A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Precise, High-throughput Analysis of Bacterial Growth
In This Article
Summary
Quantitative evaluation of bacterial growth is essential to understanding microbial physiology as a systems-level phenomenon. A protocol for experimental manipulation and an analytical approach are introduced, allowing for precise, high-throughput analysis of bacterial growth, which is a key subject of interest in systems biology.
Abstract
Bacterial growth is a central concept in the development of modern microbial physiology, as well as in the investigation of cellular dynamics at the systems level. Recent studies have reported correlations between bacterial growth and genome-wide events, such as genome reduction and transcriptome reorganization. Correctly analyzing bacterial growth is crucial for understanding the growth-dependent coordination of gene functions and cellular components. Accordingly, the precise quantitative evaluation of bacterial growth in a high-throughput manner is required. Emerging technological developments offer new experimental tools that allow updates of the methods used for studying bacterial growth. The protocol introduced here employs a microplate reader with a highly optimized experimental procedure for the reproducible and precise evaluation of bacterial growth. This protocol was used to evaluate the growth of several previously described Escherichia coli strains. The main steps of the protocol are as follows: the preparation of a large number of cell stocks in small vials for repeated tests with reproducible results, the use of 96-well plates for high-throughput growth evaluation, and the manual calculation of two major parameters (i.e., maximal growth rate and population density) representing the growth dynamics. In comparison to the traditional colony-forming unit (CFU) assay, which counts the cells that are cultured in glass tubes over time on agar plates, the present method is more efficient and provides more detailed temporal records of growth changes, but has a stricter detection limit at low population densities. In summary, the described method is advantageous for the precise and reproducible high-throughput analysis of bacterial growth, which can be used to draw conceptual conclusions or to make theoretical observations.
Introduction
Microbiological studies often start with the culture of bacterial cells and the assessment of the bacterial growth curves, which represent a fundamental phenomenon of bacterial physiology1,2,3. Basic culture principles are widely available in the published research literature and textbooks because bacterial culture is a fundamental methodology. At the bench level, substantial attention has traditionally been focused on optimizing growth media and culturing conditions, but controlling the growth rate, which would likely provide even greater understanding of microbial physiology, has not been extensively studied4. For exponentially growing bacteria, a key parameter of the cellular state is the growth rate, which has been reported to be coordinated with the genome, transcriptome, and proteome5,6,7,8. Thus, quantitative evaluation of bacterial growth is crucial for understanding microbial physiology.
To evaluate bacterial growth, the experimental methods used to estimate biomass are well established9,10 and are based on the detection of biochemical, physical, or biological parameters, such as optical turbidity. In addition, the analytical methods used to capture the dynamic properties of growth changes are commonly based on established nonlinear models11,12,13, for example, logistic equations. Growth dynamics are generally acquired by timed sampling of cell growth in culture by either measuring optical turbidity or performing colony-forming unit (CFU) assays. The limitation of these culturing and detection methods is that the data points are not a true reflection of population dynamics because the measurement intervals are often in hours and because the culture condition (e.g., changes in temperature and aeration) is disturbed at the time of sampling. Culture and analysis techniques must be updated using recent developments in technology and understanding. Recent advances in microplate readers allow the real-time observation of bacterial growth and significantly decrease labor costs. Using these advanced devices, the latest studies on bacterial growth have reported analytical methods for high-throughput measurements14,15.
The purpose of this protocol is to evaluate the precise growth dynamics in a high-throughput manner, which will be valuable for quantitative studies that ultimately address the questions of how the growth rate is determined and what factors affect the growth rate. The protocol addresses all factors that should be taken into account for the repeatable and precise quantitation of bacterial growth. The experimental method and analysis are described in detail in the main text. This method permits the precise and reproducible analysis of bacterial growth in a high-throughput manner. Microbiologists can use this protocol to derive additional quantitative results from their experimental evidence. This protocol can also be used for studies in systems biology that attempt to draw conceptual conclusions or to achieve a theoretical overview of growth.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Preparing the Growth Medium
NOTE: The chemical composition of minimal medium M63 is as follows: 62 mM K2HPO4, 39 mM KH2PO4, 15 mM (NH4)2SO4, 1.8 µM FeSO4, 15 µM thiamine-HCl, 0.2 mM MgSO4, and 22 mM glucose. M63 is made by mixing three stock solutions: Five X solution, 20% glucose and MgSO4 thiamine solution. Store all solutions at 4 °C.
- Preparing the Five X solution
- To prepare FeSO4 solution, use an electrical pipette and a disposable serological pipette to add ddH2O to a 50-mL centrifuge tube. Use a P-200 to add 0.06 mL HCl to obtain 0.01 M HCl. Add 36 mg FeSO4-7H2O and mix well.
- Measure 160 mL ddH2O in a measuring cylinder and add it to a 500-mL beaker. Add the following in this order: 10.72 g K2HPO4, 5.24 g KH2PO4, 2.0 g (NH4)2SO4, and 0.5 mL FeSO4 solution (prepared in step 1.1.1).
- Using a precision pH meter, adjust the pH to 7.0 by adding 2 M KOH. Pour the solution in a measuring cylinder and add ddH2O to a total volume of 200 mL. Sterilize the solution using a 250-mL filtration unit (PVDF, 0.22 µM).
- Preparing the 20% glucose solution
- Measure 200 mL ddH2O in a measuring cylinder and pour it to a 500-mL beaker. While mixing using a magnetic stirrer, add 50 g of glucose. Dilute the solution in a measuring cylinder to a total volume of 250 mL. Sterilize the solution as described in step 1.1.3.
- Preparing the MgSO4 thiamine solution
- Add 150 mL ddH2O to a 500-mL beaker. While mixing with a magnetic stirrer, add 1.0 g of thiamine-HCl and 10 g of MgSO4-7H2O. Dilute the solution in a measuring cylinder to a total volume of 200 mL. Sterilize the solution as described in step 1.1.3.
- Preparing minimal medium M63
- Place the Five X solution, the 20% glucose solution, the MgSO4 thiamine solution, an electronic pipette, a P-200 pipette, and 200-µL pipette tips on a clean bench.
- Measure 155.8 mL ddH2O in a measuring cylinder and pour it into a 500-mL beaker.
- Using the electronic pipette and disposable serological pipette, add 40 mL of Five X solution and 4 mL 20% glucose solution to the measured ddH2O. Then, add 0.2 mL MgSO4 thiamine solution with the P-200. Sterilize the solution as described in step 1.1.3.
2. Preparing the Glycerol Stock
- Cell culture
NOTE: The bacterial strains (e.g., W3110 and its reduced genomes) are available from strain bank organizations. The strains are usually obtained in the form of colonies on agar plates.- Place five sterilized glass tubes with silicone rubber stoppers, an electronic pipette, P-1,000, 1,000-µL pipette tips, P-200, 200-µL pipette tips, and the target strains (colonies on plates) on a clean bench.
- Expose the mouth of the glass tube to a Bunsen burner before opening the silicone rubber stopper. Expose the silicone rubber stopper to the flame after the tube is opened, and then lightly place the cap back on the glass tube.
- Use the electronic pipette and disposable serological pipette to add 5 mL M63 to one of the glass tubes and 4.5 mL M63 to the other four tubes.
- Use the P-200 tip to pick a colony and inoculate it in the glass tube containing 5 mL M63.
- Vortex the tube to make a suspension. Then, dilute the solution 10-fold by transferring 0.5 mL of this solution to one of the four tubes containing 4.5 mL M63.
- Repeat the process described in step 2.1.5 for the remaining tubes. A dilution series with five different concentrations (dilutions of 1, 10, 100, 1000, and 10,000) is now ready.
- Sterilize the mouths of the glass tubes and the silicone rubber stoppers as described in step 2.1.2. Cap the tubes with the stoppers. Avoid contamination by not wrinkling the silicone rubber stoppers.
- Place the five tubes in a pre-warmed shaking incubator at 37 °C and shake at 200 rpm. Incubate the culture overnight or for 10 to 30 h.
- Selection of the culture for the glycerol stock
- Place the pre-warmed room temperature M63 medium, P-1,000, 1,000-µL pipette tips, and a disposable cuvette on a clean bench.
- Add 1000 µL M63 to a disposable cuvette with a P-1,000. Place the disposable cuvette in a spectrophotometer, start the program at a fixed wavelength of 600 nm, and measure the blank.
- Move the five glass tubes from the shaking incubator to the clean bench.
- Discard M63 from the disposable cuvette and add 1,000 µL culture to the same disposable cuvette with a P-1,000. Measure the optical turbidity of the cell culture (OD600) as described in step 2.2.2.
NOTE: To avoid any contamination and to achieve a precise measurement, expose the glass tubes and the stoppers to the flame as described and vortex the culture before sampling. To ensure reliable results, repeated measurements are recommended, particularly when the cell density is low. - Of the five cell cultures, choose one that is in the early exponential growth phase (OD600= 0.01 - 0.05) for the glycerol stock.
NOTE: If multiple cultures have densities within the optimal range, the one closest to 0.05 is commonly selected.
- Make the glycerol stocks for repeated tests.
NOTE: This is described for preparing ten stocks. Larger or smaller amounts can be made according to the experimental requirements.- Place the sterilized 60% glycerol solution, ten sterilized 1.5-mL microtubes, P-1,000 and P-200, 1,000- and 200-µL pipette tips, and a microtube stand on a clean bench.
- Add 250 µL sterilized 60% glycerol solution and 750 µL of the selected cell culture to the 1.5-mL microtube and mix by pipetting.
NOTE: The stock volume is variable, but always maintain a 1:3 ratio of 60% glycerol to cell culture; this results in a final concentration of 15% glycerol. - Place the remaining nine microtubes in the microtube stand and dispense 100 µL of the mixture prepared in step 2.3.2 to each tube. There are now ten identical glycerol stocks for future use.
- Store the stocks in a deep freezer at -80 °C.
3. Acquiring the Growth Curves
- Setting up the microplate reader.
NOTE: The terms shown in quotation marks show the specific phrasing used on the plate reader used here (see the Table of Materials).- Open the software. Open "Protocols" in "Task Manager" and choose "Create New". Choose "Standard Protocol".
- Open "Procedure", and adjust the settings. Open "Set Temperature", and select "Incubator On". Set "Temperature" to 37 °C and "Gradient" to 0 °C. Check "Preheat" before continuing with the next step. Open "Start Kinetic", set "Run time" for 24:00:00 or 48:00:00, and "Interval" for 00:30:00 or 01:00:00.
NOTE: It takes approximately 1 min to read an entire 96 well plate. - Open "Shake" and set "Shake Mode" as "Linear". Check "Constitution Shake" and set "Frequency" at 567 cpm. Open "Read", check "Absorbance", "Endpoint/Kinetic", and "Monochromators." Set "Wavelength" to 600.
- Click "Validate" to confirm that the procedure is correct. Click "Save" to save it as a new program for future use.
- Real-time recording of growth
- Draw a 96-well plate pictogram (8 × 12 table) to indicate the positions of the inoculated culture samples on the 96-well plate. Print out the table and use it as a reference for the experiment.
NOTE: The wells located on the edges of the microplate should only contain blank medium because of evaporation. - Place a sterilized 96-well flat bottom microplate with lid, P-1000, 1000-µL pipette tips, P-200, 200-µL pipette tips, several 1.5-mL microtubes, an 8-multichannel pipette, a sterilized reagent reservoir, room temperature M63, and the glycerol stocks (prepared in step 2.3) on a clean bench.
- Add approximately 25 mL M63 to the reagent reservoir. Use this reservoir stock for all the following steps.
- Add 900 µL M63 to the microtubes in preparation for making serial dilutions.
- Thaw the glycerol stock at room temperature. Add 900 µL M63 to the thawed glycerol stock and vortex. This results in a 10-fold dilution of the original glycerol stock.
- Transfer 100 µL of the 10-fold dilution to another microtube containing 900 µL of M63 and vortex. This results in a 100-fold dilution.
- Repeat step 3.2.6 until the desired number of dilutions are achieved.
- Fill the wells at the edge of the microplate with 200 µL M63 using an 8-channel pipette (P-200).
NOTE: These wells can be used as the blank. - Load 200 µL of each diluted sample prepared in steps 3.2.4 - 3.2.7 to the microplate wells according to the reference table (step 3.2.1). Vortex the diluted samples prior to loading and load the same sample in multiple wells at varied locations on the plate.
- Place the 96-well microplate on the plate reader.
- Open "Read Now" in "Task Manager" and choose the program (section 3.1). Click "OK" to start measuring. Save the recording as a new experimental file for data analysis (section 4).
- Draw a 96-well plate pictogram (8 × 12 table) to indicate the positions of the inoculated culture samples on the 96-well plate. Print out the table and use it as a reference for the experiment.
4. Data Analysis
- Export the results of the real-time growth-rate data (section 3.2) to a USB memory stick as a text file.
NOTE: A 96-well formatted results (curves) will display on the plate reader in real-time. The hourly records (OD values) can be exported as a table; the rows and columns represent the well numbers (e.g., A1, B1) and the measuring time (e.g., 00:00:59), respectively. - Open the text file with a spreadsheet software.
- Copy the hourly OD600 reads of the 96-well microplate to a new worksheet for further analysis.
- Subtract the background reads at time zero from the hourly reads of each sample well.
NOTE: For convenience, the mean value of the blank wells containing M63 (step 3.2.8) can be used as the background value. - Calculate the mean of five consecutive OD600 reads to estimate the maximal population density.
NOTE: The largest mean value of the five consecutive OD600 reads is defined as the maximal OD600 of the corresponding growth curve. - Calculate the growth rate, µ (h-1), by applying the following equation for all pairs of consecutive values of OD600:
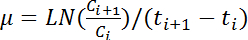
NOTE: In this equation, Ci and Ci+1 represent the OD600 values of any two consecutive time points (ti and ti+1, respectively). LN indicates natural logarithm. - Calculate the changes in the growth rate over time based on the hourly OD600 reads according to step 4.6. Calculate the mean and the standard deviation of five consecutive growth rates to estimate the maximal growth rate.
NOTE: The largest mean with the smallest standard deviation is defined as the maximal growth rate of the corresponding growth curve.
5. Confirming the Global Bias of the 96-well Reads (Optional)
NOTE: Both the plate reader and the consumable 96-well plate can cause biased measurements. To achieve highly precise and reproducible quantitative results, confirming the global bias of the 96-well plate is highly recommended.
- Prepare the 96-well plate and the plate reader for the bias test as described in section 3.2.2.
- Add 20 mL M63 to a sterilized 50-mL centrifuge tube. Add the glycerol stock to the same centrifuge tube and vortex. Transfer the suspended solution to a sterilized reagent reservoir.
- Use an 8-channel pipette to transfer 200 µL of the suspended solution to the 96-well plate.
- Place the 96-well plate on the plate reader and start the measurement.
- Record the hourly OD600 reads and analyze as described in section 4. Compare the calculated maximal growth rate and population density of each well to determine locational bias of the 96-well reads.
Access restricted. Please log in or start a trial to view this content.
Results
The described method provides a means to capture dynamic bacterial growth in a continuous, high-throughput manner by utilizing a 96-well format reader that takes multiple optical density measurements at various time intervals (from minutes to hours to days). The growth curves of an assortment of E. coli strains expressing various genomes can be precisely acquired in a single experiment (Figure 1A). In comparison to the described method, the tradition...
Access restricted. Please log in or start a trial to view this content.
Discussion
Critical steps in the protocol include the preparation of a common stock of exponentially growing cells and the replication of the same samples in multiple wells at various positions on the microplate. Previously, microbiologists started the culture from an overnight pre-culture. While this method may reduce the lag time of bacterial growth, it is difficult to achieve reproducible growth curves. As shown in Figure 2, the independent measurements using the common glycerol stocks resulted in n...
Access restricted. Please log in or start a trial to view this content.
Disclosures
We thank Kohei Tsuchiya for providing the CFU assay example. This work was partially financially supported by a Grant-in-Aid for Scientific Research (C) no. 26506003 (to BWY) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Acknowledgements
The authors have nothing to disclose.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| K2HPO4 | Wako | 164-04295 | |
| KH2PO4 | Wako | 166-04255 | |
| (NH4)2SO4 | Wako | 019-03435 | |
| MgSO4-7H2O | Wako | 138-00415 | |
| Thiamine-HCl | Wako | 201-00852 | |
| glucose | Wako | 049-31165 | |
| HCl | Wako | 080-01066 | |
| Iron (II) sulfate heptahydrate (FeSO4-7H2O) | Wako | 094-01082 | |
| KOH | Wako | 168-21815 | |
| Glycerol | Wako | 075-00611 | |
| Centrifuge tube (50 mL, sterilized) | WATSON | 1342-050S | |
| Pipette Tips, 200 µL | WATSON | 110-705Y | |
| Pipette Tips, 1,000 µL | WATSON | 110-8040 | |
| Microtube (1.5 mL) | WATSON | 131-715C | |
| 8 multichannel-pipette | WATSON | NT-8200 | |
| PASORINA STIRRER | AS ONE | 2-4990-02 | |
| Glass cylinder (200 mL) | AS ONE | 1-8562-07 | |
| Precision pH mater | AS ONE | AS800 / 1-054-01 | |
| Pipetman P-200 | GILSON | 1-6855-05 | |
| Pipetman P-1000 | GILSON | 1-6855-06 | |
| Disposable Serolocical Pipettes (10 mL) | SANPLATEC | SAN27014 | |
| Disposable Serolocical Pipettes (25 mL) | SANPLATEC | SAN27015 | |
| Microtube stand | BM Bio | 801-02Y | |
| Vortex | BM Bio | BM-V1 | |
| Corning Costar 96-well microplate with lid (Flat bottom, Clear) | Sigma-Aldrich | Corning, 3370 | |
| Corning Costar reagent reservoir (50 mL) | Sigma-Aldrich | Corning, 4870 | |
| Stericup GV PVDF (250 mL, 0.22 µM) | Merck Millipore | SCGVU02RE | |
| Pipet-Aid XP | DRUMMOND | 4-000-101 | |
| Bioshaker (BR-23UM MR) | TAITEC | 0053778-000 | |
| Disposal cell (1.5 mL) | Kartell | 1938 / 2-478-02 | |
| DU 730 Life Science UV/Vis Spectrophotometer | Beckman Coulter | A23616 | |
| EPOCH2 | BioTek | 2014-EP2-002 / EPOCH2T | |
| Beaker (500 mL) | IWAKI | 82-0008 | |
| BIO clean bench | Panasonic | MCV-B131F | |
| Glass tubes | NICHIDEN RIKA GLASS | P-10M~P-30 /101019 | |
| Silicone rubber stoppers | ShinEtsu Polymer | T-19 | |
| Bacterial strains | Strain bank organization; National Bio Resource Project (NBRP) in Japan |
References
- Kovarova-Kovar, K., Egli, T. Growth kinetics of suspended microbial cells: from single-substrate-controlled growth to mixed-substrate kinetics. Microbiol Mol Biol Rev. 62 (3), 646-666 (1998).
- Soupene, E., et al. Physiological studies of Escherichia coli strain MG1655: growth defects and apparent cross-regulation of gene expression. J Bacteriol. 185 (18), 5611-5626 (2003).
- Sezonov, G., Joseleau-Petit, D., D'Ari, R. Escherichia coli physiology in Luria-Bertani broth. J Bacteriol. 189 (23), 8746-8749 (2007).
- Egli, T. Microbial growth and physiology: a call for better craftsmanship. Front Microbiol. 6, 287(2015).
- Kurokawa, M., Seno, S., Matsuda, H., Ying, B. W. Correlation between genome reduction and bacterial growth. DNA Res. 23 (6), 517-525 (2016).
- Matsumoto, Y., Murakami, Y., Tsuru, S., Ying, B. W., Yomo, T. Growth rate-coordinated transcriptome reorganization in bacteria. BMC Genomics. 14, 808(2013).
- Nahku, R., et al. Specific growth rate dependent transcriptome profiling of Escherichia coli K12 MG1655 in accelerostat cultures. J Biotechnol. 145 (1), 60-65 (2010).
- Dai, X., et al. Reduction of translating ribosomes enables Escherichia coli to maintain elongation rates during slow growth. Nat Microbiol. 2, 16231(2016).
- Madrid, R. E., Felice, C. J. Microbial biomass estimation. Crit Rev Biotechnol. 25 (3), 97-112 (2005).
- Harris, C. M., Kell, D. B. The estimation of microbial biomass. Biosensors. 1 (1), 17-84 (1985).
- Yates, G. T., Smotzer, T. On the lag phase and initial decline of microbial growth curves. J Theor Biol. 244 (3), 511-517 (2007).
- Kargi, F. Re-interpretation of the logistic equation for batch microbial growth in relation to Monod kinetics. Lett Appl Microbiol. 48 (4), 398-401 (2009).
- Peleg, M., Corradini, M. G. Microbial growth curves: what the models tell us and what they cannot. Crit Rev Food Sci Nutr. 51 (10), 917-945 (2011).
- Sprouffske, K., Wagner, A. Growthcurver: an R package for obtaining interpretable metrics from microbial growth curves. BMC Bioinformatics. 17, 172(2016).
- Hall, B. G., Acar, H., Nandipati, A., Barlow, M. Growth rates made easy. Mol Biol Evol. 31 (1), 232-238 (2014).
- Hermsen, R., Okano, H., You, C., Werner, N., Hwa, T. A growth-rate composition formula for the growth of E.coli on co-utilized carbon substrates. Mol Syst Biol. 11 (4), 801(2015).
- Engen, S., Saether, B. E. r- and K-selection in fluctuating populations is determined by the evolutionary trade-off between two fitness measures: Growth rate and lifetime reproductive success. Evolution. 71 (1), 167-173 (2017).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved