A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Covalent Binding of Antibodies to Cellulose Paper Discs and Their Applications in Naked-eye Colorimetric Immunoassays
In This Article
Summary
In this manuscript, periodate oxidation and glutaraldehyde cross-linking methods for covalent immobilization of antibodies on paper discs are presented. The binding activity of immobilized antibodies was evaluated. Based on these results, a glutaraldehyde cross-linking method was used to develop a novel paper-based immunoassay for immunoglobulin G (IgG) detection.
Abstract
This report presents two methods for the covalent immobilization of capture antibodies on cellulose filter paper grade No. 1 (medium-flow filter paper) discs and grade No. 113 (fast-flow filter paper) discs. These cellulose paper discs were grafted with amine functional groups through a silane coupling technique before the antibodies were immobilized on them. Periodate oxidation and glutaraldehyde cross-linking methods were used to graft capture antibodies on the cellulose paper discs. In order to ensure the maximum binding capacity of the capture antibodies to their targets after immobilization, the effects of various concentrations of sodium periodate, glutaraldehyde, and capture antibodies on the surface of the paper discs were investigated. The antibodies that were coated on the amine-functionalized cellulose paper discs through a glutaraldehyde cross-linking agent showed enhanced binding activity to the target when compared to the periodate oxidation method. IgG (in mouse reference serum) was used as a reference target in this study to test the application of covalently immobilized antibodies through glutaraldehyde. A new paper-based, enzyme-linked immunosorbent assay (ELISA) was successfully developed and validated for the detection of IgG. This method does not require equipment, and it can detect 100 ng/ml of IgG. The fast-flow filter paper was more sensitive than the medium-flow filter paper. The incubation period of this assay was short and required small sample volumes. This naked-eye, colorimetric immunoassay can be extended to detect other targets that are identified with conventional ELISA.
Introduction
The point-of-care testing (POCT) diagnostic study is important for the development of new strategies for therapeutics, personalized medicine, and home care1. Cellulose papers are widely used as platforms in immunoassays, as they are cheap, accessible, and familiar to users2. In addition, the porous structure of cellulose paper possesses the power to drive liquid flow without additional energy impact. Records of paper-based bioanalysis can be found as early as the 20th century, when paper chromatography was first invented in 1952. The most prevalent example is immunochromatographic tests3, such as pregnancy and diabetes test strips. These tests provide relatively fast assay times and inexpensive analysis4. Due to their simplicity, these conventional paper strip tests have been widely used in POCT diagnostics5.
Detection methods including colorimetric6, electrochemical7, and electrochemiluminescence8 methods have been reported to measure targets in biological samples. In addition to these quantitative methods, a reliable method for immobilizing antibodies on cellulose paper is also important for the development of diagnostic devices. Non-specific adsorption is the main strategy for modifying antibodies on the surface of the paper-based devices9, 10 to ensure maximum binding capacity to their targets after immobilization. However, a previous study showed that antibodies that are adsorbed onto cellulose paper can desorb from the fibers11 by 40%. Thus, direct adsorption of antibodies onto cellulose may not provide reproducible results12. Covalent immobilization of antibodies that are grafted on the paper surfaces is an alternative method of developing effective paper-based bioassays13. Various methods have been reported for the modification of cellulose14, 15. Ideally, antibodies should maintain their original functionality after immobilization12. Carbonyldiimidazole combined with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate16; 1-fluoro-2-nitro-4-azidobenzene through a UV-based activation strategy17, 18; a chemoenzymatic strategy based on xyloglucan modification19; a 1,4-phenylenediisothiocyanate linking agent20; heteropolysaccharide oxidation21 click chemistry22; and cationic porphyrins23 have been used to covalently immobilize biomolecules on cellulose paper. Chitosan modified paper has been used to develop paper based immunodevices24-26 since it is abundant and biocompatible27. Chitosan is cationic and adheres strongly to anionic cellulose27. The capture antibodies are immobilized on the paper through chitosan coating and glutaraldehyde cross-linking. Periodate oxidation is another method for grafting the capture antibodies on the cellulose paper28. In this method, sodium periodate is spotted on the paper to convert 1,2-dihydroxyl (glycol) groups in cellulose directly to aldehyde groups. The aldehyde groups are then used to form covalent bonds between polysaccharides and antibodies28. Although the fabrication is simple, it is difficult to completely wash out sodium periodate. The unwashed sodium periodate can cause further oxidation of the antibodies that are immobilized on the cellulose paper, affecting the activity and stability of the antibodies. N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride and N-hydroxysuccinimide cross linkers are also used to covalently immobilize antibodies on electrospun poly-L-lactic acid and cellulose acetate nanofibers for the development of nanofiber-based assays29.
In this study, a silane coupling technique was used to graft amine functional groups on cellulose paper discs. This technique helps to retain the original pore size, wicking, and filtration rate of the cellulose filter papers, allowing maximum vertical flow-through in immunoassays. The silane coupling technique has been widely used in biosensors to functionalize substrate surfaces with secondary amine groups, followed by further modification using biomolecules. The grafting of amine groups on the matrix surface comprises a condensation reaction between -OH groups of the organofunctional silane agents and matrix substrate30. The cellulose paper discs were functionalized with amine groups by silane coupling through 3-aminopropyltrimethoxysilane (APS)31. This was followed by covalently immobilizing capture antibodies using two different methods. The first method involved binding of periodate oxidized capture antibodies to the amine functionalized cellulose paper discs. The second method used glutaraldehyde as a cross-linking agent to attach the capture antibodies to the amine group-functionalized cellulose paper discs. The presence of capture antibodies was confirmed by rabbit anti-human IgG-fluorescein isothiocyanate (FITC), using a fluorescence molecular imager. The binding activity of rabbit anti-human IgG-FITC to goat anti-rabbit IgG was also evaluated by peroxidase substrate. The effects of various concentrations of sodium periodate, glutaraldehyde, and capture antibodies were investigated. The application test of the immobilized capture antibody was successfully performed through the detection of IgG serum.
Protocol
1. Grafting Amine Functional Groups on Cellulose Paper Discs
- Prepare one piece of square paper with a dimension of 1 cm × 1 cm, and 100 paper discs made from grade No. 1 cellulose paper with a diameter of 6.0 mm (medium-flow filter paper) using a hole punch.
- To derive -NH2 groups on the paper discs, mix 1 ml APS and 10 ml acetone in a 50 ml glass bottle in the fume hood. Add paper discs to the freshly prepared APS reagent mixture, and incubate for 5 hr with orbital stirring (200 rpm) at room temperature32.
Caution: Handle APS and acetone in the fume hood. - Decant excess solution from the 50 ml glass bottle into an organic waste container.
- Add 10 ml of acetone to the glass bottle, mix well and decant completely to remove any unreacted APS and other impurities. Repeat this step two times.
- Spread the paper discs on the paper towel and place in a 110 °C oven for 3 hr. Allow the paper discs to cool. Store the discs in a 50 ml centrifuge tube at room temperature.
- Use Fourier transform infrared spectroscopy (FTIR) to check the grafting of amine groups on the cellulose square paper, as described below (Figure 3A).
- Turn on the computer and open the FTIR spectroscopy instrument.
- Open the software for FTIR spectroscopy.
- Go to 'Measurement → Initialize'. The rectangles for 'BS: KBr', 'Lamp: Infrared' and 'Laser' will turn green when the initialization is finished.
- Choose 'Data' below the rectangles, and select '%Transmittance', 'Happ-Genzel', '45', '4.0', and 'Min: 400, Max: 4000' for 'Measurement Mode', 'Apodization', 'No. of Scans', 'Resolution', and 'Range (cm-1)'.
- Click 'Measure'.
- Select 'Data file' for the background data. Write down the comments.
- Click 'BKG' to get the baseline for the background.
- Fix the square paper on the film sample holder.
- Select 'Data file' for the sample data. Write down the comments.
- Click 'Sample' to obtain the spectra for the sample.
- Close the FTIR spectroscopy application and turn off the computer.
- Repeat the above steps (steps 1.1 to 1.6) to prepare amine-functionalized grade No. 113 cellulose square paper and discs (fast-flow filter paper), and obtain the FTIR spectra for the grade No. 113 square paper (Figure 3B).
2. Covalent Immobilization of Antibodies on Amine-functionalized Cellulose Paper Discs
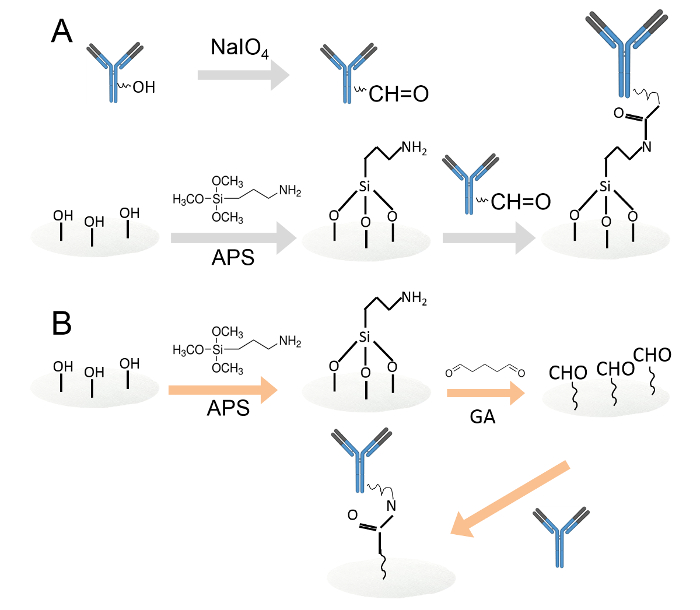
Figure 1. Covalent immobilization of antibodies by two different methods. A. Antibodies immobilized on amine-functionalized cellulose paper discs through periodate oxidation. The carbohydrate residues were oxidized by sodium periodate to produce aldehyde functional groups. Then, the oxidized antibodies were loaded onto amine-functionalized cellulose paper discs. B. The antibodies were then immobilized on amine-functionalized cellulose paper discs through glutaraldehyde. The amine functionalized cellulose paper discs were immersed in 0.05% glutaraldehyde solution to introduce aldehyde groups to the paper discs. After washing, antibodies were loaded onto the aldehyde functionalized paper discs. Please click here to view a larger version of this figure.
- Immobilize antibodies on amine-functionalized cellulose paper discs through periodate oxidation (Figure 1A).
- Mix 1 µl of 2.5 mM sodium periodate with 2 µl of 0.1 mg/ml rabbit anti-human IgG-FITC and 7 µl of 100 mM pH 5.5 acetate buffer in a 1.5 ml tube, and incubate the mixture in the dark for 30 min.
NOTE: Follow this volume ratio to prepare more oxidized antibodies if necessary. Change the volume ratio to optimize the concentration of sodium periodate and rabbit anti-human IgG-FITC. - Dilute the above antibody solution with 30 µl of 50 mM phosphate buffer saline (PBS; pH 7.4) to a final volume of 40 µl.
- Prepare three amine-functionalized medium-flow filter paper discs and three amine-functionalized fast-flow paper discs (as described in Section 1).
- Load 5 µl of the sodium periodate oxidized rabbit anti-human IgG-FITC onto each medium-flow filter paper disc and 8 µl onto each fast-flow filter paper disc. Keep these paper discs in the dark for one hour at room temperature.
- Wash each of the paper disc with 0.2 ml of washing buffer (50 mM Tris buffer with 0.15 M NaCl and 0.05% surfactant, pH 7.4). Repeat the wash three times.
- Photograph the fluorescence images through a fluorescence molecular imager to identify the presence of antibodies on each cellulose paper disc32. Use blank paper discs (treated with the same concentration of antibodies in the absence of sodium periodate) as a control (Figure S1 to Figure S3).
NOTE: For experimental optimization, change the concentration of one parameter that needs to be optimized and fix the concentrations of all other parameters. A high fluorescence intensity increases the amount of capture antibodies that are immobilized on the cellulose paper discs.
- Mix 1 µl of 2.5 mM sodium periodate with 2 µl of 0.1 mg/ml rabbit anti-human IgG-FITC and 7 µl of 100 mM pH 5.5 acetate buffer in a 1.5 ml tube, and incubate the mixture in the dark for 30 min.
- Immobilize antibodies on amine functionalized cellulose paper discs through glutaraldehyde (Figure 1B).
- Add the three APS treated medium-flow filter paper discs and the three fast-flow filter paper discs (described in Section 1) to 2 ml of 50 mM PBS (pH 7.4) that contains 0.05% glutaraldehyde for 1 hr, with orbital stirring at room temperature.
Caution: Handle glutaraldehyde in the fume hood. - Place three discs each in two 1.5 ml centrifuge tubes. Add 1 ml of deionized (DI) water to each tube and shake the tubes for 10 sec. Remove the water by aspirating with a pipette. Repeat two more times to remove any unreacted glutaraldehyde.
- Load 5 µl of 25 µg/ml rabbit anti-human IgG-FITC (capture antibody) onto each aldehyde-functionalized medium-flow filter paper disc, and add 8 µl onto each aldehyde-functionalized fast-flow filter paper disc. Incubate in the dark for approximately 20 min at room temperature. Then, add 10 µl of 50 mM PBS (pH 7.4) to each paper disc without removing the antibodies and incubate for 40 min for the amine aldehyde reaction.
- Wash the paper discs with 0.2 ml of washing buffer on top of a paper towel. Repeat the wash two times.
- Photograph the fluorescence images through a fluorescence molecular imager to check for the presence of antibodies on each cellulose paper disc33. Use blank paper discs as a control.
NOTE: In Figure 4, '0' stands for the blank paper disc that was treated with the same concentration of FITC-antibody in the absence of glutaraldehyde; in Figure 5A, the blank paper discs were treated with glutaraldehyde, but no FITC-antibodies were loaded onto the paper discs.
- Add the three APS treated medium-flow filter paper discs and the three fast-flow filter paper discs (described in Section 1) to 2 ml of 50 mM PBS (pH 7.4) that contains 0.05% glutaraldehyde for 1 hr, with orbital stirring at room temperature.
- Dry the paper discs (from Sections 2.1 and 2.2) at 37 °C for 10 min.
- Block the paper discs with 15 µl of blocking buffer (10% skimmed milk powder in 50 mM Tris buffer, pH 7.4, with 0.15 M NaCl) for 10 min at room temperature.
- Load 5 µl and 8 µl of peroxidase conjugated goat anti-rabbit IgG in PBS (1:10,000) onto medium-flow and fast-flow filter paper discs, respectively. Incubate for 30 min in the dark at room temperature.
- Wash the paper discs with 0.2 ml of washing buffer on top of a paper towel. Repeat the wash three times.
NOTE: It is not necessary to remove the washing buffer as the results are not affected by the buffer. - Load a 10 µl mixture of 3,3',5,5'-tetramethylbenzidine (TMB) and hydrogen peroxide solution onto each disc.
- Take images of the paper discs with a digital camera or smart phone after 5 min of incubation.
NOTE: In Figure 5B, '0' stands for the paper discs that were treated with glutaraldehyde, followed by loading antibody-HRP (horse radish peroxidase) conjugate in the absence of FITC-antibody.
3. Paper-based ELISA for IgG Detection
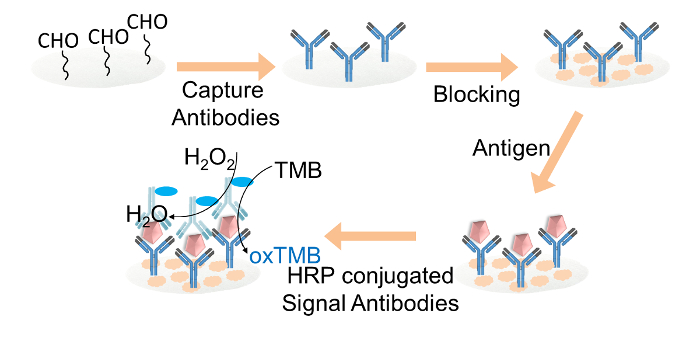
Figure 2. Schematic representation of the paper-based ELISA for IgG detection. Capture antibodies were covalently immobilized on the aldehyde-functionalized cellulose paper discs through glutaraldehyde. The cellulose paper discs were blocked with blocking buffer. Target IgG was then added to the discs, followed by the loading of HRP-conjugated signal antibodies. Finally, the TMB and hydrogen peroxide mixture solution was loaded onto each paper disc for the color readout. Please click here to view a larger version of this figure.
- Add 5 ml of 0.05% glutaraldehyde solution (prepared in 50 mM PBS buffer, pH 7.4) to a 20 ml glass bottle. Immerse 15 amine-functionalized medium-flow filter paper discs in this solution and keep for 1 hr with shaking at room temperature.
- Concurrently, repeat Step 3.1 to prepare another 15 aldehyde functionalized fast-flow filter paper discs.
Caution: Handle glutaraldehyde in the fume hood.
- Concurrently, repeat Step 3.1 to prepare another 15 aldehyde functionalized fast-flow filter paper discs.
- To remove unreacted glutaraldehyde from the paper discs, place the 15 medium-flow filter paper discs in a 15 ml centrifuge tube, and the 15 fast-flow filter paper discs in another 15 ml centrifuge tube. Add 5 ml of DI water to each tube and shake the tubes for 10 sec. Remove the water by aspirating with a pipette. Repeat two times to remove any unreacted glutaraldehyde.
- Dry the paper discs in a 37 °C oven.
- Add 5 µl and 8 µl of 0.025 mg/ml mouse IgG-Fc fragment antibodies to each of the medium-flow and fast-flow filter paper discs, respectively, and incubate for 20 min.
- Add 10 µl of 50 mM PBS (pH 7.4) to each paper disc without removing the antibodies and incubate for 40 min for the amine aldehyde reaction.
- Wash the paper discs with 0.2 ml of washing buffer on top of a paper towel. Repeat the wash three times.
- Dry the paper discs in an oven at 37 °C.
- Block the paper discs with 15 µl of blocking buffer for 10 min at room temperature.
- Wash each paper disc with 0.2 ml of washing buffer on top of a paper towel. Repeat the wash three times.
- Run IgG standards.
- Load 10 µl of various IgG concentrations (e.g., 0, 10, 125, 250, and 500 ng/ml in PBS) onto each disc in triplicate. Incubate for 1 hr at room temperature.
- Wash the paper discs with 0.2 ml of washing buffer on top of a paper towel. Repeat the wash three times.
- Load 10 µl of HRP conjugated mouse IgG-Fc fragment antibodies (1:10,000, 10 mM PBS, pH 7.4), and incubate for 1 hr at room temperature.
- Wash the paper discs with 0.2 ml of washing buffer on top of a paper towel. Repeat the wash three times.
NOTE: It is not necessary to remove the washing buffer as the results are not affected by the presence of buffer. - Load a 10 µl mixture of TMB and hydrogen peroxide onto each disc.
- Take images of all paper discs with a digital camera or smart phone after 5 min of incubation.
NOTE: In Figure 6A, '0' stands for paper discs treated with capture antibody immobilization, and the antibody-HRP/TMB solution without IgG serum. - Analyze the intensity of each paper disc in the image by Image J.
- Convert the images taken in step 3.15 to '.tif' format.
- Open 'Image J' software.
- Go to 'File → Open', choose the image to analyze.
- Choose the shape button 'Oval'.
- Go to 'Image → Type → 32 bit'.
- Go to 'Edit → Invert'.
- Go to 'Analyze → Measure'.
- Copy and analyze the data in a spreadsheet.
Results
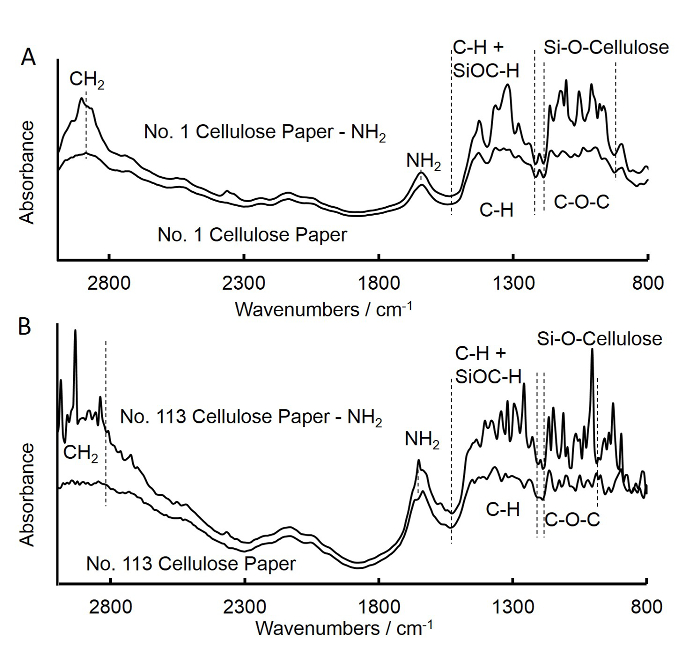
Figure 3. Fourier transform infrared (FTIR) spectra of untreated and APS-treated medium-flow filter square paper (A) and fast-flow filter square paper (B). A. The spectra for untreated medium-flow filter square paper was similar to that of APS treated medium-flow filter square paper. The increase in intensities at bands of 902-1,170 cm-1 and 1,210-1,500 cm...
Discussion
Direct coating of affinity purified goat anti-Mouse IgG-Fc capture antibody on unmodified cellulose paper discs was performed to detect IgG concentrations. The results indicated that, further fixation of the capture antibodies is required for reproducibility. The silane technique was successfully used to introduce amine functional groups to the cellulose paper discs34. The concentration of APS affects the immobilization of antibodies. Therefore, the amount of APS in acetone was also optimized. 1 ml of APS in 1...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was financially supported by the Ministry of Education, Singapore through the Translational and Innovation Grant (MOE2012-TIF-2-G-009).
Materials
| Name | Company | Catalog Number | Comments |
| Cellulose filter paper, Grade 1 (medium flow filter paper) | GE Healthcare Pte Ltd Singapore | 1001 110 | |
| Cellulose filter paper, Grade 113 (Fast flow filter paper) | Sigma-Aldrich, Singapore | 1113-320 | |
| Glutaraldehyde | Sigma-Aldrich, Singapore | G6257 | Grade II, 25% in H2O |
| Surfactant | Tween-20, Sigma-Aldrich, Singapore | P2287 | |
| Bovine serum album | Sigma-Aldrich, Singapore | A2153 | |
| Skimmed milk powder | Louis François | Packed by Kitchen Capers, Singapore | |
| Tris base | Promega | H5135 | |
| Sodium periodate | Merck | 106597 | |
| Na2HPO4 | Merck | 106585 | |
| KH2PO4 | Merck | 104873 | |
| NaCl | CALBIOCHEM | 567441 | |
| NaOH | Merck | 106462 | |
| HCl | Merck | 100317 | |
| phosphate buffer saline (PBS) | N/A | N/A | PBS, containing 137 mmol/L NaCl, 2.7 mmol/L KCl, 8.0 mmol/L Na2HPO4 and 1.5 mmol/L KH2PO4, is prepared with water and adjusted to pH 7.4 with 0.1 mol/L NaOH or 0.1 mol/L HCl |
| Acetone | Tee Hai Chem Pte Ltd Singapore | 9005-68 | |
| Mixture of TMB and hydrogen peroxide solution | 1-Step ultra TMB-ELISA solution , Thermo Scientific Pierce | 34029 | 1 L |
| Rabbit anti-human IgG-FITC | TWC/Bio Pte Ltd Singapore | sc-2278 | |
| Peroxidase conjugated goat anti-rabbit IgG | TWC/Bio Pte Ltd Singapore | sc-2030 | |
| Affinity purified goat anti-Mouse IgG-Fc coating antibody | Bethyl Laboratories, Inc | A90-131A | |
| Mouse reference serum | Bethyl Laboratories, Inc | RS10-101-5 | 9.5 mg/ml |
| HRP conjugated goat anti-mouse IgG-Fc detection antibody | Bethyl Laboratories, Inc | A90-131P | |
| Equipment | |||
| Fourier transform infrared spectrophotometer | Shimadzu IR Prestige-21 | N/A | |
| Fluorescence molecular imager | Pharos FXTM plus molecular imager, Bio-Rad, Singapore | N/A | |
| Oven | NUVE FN500 | N/A | |
| Turbo mixer VM-2000 | MYC LTD | N/A | |
| ImageJ | RGB, free download | N/A |
References
- Curtis, K. A., Rudolph, D. L., Owen, S. M. Rapid detection of HIV-1 by reverse-transcription, loop-mediated isothermal amplification (RT-LAMP). J. Virol. Methods. 151 (2), 264-270 (2008).
- Martinez, A. W., Phillips, S. T., Carrilho, E., Thomas, S. W., Sindi, H., Whitesides, G. M. Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal. Chem. 80 (10), 3699-3707 (2008).
- Lode, P. V. Point-of-care immunotesting: approaching the analytical performance of central laboratory methods. Clin. Biochem. 38 (7), 591-606 (2005).
- Posthuma-Trumpie, G. A., Amerongen, A. V., Korf, J., Berkel, W. J. V. Perspectives for on-site monitoring of progesterone. Trends Biotechnol. 27 (11), 652-660 (2009).
- Lim, D. V., Simpson, J. M., Kearns, E. A., Kramer, M. F. Current and developing technologies for monitoring agents of bioterrorism and biowarfare. Clin. Microbiol. Rev. 18 (4), 583-607 (2005).
- Li, X., Tian, J., Shen, W. Progress in patterned paper sizing for fabrication of paperbased microfluidic sensors. Cellulose. 17 (3), 649-659 (2010).
- Nie, Z., et al. Electrochemical sensing in paper-based microfluidic devices. Lab Chip. 10, 477-483 (2010).
- Delaney, J. L., Hogan, C. F., Tian, J., Shen, W. Electrogenerated chemiluminescence detection in paper-based microfluidic sensors. Anal. Chem. 83 (4), 1300-1306 (2011).
- Oh, Y. K., Joung, H. A., Kim, S., Kim, M. G. Vertical flow immunoassay (VFA) biosensor for a rapid one-step immunoassay. Lab Chip. 13 (5), 768-772 (2013).
- Cheng, C. M., et al. Paper-based ELISA. Angew. Chem. 122 (28), 4881-4884 (2010).
- Jarujamrus, P., Tian, J., Li, X., Siripinyanond, A., Shiowatana, J., Shen, W. Mechanisms of red blood cells agglutination in antibody-treated paper. Analyst. 137 (9), 2205-2210 (2012).
- Credou, J., Volland, H., Dano, J., Berthelot, T. A one-step and biocompatible cellulose functionalization for covalent antibody immobilization on immunoassay membranes. J. Mat. Chem. B. 1, 3277-3286 (2013).
- Kong, F., Hu, Y. F. Biomolecule immobilization techniques for bioactive paper fabrication. Anal. Bioanal. Chem. 403, 7-13 (2012).
- Orelma, H., Teerinen, T., Johansson, L. S., Holappa, S., Laine, J. CMC-modified cellulose biointerface for antibody conjugation. Biomacromolecules. 13, 1051-1058 (2013).
- Yu, A., et al. Biofunctional paper via covalent modification of cellulose. Langmuir. 28 (30), 11265-11273 (2012).
- Stollner, D., Scheller, F. W., Warsinke, A. Activation of cellulose membranes with 1,1′-carbonyldiimidazole or 1-cyano-4-dimethylaminopyridinium tetrafluoroborate as a basis for the development of immunosensors. Anal Biochem. 304 (2), 157-165 (2002).
- Bora, U., Sharma, P., Kannan, K., Nahar, P. Photoreactive cellulose membrane - a novel matrix for covalent immobilization of biomolecules. J. Biotechnol. 126 (2), 220-229 (2006).
- Sharma, P., Basir, S. F., Nahar, P. Photoimmobilization of unmodified carbohydrates on activated surface. J Colloid Interface Sci. 342 (1), 202-204 (2010).
- Brumer, H., Zhou, Q., Baumann, M. J., Carlsson, K., Teeri, T. T. Activation of crystalline cellulose surfaces through the chemoenzymatic modification of xyloglucan. J. Am. Chem. Soc. 126 (18), 5715-5721 (2004).
- Araujo, A. C., Song, Y., Lundeberg, J., Stahl, P. L., Brumer, H. Activated paper surfaces for the rapid hybridization of DNA through capillary transport. Anal Chem. 84 (7), 3311-3317 (2012).
- Xu, C., Spadiut, O., Araujo, A. C., Nakhai, A., Brumer, H. Chemo-enzymatic Assembly of Clickable Cellulose Surfaces via Multivalent Polysaccharides. ChemSusChem. 5 (4), 661-665 (2012).
- Filpponen, I., et al. Generic method for modular surface modification of cellulosic materials in aqueous medium by sequential "click" reaction and adsorption. Biomacromolecules. 13 (3), 736-742 (2012).
- Feese, E., Sadeghifar, H., Gracz, H. S., Argyropoulos, D. S., Ghiladi, R. A. Photobactericidal porphyrin-cellulose nanocrystals: synthesis, characterization, and antimicrobial properties. Biomacromolecules. 12 (10), 3528-3539 (2011).
- Zang, D., Ge, L., Yan, M., Song, X., Yu, J. Electrochemical immunoassay on a 3D microfluidic paper-based device. Chem. Commun. 48 (39), 4683-4685 (2012).
- Ge, L., Yan, J. X., Song, X. R., Yan, M., Ge, S. J., Yu, J. H. Three-dimensional paper-based electrochemiluminescence immunodevice for multiplexed measurement of biomarkers and point-of-care testing. Biomaterials. 33 (4), 1024-1031 (2012).
- Wang, S., et al. Paper-based chemiluminescence ELISA: lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens. Bioelectron. 31 (1), 212-218 (2012).
- Koev, S. T., et al. Chitosan: an integrative biomaterial for lab-on-a-chip devices. Lab Chip. 10, 3026-3042 (2010).
- Wang, S. M., et al. Simple and covalent fabrication of a paper device and its application in sensitive chemiluminescence immunoassay. Analyst. 137 (16), 3821-3827 (2012).
- Sadira, S., Prabhakaranc, M. P., Wicaksonob, D. H. B., Ramakrishna, S. Fiber based enzyme-linked immunosorbent assay for C-reactive protein. Sensor Actuat B-Chem. 205, 50-60 (2014).
- Koga, H., Kitaoka, T., Isogai, A. In situ modification of cellulose paper with amino groups for catalytic applications. J. Mater. Chem. 21, 9356-9361 (2011).
- Klemm, D., Heublein, B., Fink, H. P., Bohn, A. Cellulose: fascinating biopolymer and sustainable raw material. Angew. Chem., Int. Ed. 44 (22), 3358-3393 (2005).
- Fernandes, S. C. M., et al. Bioinspired antimicrobial and biocompatible bacterial cellulose membranes obtained by surface functionalization with aminoalkyl groups. ACS Appl. Mater. Interfaces. 5 (8), 3290-3297 (2013).
- . . Pharos FX plus molecular imager instructions, Catalog Number 170-9460. , (2005).
- Tee, Y. B., Talib, R. A., Abdan, K., Chin, N. L., Basha, R. K., Yunos, K. F. M. Aminosilane-grafted cellulose. BioResourses. 8 (3), 4468-4483 (2013).
- Xing, Y., et al. Bioconjugated quantum dots for multiplexed and quantitative immunohistochemistry. Nat Protoc. 2, 1152-1165 (2007).
- Guidi, A., Laricchia-Robbio, L., Gianfaldoni, D., Revoltella, R., Del Bono, G. Comparison of a conventional immunoassay (ELISA) with a surface plasmon resonance-based biosensor for IGF-1 detection in cows' milk. Biosens Bioelectron. 16, 971-977 (2001).
- Ahmed, S., Bui, M. N., Abbas, A. Paper-based chemical and biological sensors: Engineering aspects. Biosens Bioelectron. 77, 249-263 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved