1 to 2.3.5 every hour for the first 12 hr and then every 24 hr. - Use 1 ml of the old culture medium (sampled when changing the culture medium, step 2.2.4) for measuring the lactate and glucose concentrations with the blood gas analyzer.
- Use 1 ml of the fresh culture medium as a baseline level for the glucose and lactate concentrations measurements14.
Note: Repeat the steps 2.3.6 and 2.3.7 every 2 days after culture medium changing.
- Construct Harvesting for Further Mechanical and Biological Ccharacterizations
- After 1 or 2 weeks of static maturation period, transfer the static bioreactor into the cell culture hood.
- Transfer gently the mature construct from its mandrel (Supplemental Video 1) to a 100 mm diameter Petri dish containing 40 ml of fresh culture medium (Figure 5 and Figure 7A).
3. Mechanical Characterization of the Constructs in the Longitudinal and Circumferential Directions
- Install the experimental set-up consisting of the micromechanical tester equipped with a 5 or 10 N load cell and a bath containing PBS at 37 °C to keep the samples at pseudo-physiological conditions (Figure 6).
- Balance the load cell and the extensometer.
Note: Balancing is a function integrated into the micromechanical tester consisting in resetting the displayed extension value and the displayed load value while no sample is mounted onto the machine. This function allows defining the reference for both measurements. - Mounting the tubular constructs onto the mechanical apparatus: longitudinal direction.
Note: Perform longitudinal fatigue tests directly on the entire tubular constructs. Use in-house-built gripping devices to connect the gauze grips of the constructs to the load cell and to the base of the PBS bath.- Mount the tubular construct onto the gripping devices (Figure 7B), following the harvesting procedure (section 2.4).
- Wrap the gripping devices and the gauze grips together with Teflon tape to prevent any slipping of the gauze grips during the test. Mount the sample onto the micromechanical tester (Figure 7C).
- Mounting the ring-shaped constructs onto the mechanical apparatus: circumferential direction.
Note: Perform circumferential fatigue tests on ring-shaped specimens sectioned from the tubular constructs. Use two stainless steel bars as grips to hold the specimens.- Mount the tubular construct onto a plastic pipe as a support marked with 5 mm gaps (Figure 7B), following the harvesting (section 2.4).
- Cut 10 mm rings from the tubular construct.
- Measure the length of the specimen using a vernier caliper for the further analyses.
- Mount the ring-shaped specimen onto the stainless steel bars of the micromechanical tester (Figure 7C). Make sure to place the specimen at the center of the bars.
Note: The plastic pipe in the step 3.4.1 and a cutting system as shown in Figure 7B are used to avoid any damage to the construct during cutting.
- Fatigue test on constructs in the longitudinal or circumferential direction.
- Stretch the construct to its initial gauge length.
- Maintain the construct in this position for 10 min in pseudo-physiological environment.
- Apply 10% cyclic strain of the initial gauge length (30 cycles) to the construct at 5%/sec strain rate.
- Repeat step 3.5.3 at incremental steps of 10% cyclic strain until failure of the sample.
Note: The use of the pseudo-physiological environment requires taking into account the buoyancy and the inertia of the gripping system that affect the measurement of the applied load. - Record the background as follows:
- Move the load frame to the initial gauge length.
- Repeat the steps 3.5.3 and 3.5.4 without any sample mounted, and keeping the gripping devices connected to the load cell (only 1 cycle is required).
4. Luminal Endothelialization of Constructs
Note: After following the harvesting protocol (section 2.4), the constructs withstand handling to be mounted in the rotating-wall bioreactor for the further endothelialization.
- Rotating-wall Bioreactor Design
- Drill a 4.5 mm diameter hole at the center of the ventilated cap of the reservoir tube without damaging the filter membrane that covers the aerating holes.
- Press-fit a mandrel (diameter = 4.5 mm, length = 40 mm) into the hole and fix the mandrel as described in step 1.1.2.
- Prepare two C-shaped silicone support for the construct external diameter = 14 mm; internal diameter = 8 mm).
- Position a rotating motor in one end of the rotating-wall bioreactor and a bearing on other end (Figure 8B).
- Lumen Endothelialization
- Expand human umbilical vein endothelial cells (HUVECs) in 25 cm2 culture flasks with 5 ml of M199 culture medium supplemented with 10% (v/v) PS, 10% (v/v) FBS, 1% (v/v) pen-strep in Petri dish inside an incubator (T = 37 °C, 5% CO2, 100% humidity) till 90% confluence.
- Prepare 1.5 ml of the protein coating solution per construct required for optimal cell adhesion by diluting the concentrate protein mixture to 10.5 ng/ml in serum-free endothelial cell culture medium.
- Measure the length of the construct using a vernier caliper.
- Calculate the luminal volume V and the luminal area A of the construct as: V = Din2 L/4 and A = Din L respectively (where Din is the inner diameter corresponding to the mandrel diameter, and L is the length of the construct).
- Position the construct at the center of the reservoir following the harvesting procedure (section 2.4). Use C-shaped silicone support to fix the construct at both ends to the reservoir (Figure 8A).
- Fill the reservoir with 35 ml of culture medium.
- Fill 75% of the calculated luminal volume of the construct (V) with the protein coating solution prepared in step 4.2.2. Close both of the extremities of the construct to avoid any leakage of the protein coating solution (Figure 8A).
- Assemble the rotating-wall bioreactor system inside the cell culture hood.
- Place the bioreactor in a 37 °C incubator and start the rotation of the bioreactor at 4.02 x 10-5 g force for 1 hr to allow the luminal coating as shown in Figure 8B.
- Open the upper extremity of the construct and aspirate the protein coating solution from the lumen.
- Detach HUVECs (passage 2-3) by removing the culture medium from the flask of HUVECs and adding 3 ml of the trypsin solution (1x in PBS). Incubate for 5 min (T = 37 °C, 5% CO2, 100% humidity).
- Resuspend the HUVECs at a concentration of 4 x 106 cells/ml in supplemented M199 culture medium.
- Inside the cell culture hood, seed HUVECs into the lumen of the construct with a density of 1,000 cells/cm2 15. Close the upper extremities of the construct to avoid any leakage of the HUVECs solution.
- Incubate the constructs (T = 37 °C, 5% CO2, 100% humidity) hosted into the rotating-wall bioreactor (Figure 8B) and culture for 2 days at a constant rotation of 4.02 x 10-5 g force.
- Harvest the construct after 2 days of culture in sterile conditions and prepare it for further biological characterization as described in section 2.4.
Results
This work describes the fabrication of engineered tubular collagen-based constructs containing vascular cells. Already after 1 hr of early gelation, cells-and-collagen mixture was directly assembled in a 3D tubular geometry, with the external diameter equal to the diameter of the corresponding mold (around 14 mm). All along static maturation, measurements revealed the rapid reduction of the external diameter of the tubular cellularized structures, as shown in Table 1. The diameter of the cellularized collagen gels shrunk of about 60% of its initial value after 1 day of static culture, and of almost 85% within 7 days (Supplemental Video 2). SMCs within the constructs are responsible for the observed shrinking and the related mechanical reinforcement, as this phenomenon does not occur in non-cellularized collagen scaffolds. Note that no gradient of any type (thermal, biochemical, mechanical, or others) was applied. The cells-driven compaction resulted in a material with greater collagen density that could be handled and subdued to mechanical solicitations (Supplemental Videos 3 and 4).
To relate the cells-driven remodeling to the overall mechanical and viscoelastic properties, fatigue tests were performed on the constructs (Supplemental Videos 5 and 6). These tests consisted in cycling the constructs (30 times) at different constant strains (10%, 20%, and 30% of initial gauge length) and to record the stress as the response of the constructs to the mechanical solicitation over time. The representative results for one construct are shown in Figure 9. The construct withstood higher stresses in the longitudinal direction (75 kPa) than in the circumferential direction (16 kPa) when subjected to the same strain range (30% strain). Meanwhile, at each cycle, the stress peak value reached for the targeted maximum strain decreased over time. This behavior is typical of the high viscoelastic properties exhibited by these collagen-based constructs.
The biological activity of the cellularized constructs was assessed during static maturation. Hence, metabolic activity of SMCs was evaluated by measuring the glucose consumption and lactate production during static culture. Culture medium was sampled every 2 days and glucose and lactate concentrations were measured using a blood gas analyzer. The constant increase in glucose consumption and lactate production combined to the important shrinking of the constructs, attest the SMCs activity all along static culture (Figure 10).
The increased mechanical stability due to the cell-driven remodeling allowed the manipulation of the constructs and the subsequent endothelialization process. Masson’s trichrome staining performed on the endothelialized constructs showed a highly homogenous endothelium. SMCs exhibited a spindle-like shaped morphology and appeared homogenously dispersed through the wall, while HUVECs appeared well spread in the luminal side (Figure 11).
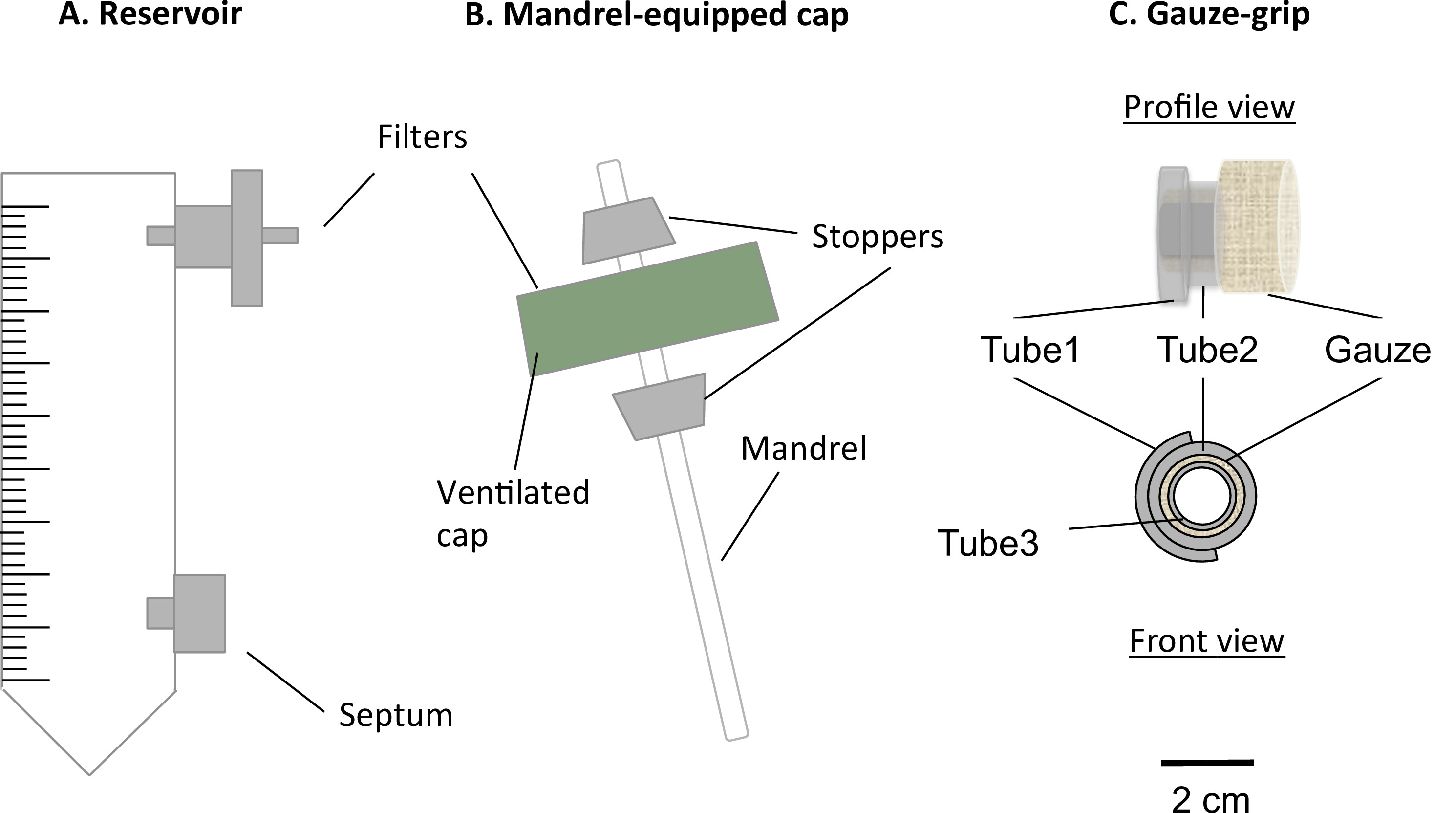
Figure 1: Components of the static bioreactor. The static bioreactor consisted of a modified 50 ml centrifuge tube (A) and a mandrel-equipped cap (B). The tube served as medium reservoir, and was equipped with a port for a 0.22 µm filter, for the gas exchange, and a septum, for the medium sampling and changing. A mandrel present in the ventilated cap allowed the fabrication of constructs in tubular shape. The gauze-grips (C) were designed and fabricated to support the gelation of the constructs over the mandrel. Moreover, these grips allowed the constructs to be handled after the static maturation and to be fixed to the mechanical apparatus. The external diameter of the mandrel was 4.7 mm.
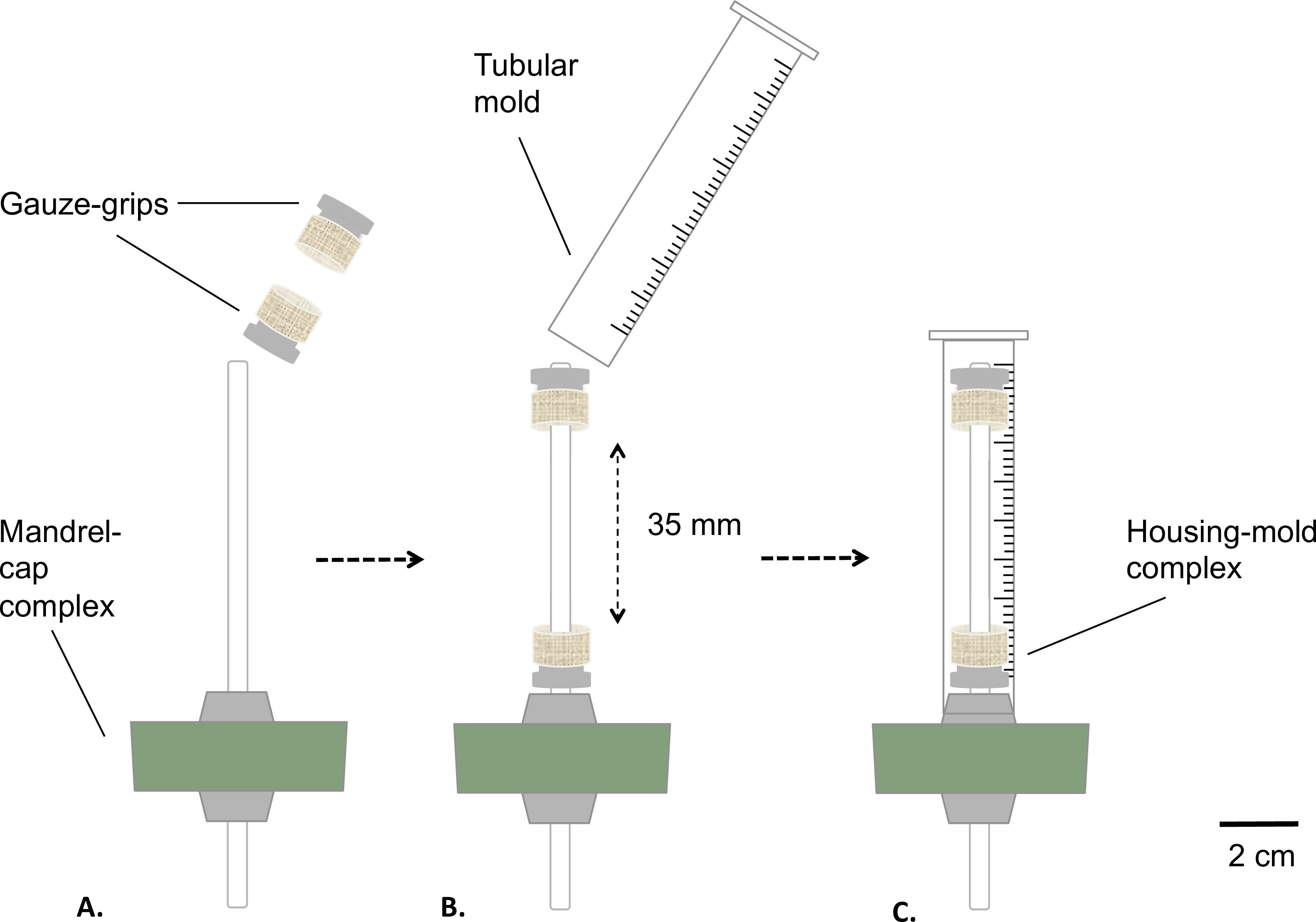
Figure 2: Assembling of the static bioreactor. Assembling phases of the bioreactor before the sterilization. The gauze-grips were mounted on the mandrel (A) at a fixed distance. A mold was inserted (B) and tightly fixed to the silicone stopper (C). The external diameter of the mandrel was 4.7 mm.
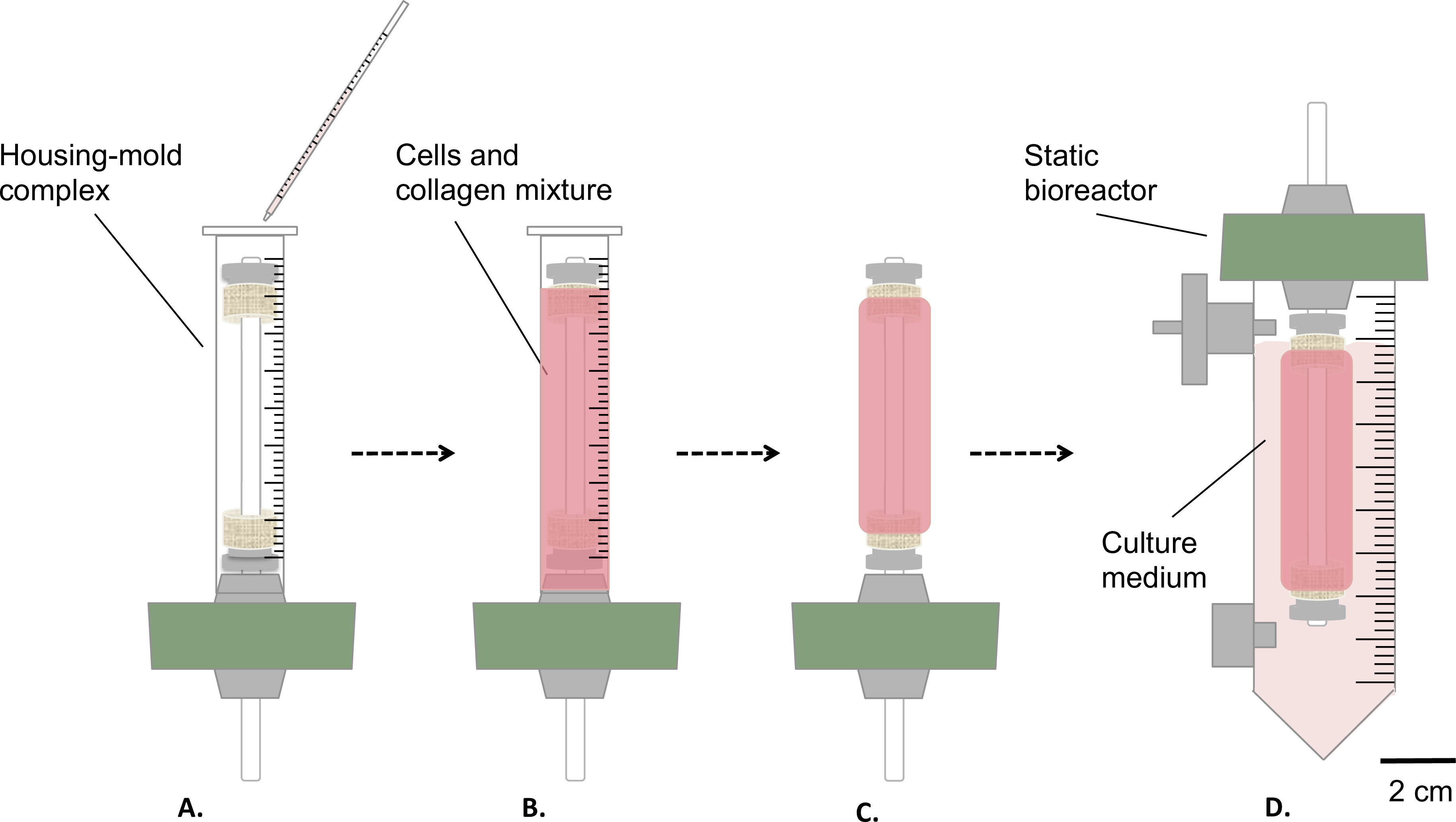
Figure 3: Fabrication of the constructs in sterile conditions. The cells and collagen mixture was poured into the housing-mold complex (A), and let gel for 1 hr at room temperature (B). Afterwards, the mold was removed (C), the static bioreactor was assembled (D) and transferred inside a reservoir for the static maturation of the construct in incubator (T = 37 °C, 5% CO2, 100% humidity). The external diameter of the mandrel was 4.7 mm.
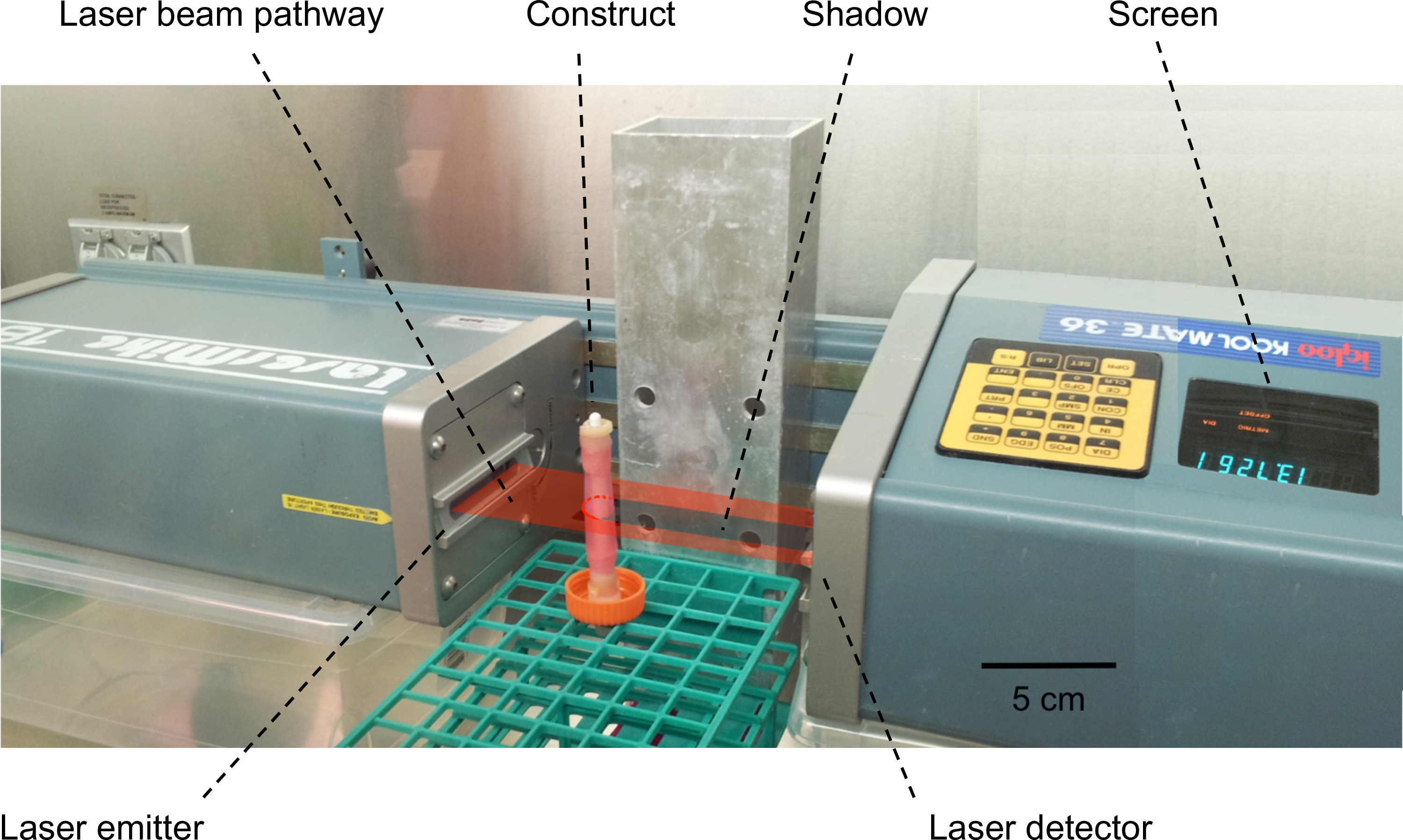
Figure 4: Measurement of the thickness/external diameter of the constructs. A laser scanning interferometer was used to perform the measurement of the external diameters of the constructs. The construct was placed into the pathway of the laser beam and generated a shadow. The width of the shadow, corresponding to the external diameter of the construct, was then measured and displayed on the screen.
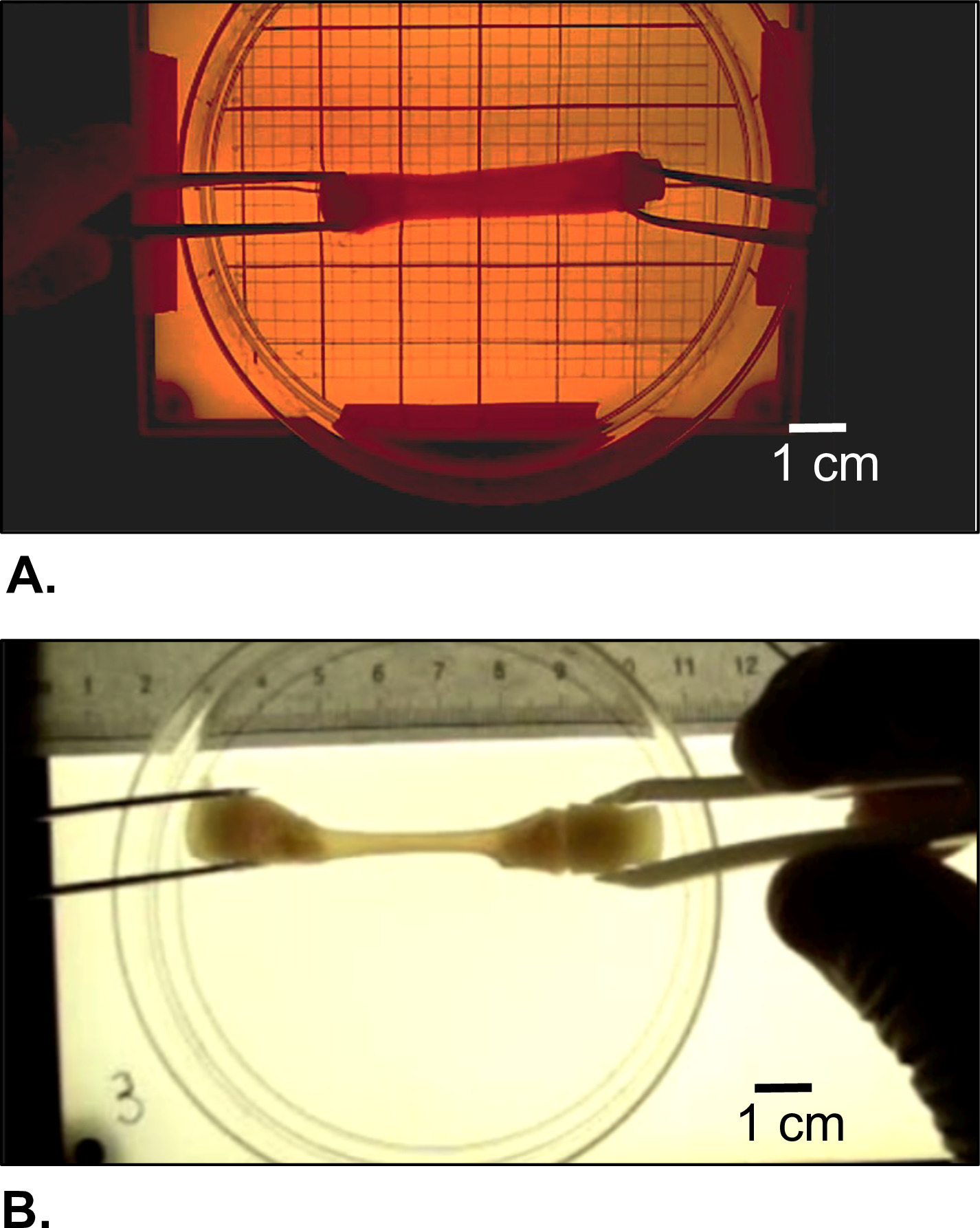
Figure 5: Morphological appearance of the harvested construct. (A) Right after gelation and (B) after cells-driven remodeling during static maturation for 2 weeks.
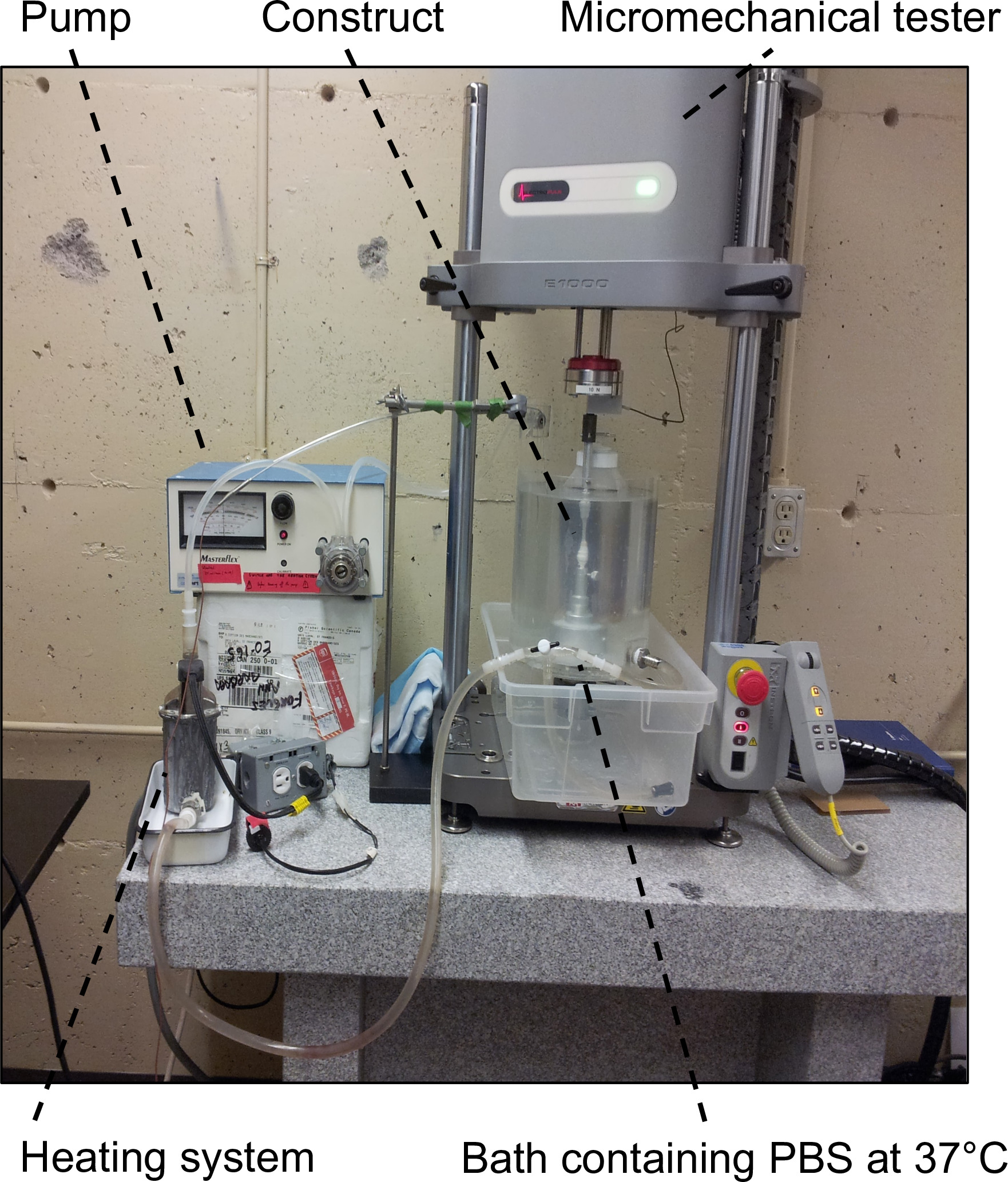
Figure 6: Experimental set-up for mechanical characterizations. It consisted of the micromechanical tester equipped with a 5 or 10 N load cell and a bath containing PBS at 37 °C to keep the samples in pseudo-physiological conditions.
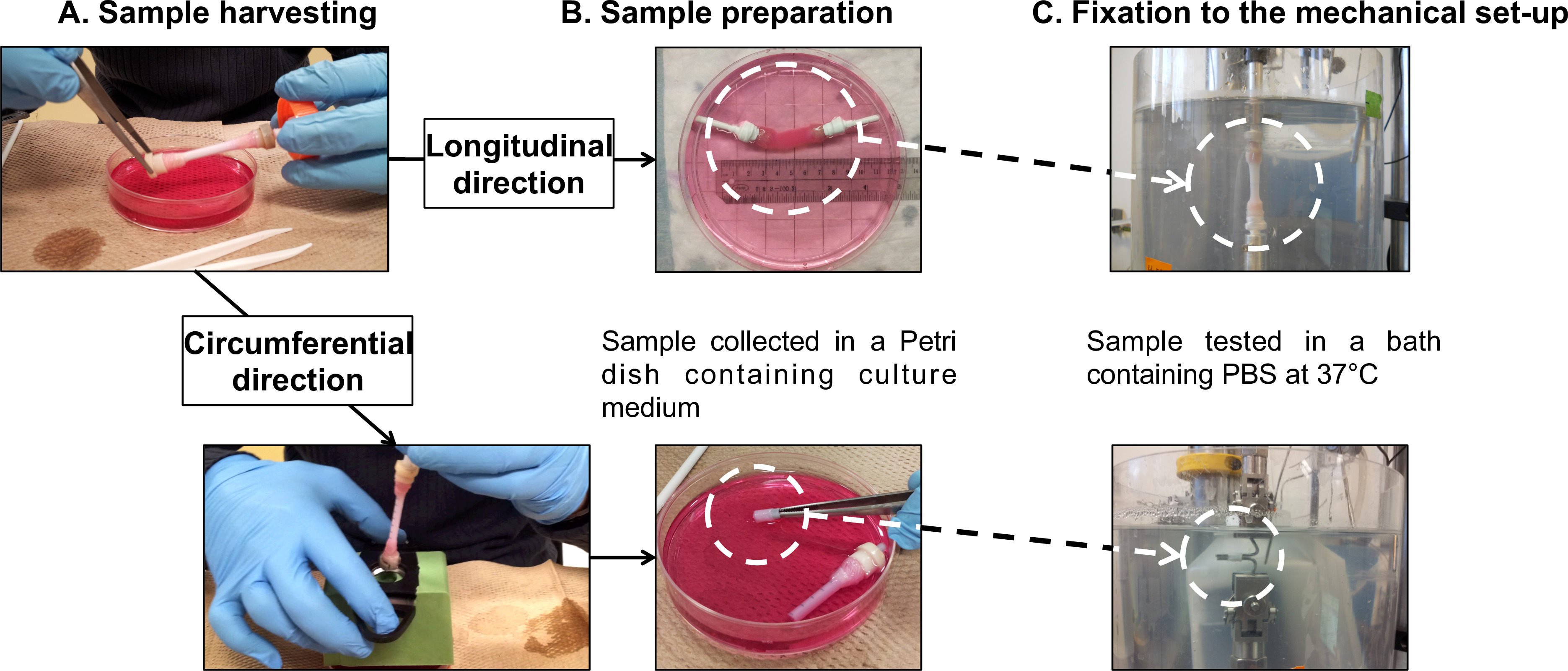
Figure 7: Sample preparation for mechanical characterizations. Sample harvesting (A) and preparation (B) for fatigue tests performed in the longitudinal and the circumferential directions (C). The external diameter of the mandrel was 4.7 mm.
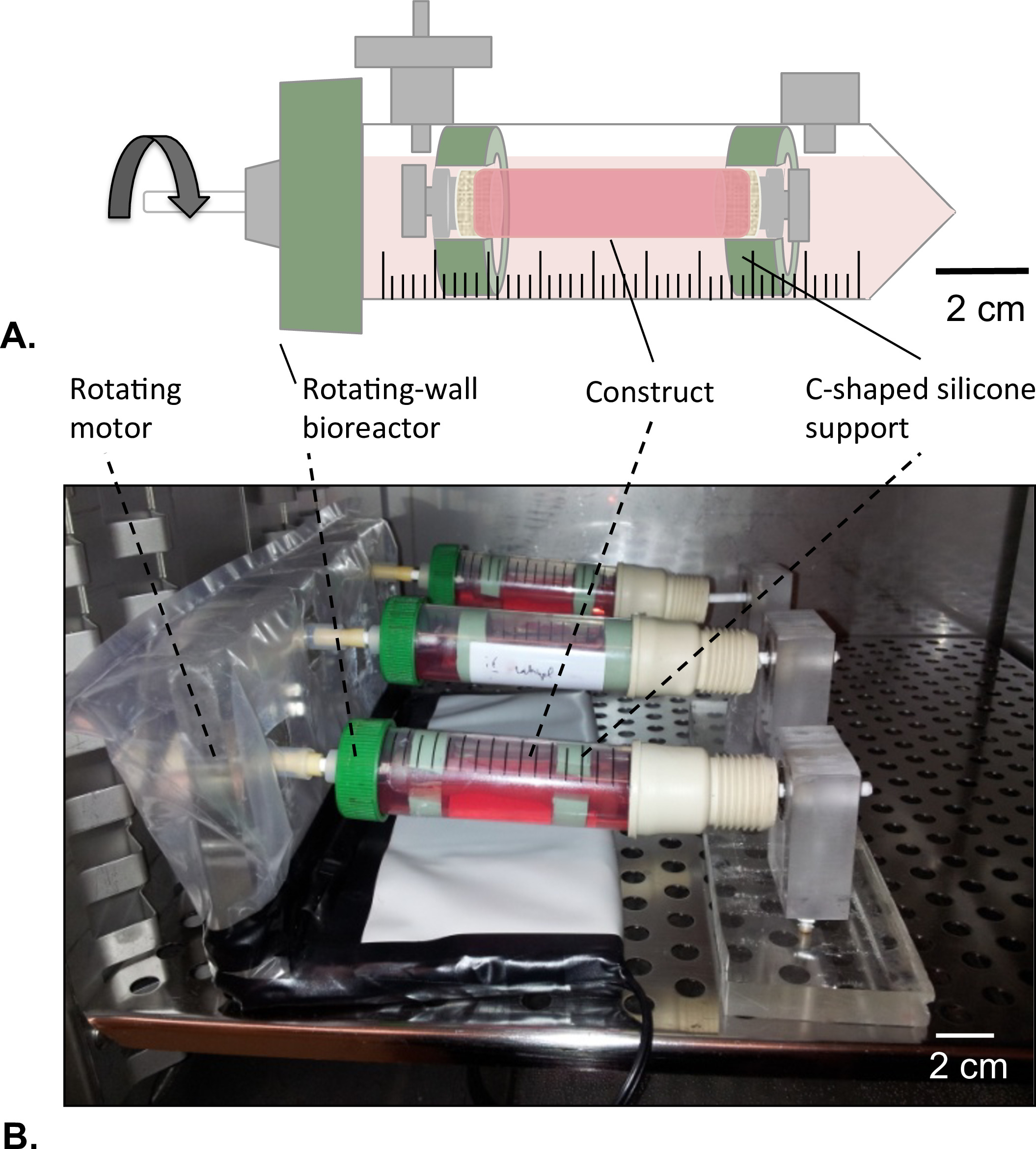
Figure 8: Rotating-wall bioreactor. (A) The tubular constructs were assembled in the center of the reservoir with the help of c-shaped silicone support. Both of the extremities of the construct were closed to avoid any leakage of the HUVECs solution. (B) The constructs were cultured in incubator (T = 37 °C, 5% CO2, 100% humidity) in rotation at 4.02 x 10-5 g force for 2 days.
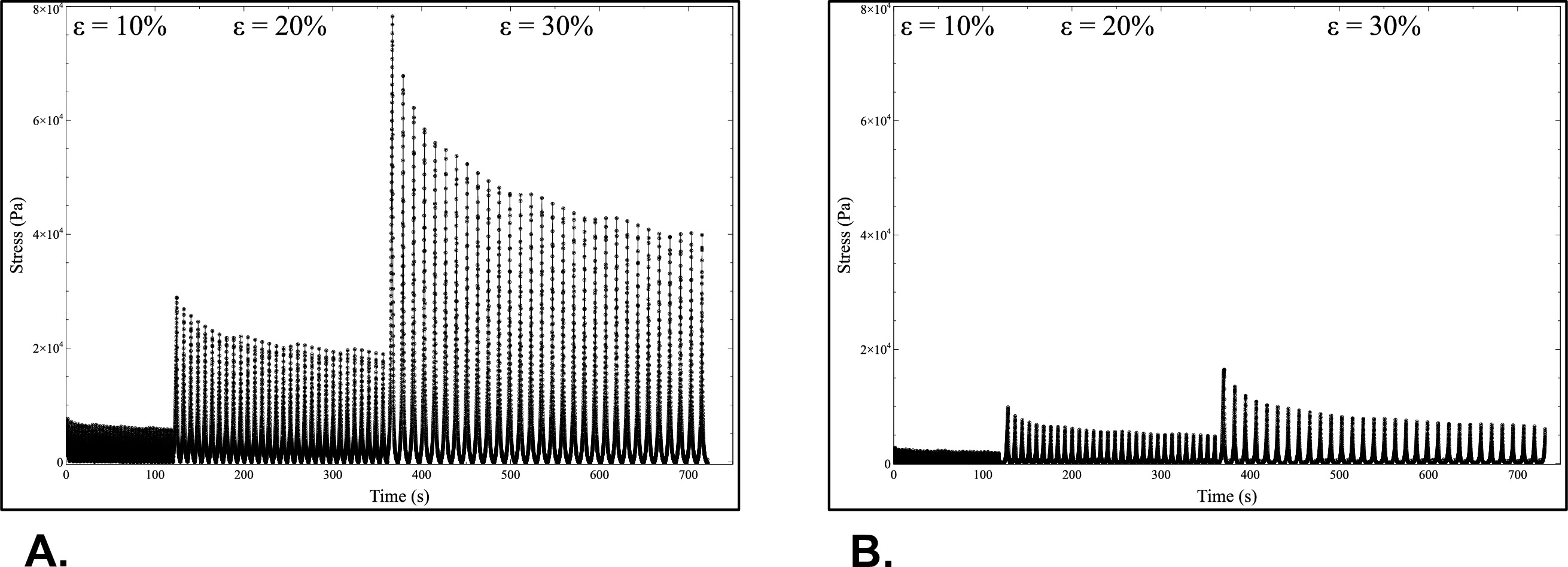
Figure 9: Mechanical characterizations. Results of fatigue tests performed on constructs in longitudinal (A) and circumferential (B) directions after cell-driven remodeling. Please click here to view a larger version of this figure.
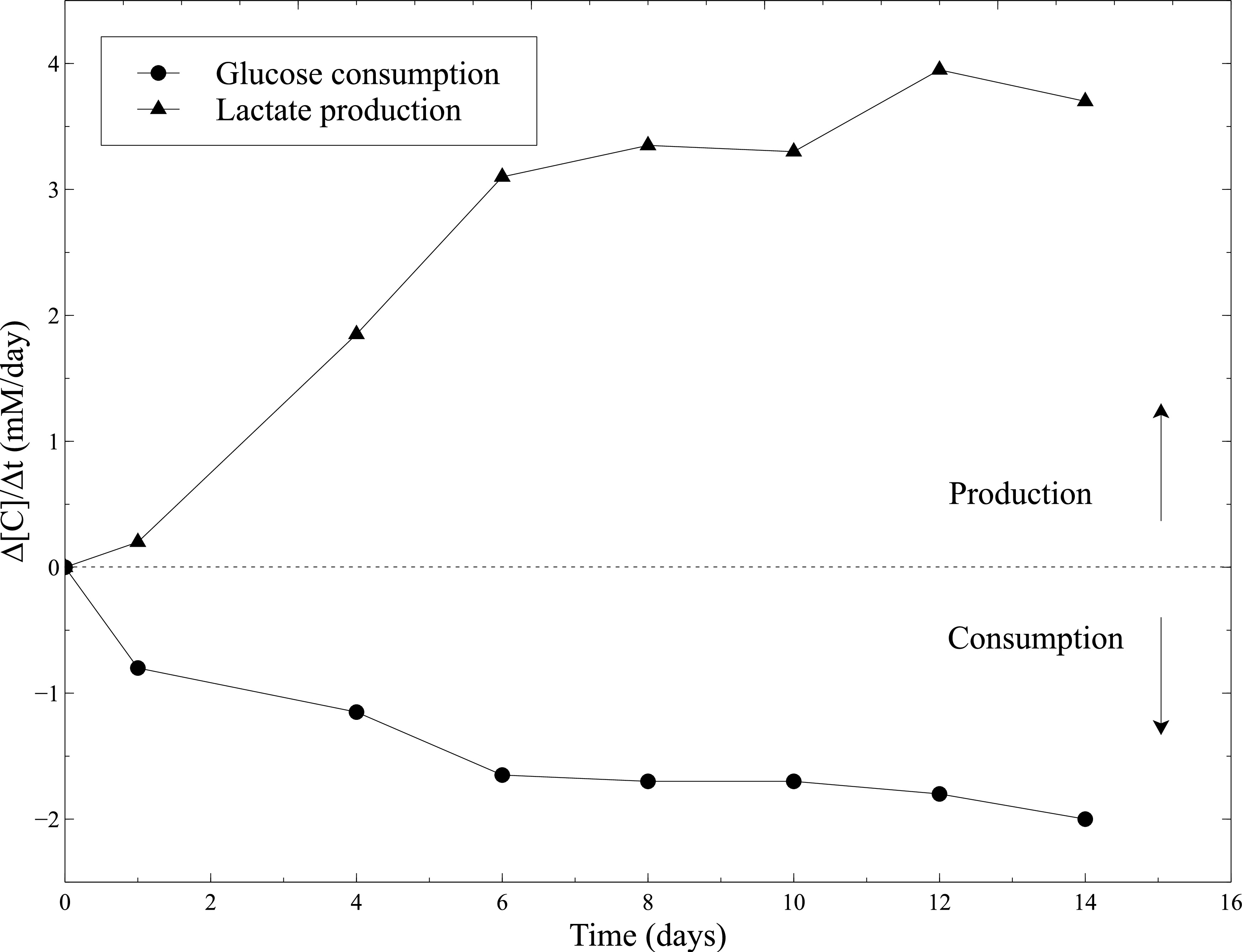
Figure 10: Metabolic activity of SMCs within the collagen gels. Measurements of glucose consumption rate and lactate production rate were performed with the blood gas analyzer every 2 days, after the culture medium changing. Fresh culture medium was used as a baseline level for the glucose and lactate concentrations measurements.
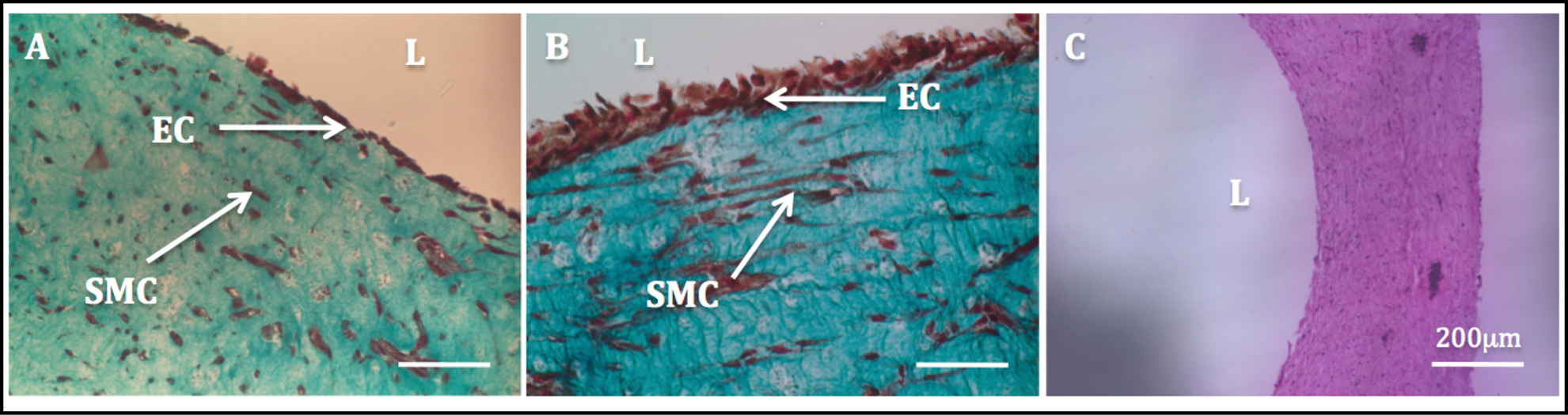
Figure 11: Lumen endothelialization. Histological images of the radial cross-sections of tubular constructs. Masson’s Trichome staining of tubular constructs cultured statically for 1 week (A) and 2 weeks (B). H & E staining of a tubular construct (C).
| Time | Thickness (mm) | Compaction (%) |
| 0 hr | 4.83 ± 0.02 | 0 ± 0 |
| 2 hr | 4.26 ± 0.02 | 12 ± 0 |
| 4 hr | 4.21 ± 0.03 | 13 ± 1 |
| 6 hr | 4.06 ± 0.10 | 16 ± 2 |
| 12 hr | 3.16 ± 0.07 | 35 ± 1 |
| 1 day | 2.08 ± 0.11 | 57 ± 2 |
| 1 week | 0.68 ± 0.07 | 86 ± 1 |
| 2 weeks | 0.36 ± 0.00 | 93 ± 0 |
Table 1: Rapid compaction of construct diameter during the static maturation. Wall thickness of the constructs and the compaction rate as a function of time of static culture. Compaction was measured by determining the external diameter of the tubular constructs with a scanning laser interferometer (Series 183B, LaserMike 136). After 24 hr, the constructs compacted to 57% ± 2% of their molded dimensions. Data are expressed as mean ± SD (n = 3). The presence and the activity of living smooth muscle cells was the only responsible for such major changes.
Supplemental Video 1: Harvesting of the non-remodeled tubular collagen gels. Please click here to view this video.
Supplemental Video 2: Cells-driven compaction of tubular collagen gels. Please click here to view this video.
Supplemental Video 3: Manipulation of the non-remodeled tubular collagen gels. Please click here to view this video.
Supplemental Video 4: Manipulation of the cells-remodeled tubular collagen gels. Please click here to view this video.
Supplemental Video 5: Longitudinal fatigue test (at 30%) on cells-remodeled tubular collagen gels. Please click here to view this video.
Supplemental Video 6: Circumferential fatigue test (at 30%) on cells-remodeled tubular collagen gels. Please click here to view this video.
Discussion
Among the community of vascular tissue engineers, tremendous efforts have been done to reproduce the tunica media layer responsible for the mechanical stability of blood vessels16. Since the pioneering work of Weinberg and Bell17, collagen has been widely used as a scaffold for vascular tissue engineering because of its biocompatibility, non-immunogenic properties and availability. However, the use of collagen represents a big challenge for researchers, as this material is not easy to handle, due to the intrinsic lack of mechanical stiffness. Manipulations during scaffold preparation may damage the scaffolds, compromising them for further use.
The technique described in this work allows: i) to engineer cellularized collagen gels into a tubular-shaped geometry; ii) to engineer biological tissues strong enough to be handled after a short static maturation period (1 or 2 weeks); iii) to assess mechanical and viscoelastic properties of such tubular-shaped biological tissues in 2 directions. Cells in the gel play a key role in the collagen matrix remodeling. During the maturation period, contractile SMCs led to the compaction of the gels yielding a construct with higher mechanical stability that could be assessed in the longitudinal and circumferential directions. Afterwards, HUVECs seeded in the luminal side of the constructs generated a homogenous and viable endothelium, thus demonstrating the suitability of the collagen gels for vascular tissue engineering applications.
The bioreactor presented in this work was specifically designed to provide an optimal environment for cell growth during static maturation. In addition, the devices developed for the characterization of the mechanical and viscoelastic properties of the constructs were designed with the aim to reduce any potential damage inherent to the manipulation of such delicate materials. Hence, the static bioreactor was equipped of a 0.22 µm filter and a filter membrane on the cap (step 1.1.2, Figure 1A and B) that allowed gas exchange between culture medium inside the reservoir and the incubator, while keeping a sterile culture environment. The luer septum at the bottom was used as a port for culture medium sampling and changing during static culture. Some critical steps have to be considered during construct fabrication and characterizations. All the manipulations (performed in the step 2.1.1 and in the subsequent steps) that might alter the sterility of the system were performed in a sterile biological hood. Cells and collagen gel mixture preparation was handled on ice in order to delay the gelation process (steps 2.1.4 to 2.1.7). At step 2.1.7, any air-bubbles entrapped in the mixture prior to gelation are potential stress concentration areas that can compromise the stability of the constructs. Therefore, removal of such air-bubbles requires slightly shaking the assembly or using medical vacuum for 3 min for degasing in sterile conditions. Finally, the grips were specifically designed for maintaining the axis of the mandrel central in the tubular mold during gelation and for allowing delicate manipulation of the constructs during harvesting (removal of the mandrel, section 2.4), for endothelialization, and for facilitating the mounting onto the mechanical system (longitudinal tests).
The present protocol proposes an original easy-to-process alternative approach of reinforcement of collagen gels constructs based on the natural inherent contractile potential of SMCs. Common techniques of collagen matrices reinforcement involve the use of physical and chemical crosslinking agents that can have deleterious effects on cells-matrix interactions18–20. The fabrication technique presented in this work allows directing this cells-driven remodeling process to yield a tissue-engineered construct with targeted mechanical properties without any physical or chemical treatment.
Characterization of mechanical and viscoelastic properties of hydrated collagen gels is a great challenge. In this perspective, the present protocol describes an original simple and efficient method for assessing the mechanical properties of tubular soft tissues. This characterization can be performed not only in the circumferential direction, but also in the longitudinal direction, directly on the whole tubular structure. During mechanical characterization, temperature, aqueous environment, pH and ionic strength are some of the environmental factors that are known to drastically affect the mechanical behavior of biological tissues21. Hence, the present work suggests an original set-up and protocol for the mechanical characterization of biological tissues in a highly reproducible pseudo-physiological environment (saline solution at 37 °C and pH 7.4). To the best of our knowledge, this kind of characterization has never been reported elsewhere.
In conclusion, the technique proposed in this work demonstrates the high potential of direct mixing of cells with collagen for vascular tissue engineering applications. This method together with the mechanical characterization and endothelialization process constitute high polyvalent protocols. Hence, through slight modifications of the set-ups and protocols while keeping the same rationale, main requirements for engineering vascular tissue equivalents can be addressed such as rapid and uncomplicated processing, including endothelialization, and the possibility to be transposed to a wide range of soft tissues with various lengths and diameters. Furthermore, different adherent cell types, ECM proteins and molded geometries can be investigated for a number of targeted applications, such as engineering tendons, skin grafts, cardiac patches, nerves, among others. Although the mechanical properties of the constructs are encouraging, they are still lower than those of native tissues. In this context, we strongly believe that a very short static maturation period is a crucial step toward the dynamic stimulation into a bioreactor, thus leading to a higher structural integrity and mechanical stability. However, the possibility to rapidly produce tissue-engineered cellularized collagen-based constructs suitable for mechanical and histological analyses makes the static bioreactor described herein a useful and promising tool to provide insight into the interplay between cells and ECM during growth and remodeling, or even to be used as a model for therapies and drug delivery systems.
Disclosures
No funding was received from organizations or agencies with potential conflict of interests.
Acknowledgements
This research was funded by the Natural Science and Engineering Research Council of Canada, the Canadian Institute for Health Research and the CHU de Québec Research Center.
Materials
| Name | Company | Catalog Number | Comments |
| CellTreat 50 ml Bio-Reaction tubes | CELLTREAT Scientific Products | 229-475 | Centrifuge tube |
| Male luer with lock ring x 1/8" hose barb. PP. 25/pk | Cole Parmer | RK-45503-04 | Luer fittings for "gas-exchange port" |
| Female luer x 1/8" hose barb adapter. PP. 25/pk | Cole Parmer | RK-45500-04 | Luer fittings for the "medium sampling port" |
| Masterflex platinum-cured silicone tubing L/S 17. 25 ft. | Cole Parmer | RK-96410-17 | Tube 1 and 2 for the gauze grippers |
| Masterflex platinum-cured silicone tubing L/S 16. 25 ft. | Cole Parmer | RK-96410-16 | Tube 3 for the gauze grippers |
| Silastic Medical adhesive silicone, type A | Dow Corning | - | Silicon glue for the fabrication of the static bioreactor |
| Polyvent 4 Vessel venting filters | Whatman | 6713-0425 | Filter for "gas exchange port" |
| Rod. PP. stirring. 8’’ | Scienceware | 377660008 | Mandrel |
| Stopper silicone rubber 00 PK12 | VWR | 59590-084 | Stopper for the insertion of the mandrel to the vented cap of the centrifuge tube |
| Krytox PFPE/PTFE Greases | Dupont | GPL 202 | Medical grade grease for covering the mandrel |
| Trypsin-EDTA (0.5%) | Gibco | 15400-054 | Cell culture |
| Xiameter RTV-4130-J base and curing agent | Dow Corning | - | C-shaped silicone support for endothelialization |
| Dulbecco’s modified Eagle medium, DMEM, high glucose, pyruvate | Gibco (Life Technology) | 11995-065 | Cell culture |
| Pure acetone (99%) | Laboratoire Mat Inc. | AP0102 | Chemical for collagen extraction |
| Isopropyl alcohol (HPLC grade, 99.9%) | Fisher Scientific | AC610080040 | Chemical for collagen extraction |
| 0.02 N acetic acid (glacial acetic acid, HPLC grade, 99%) | Fisher Scientific | FL070494 | Chemical for collagen extraction |
| Chloroform solution (99%) | Laboratoire Mat Inc. | CR 0179 | Chemical for collagen extraction |
| Hepes | Sigma-Aldrich | 163716 | Chemical for construct preparation |
| NaOH | Laboratoire Mat Inc. | SR-0169 | Chemical for construct preparation |
| LaserMike 136 | LaserMike | Series 183B | Scanning laser interferometer |
| ElectroPulse MicroTester | Instron Corporation | - | Micromechanical Tester |
| HyClone Media M199/EBSS, 500 ml | GE Healthcare Life Sciences | SH30253.01 | Component of cell culture medium |
| Fetal bovine serum HI - 500 ml | Gibco | SH 30396.03 | Component of cell culture medium |
| Porcine serum (PS) | Sigma-Aldrich | P9783 | Component of cell culture medium |
| Penicillin-Streptomicin | Gibco | 15140-122 | Component of cell culture medium |
| Phosphate buffered saline (PBS) | Fisher Scientific | BP661-50 | Saline solution |
| Tissue culture flask T17CN Vent Cap Red | Sarstedt Inc. | 83.1812.002 | Cell culture |
| ColorpHast- pH-indicator strips (pH = 6.5-10.0) | EMD | 9583 | pH measurements |
| Matrigel Basement Membrane Matrix Growth Factor Reduced, 5 ml vial | BD Biosciences - Discovery Labware | 356230 | Concentrate protein mixture for endothelialization process |
| LifeCam VX-3000 | Microsoft | - | Thickness measurement |
| Biochemical analyzer, DxC600 | Beckman Coulter Unicell Synchron | - | Glucose and lactate concentrations measurements |
| Collagen fibers | Rat tails | - | Collagen was extracted in the laboratory |
| Porcine smooth muscle cells (pSMCs) | Porcine aortas | - | pSMCs were isolated in the laboratory |
| Human umbilical vein endothelial cells (HUVECs) | Human umbilical veins | - | HUVECs were isolated in the laboratory |
References
- Kim, B. S., Nikolovski, J., Bonadio, J., Smiley, E., Mooney, D. J. Engineered smooth muscle tissues: regulating cell phenotype with the scaffold. Experimental cell research. 251, 318-328 (1999).
- Heureux, N., McAllister, T. N., de la Fuente, H. L. Tissue-engineered blood vessel for adult arterial revascularization. The New England journal of medicine. 357 (14), 1451-1453 (2007).
- Syedain, Z. H., Meier, L. A., Bjork, J. W., Lee, A., Tranquillo, R. T. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials. 32 (3), 714-722 (2011).
- Lee, C., Singla, A., Lee, Y. Biomedical applications of collagen. International journal of pharmaceutics. 221 (1-2), 1-22 (2001).
- Couet, F., Rajan, N., Mantovani, D. Macromolecular biomaterials for scaffold-based vascular tissue engineering. Macromolecular bioscience. 7 (5), 701-718 (2007).
- Christiansen, D. L., Huang, E. K., Silver, F. H. Assembly of type I collagen: fusion of fibril subunits and the influence of fibril diameter on mechanical properties. Matrix Biology. 19 (5), 409-420 (2000).
- Eppell, S. J., Smith, B. N., Kahn, H., Ballarini, R. Nano measurements with micro-devices: mechanical properties of hydrated collagen fibrils. Journal of the Royal Society, Interface / the Royal Society. 3 (6), 117-121 (2006).
- Lai, V. K., Lake, S. P., Frey, C. R., Tranquillo, R. T., Barocas, V. H. Mechanical behavior of collagen-fibrin co-gels reflects transition from series to parallel interactions with increasing collagen content. Journal of biomechanical engineering. 134 (1), 011004 (2012).
- Seliktar, D., Nerem, R. M., Galis, Z. S. The Role of Matrix Metalloproteinase-2 in the Remodeling of Cell-Seeded Vascular Constructs Subjected to Cyclic Strain. Annals of Biomedical Engineering. 29 (11), 923-934 (2001).
- Rajan, N., Habermehl, J., Coté, M. -. F., Doillon, C. J., Mantovani, D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nature protocols. 1 (6), 2753-2758 (2006).
- Kumar, V. A., Caves, J. M., et al. Collagen-Based Substrates with Tunable Strength for Soft Tissue Engineering. Biomaterials science. 1 (11), 1193-1202 (2013).
- Li, S., Van Den Diepstraten, C., D’Souza, S. J., Chan, B. M. C., Pickering, J. G. Vascular smooth muscle cells orchestrate the assembly of type I collagen via alpha2beta1 integrin, RhoA, and fibronectin polymerization. The American journal of pathology. 163 (3), 1045-1056 (2003).
- Badylak, S. F., Freytes, D. O., Gilbert, T. W. Extracellular matrix as a biological scaffold material: Structure and function. Acta biomaterialia. 5 (1), 1-13 (2009).
- Engbers-Buijtenhuijs, P., Buttafoco, L., et al. Biological characterisation of vascular grafts cultured in a bioreactor. Biomaterials. 27 (11), 2390-2397 (2006).
- Cheung, A. L. Isolation and culture of human umbilical vein endothelial cells (HUVEC). Current protocols in microbiology. 4, 4B (2007).
- Seifu, D. G., Purnama, A., Mequanint, K., Mantovani, D. Small-diameter vascular tissue engineering. Nature reviews. Cardiology. , (2013).
- Weinberg, C., Bell, E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 231 (4736), 397-400 (1986).
- Meghezi, S., Chevallier, P., Mantovani, D. Why Mechanical Properties of Collagen Scaffolds Should Be Tested in a Pseudo-Physiological Environment. Advanced Materials Research. 409, 158-163 (2011).
- Tirella, A., Liberto, T., Ahluwalia, A. Riboflavin and collagen: New crosslinking methods to tailor the stiffness of hydrogels. Materials Letters. 74, 58-61 (2012).
- Madhavan, K., Belchenko, D., Tan, W. Roles of genipin crosslinking and biomolecule conditioning in collagen-based biopolymer: Potential for vascular media regeneration. Journal of biomedical materials research. Part A. , 16-26 (2011).
- Meghezi, S., Couet, F., Chevallier, P., Mantovani, D. Effects of a pseudophysiological environment on the elastic and viscoelastic properties of collagen gels. International journal of biomaterials. 2012, 319290 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved