A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
An Optimized Hemagglutination Inhibition (HI) Assay to Quantify Influenza-specific Antibody Titers
* These authors contributed equally
In This Article
Summary
The presented protocols describe how to perform a hemagglutination inhibition assay to quantify influenza-specific antibody titers from serum samples of influenza vaccine recipients. The first assay determines optimal viral antigen concentrations by hemagglutination. The second assay quantifies influenza-specific antibody titers by hemagglutination inhibition.
Abstract
Antibody titers are commonly used as surrogate markers for serological protection against influenza and other pathogens. Detailed knowledge of antibody production pre- and post-vaccination is required to understand vaccine-induced immunity. This article describes a reliable point-by-point protocol to determine influenza-specific antibody titers. The first protocol describes a method to specify the antigen amounts required for hemagglutination, which standardizes the concentrations for subsequent usage in the second protocol (hemagglutination assay, HA assay). The second protocol describes the quantification of influenza-specific antibody titers against different viral strains by using a serial dilution of human serum or cell culture supernatants (hemagglutination inhibition assay, HI assay).
As an applied example, we show the antibody response of a healthy cohort, which received a trivalent inactivated influenza vaccine. Additionally, the cross-reactivity between the different influenza viruses is shown and methods to minimize cross-reactivity by using different types of animal red blood cells (RBCs) are explained. The discussion highlights advantages and disadvantages of the presented assays and how the determination of influenza-specific antibody titers can improve the understanding of vaccine-related immunity.
Introduction
Infection with influenza virus is associated with considerable morbidity, mortality, and high healthcare costs1,2,3,4. In particular, elderly, newborns, pregnant women, and patients with chronic disease are at risk for more severe clinical outcomes. Therefore, vaccination against circulating influenza virus strains is the primary measure to decrease the burden of disease in these high-risk populations. The increase of the individual immune response after vaccination, e.g., influenza-specific antibodies above a protective threshold, reduces the individual risk of infection and in general the likelihood of viral transmission within a population5. A detailed understanding of the vaccine-induced humoral immune response in different populations and across various age groups is a key element to answer important clinical questions6,7,8,9, such as: Why do some elderly patients have infections despite previous vaccination? What is a "good" and "sufficient" vaccine-induced protection? How often should a vaccine be applied to an immunosuppressed patient to reach protective titers? What is the most effective dosage? What is the impact of a novel adjuvant on post-vaccination antibody titers? The measurement of the vaccine-specific antibody production may help answer these important questions and improve vaccination outcomes.
The quantification of virus-specific antibody titers can be performed with various immunological methods. This includes solid-phase10 or bead-based ELISA11 assays, the HI assay12, and neutralizing assays13. ELISA-based methods allow the screening of relatively large amounts of serum samples against various antigens. Also, pathogen-specific Immunoglobulin (Ig)M and IgG can be separately explored. Although the characteristics of an antigen, e.g., the linear amino acid sequence or virus-like particle may influence the binding of antibodies, the spectrum of potential epitopes is very broad and does not provide information on whether an antibody response has functional relevance.
In contrast, the neutralization assay determines the potential of antibodies to functionally inhibit the infection of cells and therefore reflects the neutralization potential. However, this method is very labor intensive, requires culturing of specific cell lines and live viruses, and therefore, it is time-consuming, expensive, and requires special equipment.
This article describes a step-by-step the World Health Organization (WHO)-based HI protocol12 to quantify influenza-specific antibody titers. Hemagglutination is a characteristic effect of some viruses leading to the agglutination of erythrocytes. The inhibition of this effect with patient sera allows the measurement of inhibitory antibody concentrations, which reflects a neutralizing effect.
We have modified the workflow of the WHO-protocol to allow a more efficient handling of multiple samples at the same time and thereby reducing the required time. The first protocol describes the determination of the agglutination potential of a particular influenza antigen. In doing so, the correct influenza antigen concentration is determined for the second protocol. This part should be repeated with every new viral antigen, as well as each batch of blood.
The second protocol describes the determination of influenza-specific antibody titers. The presented protocols are optimized for the investigation of influenza virus and human serum samples however, it can also be applied for mouse serum samples or cell-culture supernatants from stimulated immune cells, e.g., virus-specific B-cells. Results can be determined as absolute measured titers. In many vaccine studies, the geometric mean titers and the 95% confidence interval are shown for each particular population. For interpretation, seroprotection or seroconversion are often used to describe the susceptibility of a population to a certain virus. Seroprotection is defined as a titer of ≥1:40, and seroconversion as a more than 4-fold titer increase with achievement of seroprotective titers between two time points (most commonly pre-vaccination and 30 days post-vaccination are used).
Both protocols are easy to use and they can be adapted to a broad range of research questions. In particular, they can be used to determine reliably and quickly the antibody titers against various other viruses with the capacity for hemagglutination, such as measles, polyomaviruses, mumps, or rubella14,15,16.
Protocol
The study protocols were approved through the local ethical review board (www.EKNZ.ch) and written informed consent was obtained from all participants.
1. Serum Collection
- Collect serum samples from humans at time points of interest. For this study, we collected sera at days 0 (time of influenza vaccination), +7, +30, +60, and +180 after vaccination.
- To obtain the serum, centrifuge the sample tubes at 1,200 x g for 10 min at room temperature (20 - 25 °C).
NOTE: Non-centrifuged blood samples should be stored at 4 °C, and for no longer than 24 h. - Aliquot the serum into different tubes (cryo-vials) and freeze at -80 °C until use.
- Perform the subsequent assays batch-wise, including all the time-points of one person to reduce variability within a patient.
2. Preparation of Antigens
CAUTION: Five different antigens are used (see Table of Materials). Prepare antigens in a Biosafety Level 2 (BSL-2) laboratory.
- According to the manufacturer's instructions, reconstitute the total contents of one lyophilized influenza antigen ampoule with 1.0 mL of distilled water and allow the dissolved antigen to stand for a minimum of 5 min at room temperature before proceeding.
- Aliquot the antigen solution to 1.5 mL tubes and freeze at -80 °C until further use.
3. Preparation of Cholera Filtrate
NOTE: Cholera filtrate is used as a receptor destroying enzyme (RDE) according to the WHO protocol12. This removes innate inhibitors from the serum which would interfere with the assay17.
- Reconstitute the lyophilized RDE according to the manufacturer's instructions.
- Store the RDE solution in a 15 mL tube at 4 °C until further use.
4. HA Assay
NOTE: To ensure that the HI assays are comparable between several plates, the same amount of virus particles must be used for each plate. The HA assay (also called HA titration) is performed to quantify the virus particles necessary for hemagglutination, and is recorded in HA units. A "unit" of hemagglutination is an operational unit dependent on the method used for HA titration and is not a measurement of an absolute amount of virus. Thus, an HA unit is defined as the amount of virus needed to agglutinate an equal volume of a standardized RBC suspension. According to the WHO, the standard amount used for the HI assay is 4 HA units per 25 µL. For an illustration of the principle of the HA assay see Figure 1.

Figure 1: Principle of hemagglutination and hemagglutination inhibition. No hemagglutination occurs in a negative control situation without viruses and antibodies (left column), and erythrocytes hemagglutinate only in the presence of influenza virus (middle column). However, when the hemagglutinin of the influenza virus is blocked by virus-specific antibodies then no hemagglutination can occur (right column). Please click here to view a larger version of this figure.
NOTE: The RBCs used are dependent on the type of influenza virus in the assay (Table 1). Further, for various types of 96-well micro titer plates, the incubation time as well as the appearance of the non-agglutinated cells differ (Table 2).
| Influenza antigen | A/California/7/09 (H1N1) | A/Switzerland/9715293/2013 (H3N2) | A/Texas/50/2012 (H3N2) | B/Brisbane/60/08 | B/Massachusetts/02/2012 | ||
| RBC species | Chicken | Guinea Pig | Guinea Pig | Turkey | Turkey | ||
Table 1: Influenza antigens and corresponding species of RBCs. According to the manufacturer's instructions (NIBSC).
| RBC species | Chicken | Turkey | Guinea pig | Human type O |
| Concentration of RBCs (v/v) | 0.75% | 0.75% | 1% | 1% |
| Type of microtiter plate | V bottom | V bottom | U bottom | U bottom |
| Incubation time, RT | 30 min | 30 min | 1 hour | 1 hour |
| Appearance of non-agglutinated cells | Button* | Button* | halo | halo |
Table 2: Assay conditions with different species of RBCs. According to the WHO protocol. (* flows when tilted).
- Preparation of the RBC Suspension
- Dilute the RBC stock suspension (10%, v/v; except human type O) (see Table of Materials) with phosphate buffered saline (PBS) to make the proper concentrations for avian and mammalian RBCs of 0.75% and 1%, respectively.

Figure 2: Plate design of the HA assay. The HA titration is performed in duplicates. No antigen was added to the control rows. Also see Figure 4 for the determination of the best antigen concentration. Please click here to view a larger version of this figure.
- Preparation of the 96-well Micro Titer Plate
NOTE: See Figure 2 for an overview of the plate design.- Add 25 µL of PBS to wells 1 to 12 of each used row of a 96-well micro titer plate by using a multichannel pipette (Figure 2). Use the V-shaped micro titer plate when working with avian RBCs, like chicken and turkey. Use the U-shaped micro titer plate when working with mammalian RBCs, like guinea pig and human type O (Table 2).
- Add 25 µL of influenza antigen to the first well of the antigen-rows, which are arranged in duplicates. No antigen is added to the control rows. The control rows should not show a hemagglutination effect and serve as negative controls (Figure 2).
- Perform a serial 2-fold dilution by transferring 25 µL from the first well of the antigen-rows to successive wells by using a multichannel pipette. Mix each dilution step by pipetting up and down gently 10 times.
- Discard the final 25 µL of the last wells.
- Add 25 µL of PBS to wells 1 to 12 of each used row by using a multichannel pipette, in order to reach a total volume of 50 µL per well.
- Add 50 µL of the RBC suspension to each used well by using a multichannel pipette.
- Tap the plate carefully 10 times on all four sides to mix.
- Cover the plate with a lid and incubate at room temperature for the appropriate amount of time depending on the RBC species used (see Table 2). Do not move the plate while incubating.
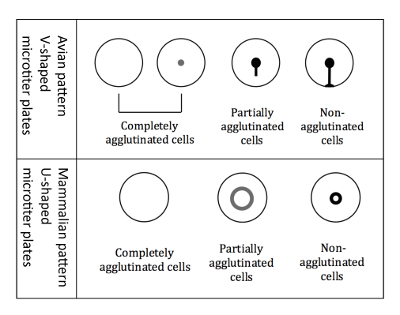
Figure 3: Agglutination patterns of avian and mammalian RBCs. V-shaped micro titer plates are used when working with avian RBCs. The readout is performed in a tilted plate position, and non-agglutinated RBCs start to run down forming a tear-like shape. U-shaped microtiter plates are used when working with mammalian RBCs. The readout is then performed in a non-tilted position, and non-agglutinated RBCs form a small halo. Please click here to view a larger version of this figure.
- Reading the plate
NOTE: The readout is slightly different when using avian RBCs compared to mammalian RBCs, because of the different shaped micro titer wells (Figure 3).- Readout of avian RBCs
- Tilt the plate 90° for 25 s.
NOTE: Tilting the plate is crucial for the differentiation of avian patterns, because all three different types of agglutination patterns (completely agglutinated, partially agglutinated, and non-agglutinated) appear as a button when not tilted. - Mark the results immediately, while the plate is still in a tilted position, on a printed scheme of the 96-well plate. The agglutination patterns of the avian RBCs are shown in Figure 3.
- Tilt the plate 90° for 25 s.
- Readout of mammalian RBCs
- Mark the results on a printed scheme of the 96-well plate, without tilting the plate (horizontal position on the bench).
NOTE: When hemagglutination occurs, the agglutinated cells do not settle to the bottom, whereas non-agglutinated cells appear as a halo at the bottom of the well. The halo of the partially agglutinated cells is less intense and has a larger diameter (Figure 3).
- Mark the results on a printed scheme of the 96-well plate, without tilting the plate (horizontal position on the bench).
- Determination of 4 HA units.
NOTE: The HA titration end point is the last well where complete hemagglutination occurs. This well contains 1 HA unit of virus. Because of the 2-fold dilutions of the antigen, two wells ahead of the HA titration endpoint is the well that contains 4 HA units of virus (Figure 4).
- Readout of avian RBCs

Figure 4: Readout of the HA titration with avian RBCs to determine the titer of 4 HA units. The optimal antigen amount required for hemagglutination is measured by the hemagglutination assay (antigen titration assay). The last well where complete hemagglutination occurs is the HA titration endpoint and contains 1 HA unit. Because of the 2-fold dilutions of the antigen, two wells ahead of the HA titration endpoint, the titer corresponds to 4 HA units. Please click here to view a larger version of this figure.
5. HI Assay
NOTE: The work-flow of the protocol has been optimized to allow a more efficient handling of multiple samples at the same time, by using PCR tube stripes and a thermo cycler (see below).
- Preparation of the Serum Samples
NOTE: Prepare serum samples in a BSL-2 laboratory.- Thaw the frozen serum samples of each time point of every person (see step 1.2) at room temperature.
- Add an aliquot of 10 µL of each thawed serum sample to a tube of a PCR tube strip (10-tubes in one strip).
NOTE: The big advantage of using PCR tube strips is that a multichannel pipette can be used for the following steps in the HI assay; this saves a lot of time when testing a large amount of serum samples and when performing repeated measures of the same samples for antibody titers against different virus strains. - Store the aliquoted serum samples in the PCR tube strips at -80 °C until use.
- One day before the HI assay is performed, thaw the serum sample aliquots of interest at room temperature.
- Add 10 µL of the appropriate anti-serum to an empty PCR tube.
NOTE: To serve as a positive control, the anti-serum against a specific virus must match the used virus. The positive control allows for standardization of plate performance over multiple plates. - Add 30 µL of cholera filtrate solution to each serum aliquot and to the anti-serum (3 volumes of cholera filtrate to 1 volume of serum) by using a multichannel pipette.
- Keep the PCR tubes in a PCR 96-well rack or an empty tip-box and vortex for 5 s.
- Incubate the samples overnight at 37 °C using a thermo cycler.
- Incubate the samples at 56 °C for 30 min to inactivate the cholera filtrate using a thermo cycler.
NOTE: Depending on the thermo cycler, this step can be programmed to further automate the process. - Keep the PCR tubes in a PCR 96-well rack or an empty tip-box and vortex for 5 s.
- Store the samples at 4 °C in the fridge until use for the HI assay.
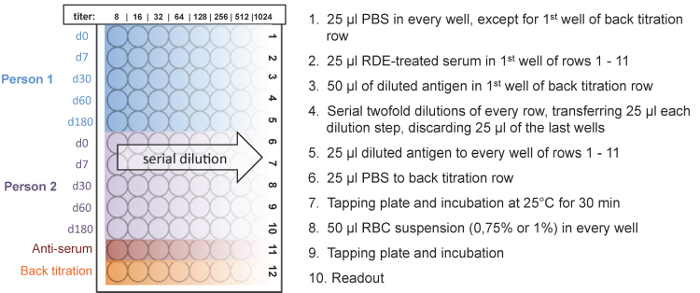
Figure 5: Plate design and workflow of the HI assay. Five time points of two people can be measured on one plate. The HI titer ranges from 8 to 1,024. An anti-serum of the used antigen served as a positive control and a back titration was performed to check if the antigen dilution equals 4 HA units. The serial dilution of the serum sample is shown for 2 individual vaccine recipients. Please click here to view a larger version of this figure.
- HI assay
NOTE: For an illustration of the principle of the HI assay see Figure 1. Depending on the virus, different species of RBCs are used for the assay (Table 1). The different species of RBCs are used in various types of 96-well plates, and the incubation time as well as the appearance of the non-agglutinated cells differs (Table 2). For the HI assay, 4 HA units of virus or antigen are added to the 2-fold dilution series of the samples.- Preparation of the antigen solution
- Calculate the volume of antigen solution needed according to the number of 96-well plates used (25 µL antigen per well × 96 = 2,400 µL antigen per 96-well plate; add 100 µL per plate extra due to the usage of a reservoir for the multichannel pipette; a total 2.5 mL of antigen per plate).
NOTE: For example, if measuring 100 serum samples then 10 plates are needed (10 samples per plate): 2.5 mL x 10 = 25 mL of antigen solution needed in total. - Prepare the proper dilution of 4 HA units for the calculated volume using PBS.
NOTE: 4 HA units are determined for the HA assay. For the appropriate amount of antigen, divide the calculated volume by the titer corresponding to 4 HA units. For example, 4 HA units correspond to a dilution of 1/64, and we needed 15,000 µL of antigen solution are needed: 15,000/64 = 234.4 µL of the dissolved lyophilized influenza antigen are added.
- Calculate the volume of antigen solution needed according to the number of 96-well plates used (25 µL antigen per well × 96 = 2,400 µL antigen per 96-well plate; add 100 µL per plate extra due to the usage of a reservoir for the multichannel pipette; a total 2.5 mL of antigen per plate).
- Preparation of the RBC suspension
- Calculate the volume of RBC suspension needed according to the number of 96-well micro titer plates used (50 µL RBC suspension per well × 96 = 4,800 µL RBC suspension per 96-well plate; add 200 µL per plate extra due to the usage of a reservoir for the multichannel pipette).
- Dilute the RBC stock suspension (normally 10%, v/v; except human type O) with PBS to make the proper concentrations for avian and mammalian RBCs of 0.75% and 1%, respectively.
- Preparation of the 96-well micro titer plate
- Label the 96-well micro titer plates (sample ID, positive control, and back titration). Please check the plate orientation in Figure 5 carefully.
- Add 25 µL of PBS to every well except to the first well of the "back titration" row (Figure 5, 12th row) using the multichannel pipette.
NOTE: A back titration was performed to check if the used antigen dilution equals 4 HA units. An antigen titer of 4 HA units is indicated if hemagglutination occurs in the first three wells of the "back titration" row, but not in the fourth well. - Add 50 µL of the prepared antigen solution (described in 5.2.1) to the first well of the "back titration" row (12th row).
- Add 25 µL of the RDE-treated serum samples to the first wells of rows 1 to 10 on each plate, using the multichannel pipette.
- Add 25 µL of the appropriate anti-serum to the first well of the 11th row as a positive control.
- Perform serial 2-fold dilutions by transferring 25 µL from the first well of each row (1 - 12) to successive wells by using a multichannel pipette. Mix by pipetting up and down 10 - 15 times for each dilution step. The same tips can be used for each dilution step per sample.
- Discard the final 25 µL of the last wells.
- Add 25 µL of the antigen solution by using a multichannel pipette to each well of rows 1 to 11 (serum samples and anti-serum). The same tips can be used if they do not touch the wells.
- Add 25 µL of PBS instead of antigen to each well of the "back titration" row (12th row).
- Tap the plate carefully 10 times on all four sides to mix.
- Cover the plate with a lid and incubate at room temperature for 30 min. Do not move the plate while incubating.
- Add 50 µL of the RBC suspension to every well.
- Tap the plate carefully 10 times on all 4 sides to mix.
- Cover the plate with a lid and incubate at room temperature for the appropriate amount of time depending on the RBC species used (see Table 2). Do not move the plate while incubating.
- Reading the plate
NOTE: The HI titer is the reciprocal of the last dilution of (anti-) serum that completely inhibits hemagglutination. It is important to consider that the RDE-treated sera were already diluted 1:4 and after the serial dilution step, the sera in the first wells are diluted 1:8, which corresponds to a HI titer of 8.- Readout of avian RBCs
- Tilt the plate 90° for 25 s.
NOTE: Tilting the plate is crucial for the differentiation of avian patterns, because all three different types of agglutination patterns (completely agglutinated, partially agglutinated, and non-agglutinated) appear as a button when not tilted. - Mark the results immediately, while the plate is still in a tilted position, on a printed scheme of the 96-well plate. The agglutination patterns of avian RBCs are shown in Figure 3.
- Tilt the plate 90° for 25 s.
- Readout of mammalian RBCs
- Mark the results on a printed scheme of the 96-well plate, without tilting the plate.
NOTE: When hemagglutination occurs, the agglutinated cells do not settle down whereas non-agglutinated cells appear as a halo at the bottom of the well. The halo of the partially agglutinated cells is less intense and has a larger diameter (Figure 3). - Determine the HI of each sample and transfer it to a computer-based table (Figure 6)
- NOTE: Partially agglutinated wells were determined as a lower titer. For example, if a serum sample completely inhibits hemagglutination up to the 4th well (1:64 dilution) and the 5th well (1:128 dilution) is partially agglutinated, then the HI titer is set to the lower titer 64 for the final analysis (Figure 6, 4th row).
- Mark the results on a printed scheme of the 96-well plate, without tilting the plate.
- Readout of avian RBCs
- Preparation of the antigen solution

Figure 6: Readout of the HI assay with avian RBCs. The pre- and post-vaccination induced influenza specific antibody response is determined by HI assay. In this example, person one has higher HI titers than person two. Both persons show an antibody response after vaccination; 180 days after the vaccination the antibody titers of both persons are decreased again. Please click here to view a larger version of this figure.
Results
Pre- and post-vaccination induced antibody response against Influenza A H3N2
The vaccine-induced antibody response was assessed in 26 healthy volunteers who received an inactivated trivalent subunit influenza vaccine containing Influenza A/H1N1/California/2009, A/H3N2/Texas/2012, and B/Massachusetts/02/2012 prior to the 2014/2015 influenza season. Figure 6 shows a representative example of 2 vaccine recipients. Interestingly, during tha...
Discussion
Quantification of pre- and post-vaccination influenza virus specific antibody titers is an important tool necessary for vaccine studies. Based on the surrogate measures of protection against virus infection, such as seroprotection (>1:40) or seroconversion (4-fold titer increase), vaccination strategies can be optimized9. Using the provided protocols can determine: (i) the hemagglutination potential of a particular virus, and (ii) the antibody titers for a virus of interest.
Disclosures
A.E. was supported by a research grants from the "SNSF Ambizione Score" program (PZ00P3_154709), "Forschungsfond, Förderung strategischer Projekte" University of Basel, Stiftungsinfektionskrankheiten Basel, and Bangeter Rhyner Stiftung. L.K. was supported by a grant of the Technical University of Graz, Austria. J.L. acknowledges support by an iPhD fellowship of the SystemsX.ch initiative in systems biology program (9th call).
Acknowledgements
none.
Materials
| Name | Company | Catalog Number | Comments |
| 25 ml Disposable Multichannel Pipette Reservoirs | Integra | 4312 | |
| 8-well PCR tubes | Brand GMBH | 781332 | For serum aliquots |
| 96-well microtiter plate, U-shaped | TPP | 92097 | For HI assay when using mammalian RBCs |
| 96-well microtiter plate, V-shaped | Corning Costar | 3897 | For HI assay when using avian RBCs |
| Aqua ad iniect. Steril | Bichsel AG | 1000004 | For preparing influenza antigen and cholera filtrate solutions |
| Chicken RBC (10%) | Cedarlane | CLC8800 | 10% suspension of chicken red blood cells in Alsever's solution |
| Cholera filtrate | Sigma-Aldrich | C8772 | Used as receptor destroying enzyme (RDE) |
| Dulbecco's PBS | Sigma-Aldrich | D8537 | For diluting the serum samples, RBCs and antigens |
| Eppendorf Multichannel pipette, 12-channel, 10-100 µl | Sigma-Aldrich | Z683949 | |
| Eppendorf Multichannel pipette, 8-channel, 10-100 µl | Sigma-Aldrich | Z683930 | |
| Guinea Pig RBC (10%) | Cedarlane | CLC1800 | 10% suspension of guinea pig red blood cells in Alsever's solution |
| Influenza Anti-A/California/7/09 HA serum | NIBSC | 14/134 | Used as positive control at the HI assay |
| Influenza Anti-A/Switzerland/9715293/2013-like HA serum | NIBSC | 14/272 | Used as positive control at the HI assay |
| Influenza Anti-A/Texas/50/2012-Like HA Serum | NIBSC | 13/178 | Used as positive control at the HI assay |
| Influenza Anti-B/Brisbane/60/2008-HA serum | NIBSC | 13/254 | Used as positive control at the HI assay |
| Influenza Anti-B/Massachusetts/02/2012 HA serum | NIBSC | 13/182 | Used as positive control at the HI assay |
| Influenza antigen A/California/7/09 (H1N1)(NYMC-X181) | NIBSC | 12/168 | Inactivated, partially purified A/California/7/09 (H1N1)(NYMC-X181) virus (ca. 46µgHA/ml) |
| Influenza antigen A/Switzerland/9715293/2013 (NIB88) | NIBSC | 14/254 | Inactivated, partially purified A/Switzerland/9715293/2013 (NIB88) virus (ca. 55µgHA/ml) |
| Influenza antigen A/Texas/50/2012 (H3N2)(NYMCX-223) | NIBSC | 13/112 | Inactivated, partially purified A/Texas/50/2012 (H3N2)(NYMCX-223) virus (ca. 74µgHA/ml) |
| Influenza antigen B/Brisbane/60/2008 | NIBSC | 13/234 | Inactivated, partially purified B/Brisbane/60/2008 virus (ca. 42µgHA/ml) |
| Influenza antigen B/Massachusetts/02/2012 | NIBSC | 13/134 | Inactivated, partially purified B/Massachusetts/02/2012 virus (ca. 35µgHA/ml) |
| Serum-Tubes | S-Monovette, Sardstedt | 01.1601.100 | For serum extraction with clotting activator |
| Single Donor Human RBC, Type 0 | Innovative Research | IPLA-WB3 | Suspension of single donor human red blood cells in Alsever's solution (ca. 26%) |
| Turkey RBC (10%) | Cedarlane | CLC1180 | 10% suspension of turkey red blood cells in Alsever's solution |
| Phosphate Buffered Saline (PBS) | Gibco |
References
- . Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices--United States, 2013-2014. MMWR Recomm Rep. 62, 1-43 (2013).
- Dominguez-Cherit, G., et al. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 302 (17), 1880-1887 (2009).
- Fox, B. D., et al. Pandemic influenza (H1N1): impact on lung transplant recipients and candidates. J Heart Lung Transplant. 29 (9), 1034-1038 (2010).
- Piercy, J., Miles, A., Krankheiten, S. B. f. G. S. V., Values, M. . The Economic Impact of Influenza in Switzerland: Interpandemic Situation. , (2003).
- Barclay, V. C., et al. Positive network assortativity of influenza vaccination at a high school: implications for outbreak risk and herd immunity. PLoS One. 9 (2), 87042 (2014).
- Baluch, A., et al. Randomized controlled trial of high-dose intradermal versus standard-dose intramuscular influenza vaccine in organ transplant recipients. Am J Transplant. 13 (4), 1026-1033 (2013).
- Haralambieva, I. H., et al. The Impact of Immunosenescence on Humoral Immune Response Variation after Influenza A/H1N1 Vaccination in Older Subjects. PLoS One. 10 (3), 0122282 (2015).
- Egli, A., et al. Vaccine adjuvants--understanding molecular mechanisms to improve vaccines. Swiss Med Wkly. 144, 13940 (2014).
- O'Shea, D., Widmer, L. A., Stelling, J., Egli, A. Changing face of vaccination in immunocompromised hosts. Curr Infect Dis Rep. 16 (9), 420 (2014).
- Meulemans, G., Carlier, M. C., Gonze, M., Petit, P. Comparison of hemagglutination-inhibition, agar gel precipitin, and enzyme-linked immunosorbent assay for measuring antibodies against influenza viruses in chickens. Avian Dis. 31 (3), 560-563 (1987).
- Martins, T. B. Development of internal controls for the Luminex instrument as part of a multiplex seven-analyte viral respiratory antibody profile. Clin Diagn Lab Immunol. 9 (1), 41-45 (2002).
- Webster, R., Cox, N., Stöhr, K. WHO Animal Influenza Manual. WHO/CDS/CSR/NCS. 2002.5, 1-99 (2002).
- Mittelholzer, C. M., et al. Human cell lines used in a micro neutralization test for measuring influenza-neutralizing antibodies. Scand J Immunol. 63 (4), 257-263 (2006).
- Hamilton, R. S., Gravell, M., Major, E. O. Comparison of antibody titers determined by hemagglutination inhibition and enzyme immunoassay for JC virus and BK virus. J Clin Microbiol. 38 (1), 105-109 (2000).
- Kumakura, S., et al. Comparison of hemagglutination inhibition assay and enzyme immunoassay for determination of mumps and rubella immune status in health care personnel. J Clin Lab Anal. 27 (5), 418-421 (2013).
- Ogundiji, O. T., Okonko, I. O., Adu, F. D. Determination of measles hemagglutination inhibiting antibody levels among school children in Ibadan, Nigeria. J Immunoassay Immunochem. 34 (2), 208-217 (2013).
- Cwach, K. T., Sandbulte, H. R., Klonoski, J. M., Huber, V. C. Contribution of murine innate serum inhibitors toward interference within influenza virus immune assay. Influenza Other Respir Viruses. 6 (2), 127-135 (2012).
- Lee, P. S., et al. Receptor mimicry by antibody F045-092 facilitates universal binding to the H3 subtype of influenza virus. Nat Commun. 5, 3614 (2014).
- Blumel, B., et al. Age-related prevalence of cross-reactive antibodies against influenza A(H3N2) variant virus, Germany, 2003 to 2010. Euro Surveill. 20 (32), 16-24 (2015).
- Reber, A. J., et al. Seasonal Influenza Vaccination of Children Induces Humoral and Cell-Mediated Immunity Beyond the Current Season: Cross-reactivity with Past and Future Strains. J Infect Dis. , (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved