A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Fatty Acid 13C Isotopologue Profiling Provides Insight into Trophic Carbon Transfer and Lipid Metabolism of Invertebrate Consumers
In This Article
Summary
The fatty acid trophic marker approach, i.e., the assimilation of fatty acids as entire molecule and transfer into consumer tissue with no or minor modification, is hampered by knowledge gaps in fatty acid metabolism of small soil invertebrates. Isotopologue profiling is provided as a valuable tool to disentangle trophic interactions.
Abstract
Fatty acids (FAs) are useful biomarkers in food web ecology because they are typically assimilated as a complete molecule and transferred into consumer tissue with minor or no modification, allowing the dietary routing between different trophic levels. However, the FA trophic marker approach is still hampered by the limited knowledge in lipid metabolism of the soil fauna. This study used entirely labelled palmitic acid (13C16:0, 99 atom%) as a tracer in fatty acid metabolism pathways of two widespread soil Collembola, Protaphorura fimata and Heteromurus nitidus. In order to investigate the fate and metabolic modifications of this precursor, a method of isotopologue profiling is presented, performed by mass spectrometry using single ion monitoring. Moreover, the upstream laboratory feeding experiment is described, as well as the extraction and methylation of dominant lipid fractions (neutral lipids, phospholipids) and the related formula and calculations. Isotopologue profiling does not only yield the overall 13C enrichment in fatty acids derived from the 13C labeled precursor but also produces the pattern of isotopologues exceeding the mass of the parent ion (i.e., the FA molecular ion M+) of each labeled FA by one or more mass units (M+1, M+2, M+3, etc.). This knowledge allows conclusions on the ratio of dietary routing of an entirely consumed FA in comparison to de novo biosynthesis. The isotopologue profiling is suggested as a useful tool for evaluation of fatty acid metabolism in soil animals to disentangle trophic interactions.
Introduction
In a cryptic habitat such as soil, trophic relationships are difficult to address and are further restricted by the small size of the fauna. The last decade has seen advances in biochemical ecology, particularly in the use of fatty acids as biomarkers for defining feeding strategies of the soil fauna under field conditions1,2,3. This is based on the fact that fatty acids from resources can be incorporated in consumer tissue as entire molecules, a process termed dietary routing4. Transfer of fatty acids has been reported over three trophic levels, i.e., from fungi to nematodes to Collembola5. Recently, the predatory fauna was considered6,7 and the first reviews on fatty acids as trophic markers in soil food webs have been published8,9.
More detailed information on trophic interactions is attained by fatty acid stable isotope probing (FA-SIP). The determination of 13C/12C ratios in fatty acids in diets and consumers can ascribe binary links and estimate the associated carbon flow, and has been employed in terrestrial, fresh water, and marine food webs10,11,12,13. The basic assumption is that dietary routed fatty acids are not subject to enzymatic processes; therefore, their 13C signal, i.e., the 13C/12C ratio of the fatty acid, in the consumer is similar to that in the diet1. However, a gradual depletion of the 13C signature up the food chain has been reported in aquatic systems, thereby hindering broad application of FA-SIP in trophic studies14,15,16. Moreover, knowledge in the lipid metabolism in most invertebrates in terrestrial food webs is still limited.
An understanding of the lipid metabolism pathways in consumers is essential for the usage of trophic marker fatty acids as means for the determination of the quantitative carbon flow in food web ecology. With this in mind, 13C-isotopologue profiling, which in principle can be applied for investigation of the carbon metabolism of any biological system17, is a promising method. Following the introduction of a 13C-labelled carbon substrate, the distribution of the 13C in the metabolic network is traceable since the generated metabolic products in the consumer show a specific isotopologue distribution. This can be assessed by quantitative nuclear metabolic resonance spectroscopy18,19 or mass spectrometry20,21, with the latter favored in biological samples with low biomass due to its higher sensitivity.
Although isotopologue profiling has been successfully applied to amino acids and provided insight into the in vivo carbon metabolism of bacterial pathogens17,22,23, its implementation in fatty acids has lagged behind. The first detailed analysis on the fate of a stable isotope labelled precursor fatty acid, its dietary routing or degradation via β-oxidation, in soil invertebrate consumers, was recently performed by Menzel et al.24. Here, the methodological basics for incorporation experiments with 13C labelled fatty acids followed by isotopologue analysis of key descendants in frequent soil invertebrates, the Collembola, are provided. These microarthropods are a good model group as they form important components of the soil food web and are well investigated for their trophic marker fatty acids8,25.
An understanding of the lipid metabolism pathways in consumers is essential for the usage of trophic marker fatty acids as means for the determination of the quantitative carbon flow in food web ecology. The present protocol gives the design and set up for a laboratory feeding experiment, and the biochemical procedures for extraction and methylation of dominant lipids fractions (neutral lipids, phospholipids) from Collembola. It demonstrates how the isotopologue composition of fatty acids is analyzed by mass spectrometry and describes the related formula and calculations. This procedure results in: (i) the ratios of isotopologues exceeding the mass of the parent ion (i.e., the fatty acid molecular ion M+) by one or more mass units (M+1, M+2, M+3, etc.) and (ii) the overall 13C enrichment in fatty acids derived from the 13C labelled precursor. Although used for Collembola, this approach can generally be applied to any other predator-prey interaction on the premise that these are culturable in sufficient quantity under controlled conditions to ensure a successful label uptake and subsequent verification.
Protocol
The described protocol does not fall under the competence of Animal Ethics. However, when people adapt the described protocols to higher animals, take care that the institutional Animal Ethics committee approved the protocol for animal handling.
1. Cultivation of Animals
NOTE: All explained experimental steps are based on well-established protocols26,27,28. Biotests in the laboratory need a continuous supply of easily culturable organisms. Here, the Collembola species Protaphorura fimata (Gisin, 1952) and Hetermurus nitidus (Templeton, 1835) have been used. Both species are simple to maintain as productive laboratory cultures fed with baker's yeast.
- In plastic microcosms with tight fitting lids (diameter 7 cm, height 4.5 cm), add a mixture of activated charcoal, plaster of Paris, and distilled water to provide a highly moist breeding substrate (Figure 1A).
- When preparing microcosms mix enough substrate, e.g., for 10 microcosms in a batch. Mix 9 parts of plaster of Paris (225 g) and 1 part of dry activated charcoal (25 g) together in a plaster pot, add carefully about 10 parts of distilled water (250 mL) and allow to sit for 5 minutes without stirring at room temperature.
- Stir with a lab spoon at moderate speed in clockwise direction to avoid air bubbles until a thick soupy consistency is achieved. Pour immediately into microcosms to a height of about 1 cm.
- Smooth the plaster by gentle tapping on the bench and swirling. Note that holes (random products of air bubbles) and furrows (actively added with a sterile spatula) may encourage fertile Collembola to lay eggs there. This study avoided holes and furrows in favor of having the same reproducible conditions. However, for demonstration purposes Figure 1B presents some holes.
- Allow drying for about 1 - 2 days at room temperature; incubation at 60 °C may reduce that time to 1 - 2 h.
- Moisten microcosms before use by adding tap water with a pipette until the substrate is slightly damp. Keep microcosm moist by regularly adding distilled water as Collembola have a soft cuticle and is susceptible to desiccation.
- Transfer Collembola easily to the plaster base using a simple suction tube, i.e., a long silicon tube about 25 cm long with a pipette tip fitted with a small mesh to prevent suction of animals into the tube. Alternatively, transfer animals by allowing them to adhere on the bristles of a small brush.
- Transfer (see step 1.2) 30 freshly hatched Collembola into new microcosms and provide granulated dry Baker's yeast as food (about a knife tip) (Figure 1B); renew at least twice a week. Plan three independent replicates per sampling day; in this study days 0 - 7 and 14. Incubate at 15 °C in darkness. Maintaining a constant temperature is essential, as fatty acids are altered by animal metabolism to meet the requirements for membrane fluidity.
- Feed Collembola with baker's yeast for four weeks before starting the exposure experiments to get a homogenous 13C/12C signal and pattern in fatty acids. Use a suction tube or a brush to remove all eggs (Figure 1C), fecal pellets, and exuviae regularly as animals may feed on them thereby altering their lipid profile.
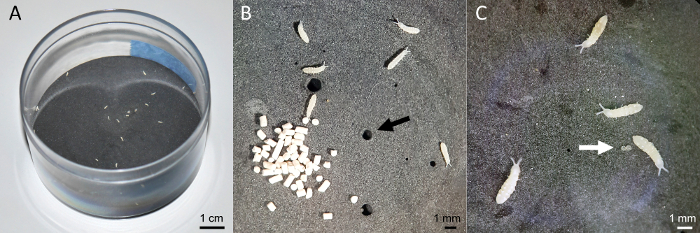
Figure 1: Cultivation of Collembola. (A) Microcosm filled with breeding substrate, a dried mixture of plaster of Paris, activated charcoal and distilled water. (B) and (C) Representative specimen of a Protaphorura fimata culture; note the small nuggets of dry Baker's yeast used as the food source and also as holes in the breeding substrate (black arrow) (B) as well as two eggs (white arrow) (C). Please click here to view a larger version of this figure.
2. Labelling Diet, Harvest, and Sample Handling
- Labelling
- Four weeks after establishing the Collembola cultures, place 30 individuals each into new microcosms and incubate at 15 °C in darkness.
- Introduce the pulse label by feeding them bakers' yeast containing an entirely 13C-labeled palmitic acid (13C16:0, 99 atom%) by mixing 13C-labeled palmitic acid with baker's yeast with a spatula at a ratio 0.5:1, e.g., 5 g 13C16:0 and 10 g dry baker's yeast. Place about a knife tip on each microcosm.
- After 6 h, replace this labeled food with completely unlabeled baker's yeast.
- Further cultivation
- During the course of the experiment renew the yeast diet every three days and add amounts higher than what Collembola will consume within that period. Most importantly, use a suction tube or brush to remove Collembolan eggs, fecal pellets and exuviae regularly to ensure exclusive feeding by the animals on the provided food.
- Harvest
- Sample microcosms destructively daily until day 7. Then on day 14, collect three independent replicates at each sampling time; different sampling times are possible.
- Prepare 10 mL glass tubes equipped with Teflon coated screw caps, one for each sample. Beforehand clean these tubes in a glassware washer and rinse twice with deionized water afterwards. Finally, to remove any traces of hydrophobic pollutants wash twice by adding 2 mL of chloroform (HPLC grade), vortex roughly and discard the solvent.
- For control samples (day 0), take 3 30 non-exposed Collembola from the pre-cultures as day 0 samples.
- For exposed samples (day 1 and onwards), take for the daily samples in each case 3 30 exposed Collembola from the cultures intermediately fed with the mix of 13C-labeled palmitic acid and baker's yeast.
- Record Collembola fresh weight by an ultra-microbalance. Transfer animals using an exhauster or brush to appropriate scale-pans. To ensure easy handling of Collembola during weighing in the scale-pans, shock-cool animals before (-80 °C for 2 h). Alternatively, a CO2 stream for 10 min can safely stun animals.
- Directly after weighing put animals from the scale-pans carefully into 10 mL glass test-tubes. Fill the tubes with 1 mL of methanol (HPLC grade) and store at -20 °C until analysis.
NOTE: From this step onwards, avoid sample handling with plastic equipment as organic solvents are involved; instead use dispensers and pipettes that are suitable for solvents as well as glass vessels.
3. Lipid Extraction from Animal Tissue and Methanolysis
- Prepare three glass test-tubes (equipped with Teflon coated screw caps) per batch containing only 1 mL of methanol as blank values. Importantly, add or transfer any solvent used in this protocol only by glass pipettes or chloroform/methanol rinsed solvent-resistant dispensers.
- At the beginning of the lipid extraction process, reduce the methanol applied for storage (or blanking) by evaporation; a compact benchtop Rotational Vacuum Concentrator (RVC) equipped with a vacuum pump and a cold trap is recommended. Transfer the open tubes into the RVC and evaporate until dry at 50 °C and vacuum pressure of 200 hPa for 20 min.
- Add 5 mL of single-phase extraction solvent (chloroform/methanol/0.05 M phosphate buffer 1:2:0.8, pH 7.4) to each sample (including blanks) and extract Collembolan lipids at room temperature by shaking overnight (~ 200 rpm).
- Transfer the solvent to new tubes and re-extract samples by shaking for 3 h with an additional 2.5 mL of extraction solvent. After that, combine extracts from both steps; the use of glass Pasteur pipettes is recommended. Add 0.8 mL of chloroform and 0.8 mL of distilled water, then mix and centrifuge at 2,000 g at 20 °C for 5 min. Finally, allow samples to stand for 5 min for the separation of aqueous and chloroform phases.
- For fatty acid pattern analysis, divide the total cellular lipids of Collembola into neutral lipid, glycolipid, and phospholipid fractions.
- For each sample, prepare a silica acid column (commercial column with 0.5 g silicic acid, mesh size 100-200 µm, see Table of Materials) by adding 1 mL of chloroform (preconditioning). To speed up this process, mount the columns on a vacuum block as commonly used in chromatography for solid phase extraction. Do not use nylon needles; use stainless steel above the tubes.
- After the chloroform used for preconditioning has passed through the column transfer the complete lower chloroform phase of each sample to an individual column. To simplify this procedure, the upper aqueous phase can be removed beforehand. Use Pasteur pipettes of glass, however, take care that the columns do not dry out.
- Successively elute lipid fractions with 5 mL of chloroform (including neutral lipid fatty acids, NLFAs), 10 mL of acetone (glycolipids - not analyzed in this project) and 5 mL of methanol (including phospholipid fatty acids, PLFAs). Collect each fraction in individual glass vessels.
- At the end of extraction, reduce the chloroform (NLFAs) and the methanol (PLFAs) via evaporation in an RVC. Transfer the open tubes to the RVC and evaporate until dry, ~ 90 min at 60 °C and a vacuum of 24 hPa.
- Start saponification of lipids (NLFA and PLFA fractions) following the protocol from Welch (1991)29 with addition of 1 mL of sodium hydroxide-methanol solution (45 g of sodium hydroxide, 150 mL of methanol, and 150 mL of distilled water) and incubate at 100 °C for 30 min in a water bath. Cool the sample in icy water for 2 min, then put the samples back on the bench and continue working at room temperature.
- Add the internal standard to each sample including the blanks. Choose a fatty acid not common in the experimental organisms; also use a saturated fatty acid to minimize loss by cleavage and select a molecule with intermediate chain-length. For many purposes, the odd-numbered nonadecanoic acid (19:0) works well. So, add 30 µL of a 0.74 mM solution in isooctane. The exact quantity is very important - be sure to have checked the precision of your pipette with a microbalance in advance.
- Add 2 mL of hydrochloric acid-methanol (mix 325 mL of 6.0 N hydrochloric acid with 275 mL of methanol), incubate at 80 °C for 10 min in a water bath and cool rapidly on ice for 2 min. This step is time and temperature sensitive; use 80 ± 1 °C and 10 ± 1 min. Check with how many samples can go into the water bath at once to keep the 80 °C.
NOTE: This procedure results in fatty acids methyl esters (FAMEs), i.e., fatty acid analytes stabilized for vaporization in gas chromatography. - Finally, add 1.25 mL of hexane/methyl tertiary butyl ether (1:1) and rock gently for 10 min, then centrifuge at 2,000 g for 5 min. Remove the bottom phase and keep the top phase comprised of FAMEs; use glass Pasteur pipettes. Add 3 mL of aqueous sodium hydroxide (10.8 g of NaOH dissolved in 900 mL of distilled water) for a washing step.
- Rock and centrifuge again.
- Take the complete upper lipid-containing phase using a glass Pasteur pipette and transfer to a gas chromatography sample vial equipped with a Teflon septum. Avoid including even small amounts of the aqueous phase, as this will cause problems with the GC measurement. Encapsulate vial and store at -20 °C until analysis.
4. Quantification of Fatty Acids by GC-FID
- Use gas chromatography to identify and quantify the FAMEs in the NFLA and PLFA fractions of Collembola (animal) lipids. A gas chromatograph (GC) equipped with flame ionization detector (FID) is an established and proven equipment30.
- For identification of FAMEs, compare retention time of peaks in the sample to those in a FAME standard. These are qualitative or quantitative standard mixture of key FAMEs covering a variety of food products and organisms.
- Employ FAME standard mixtures comprising representative FAs for the investigated organism group and experimental diet. In Collembola, these are fatty acids characteristic for eukaryotes, e.g., long-chain polyunsaturated fatty acids such as arachidonic acid (20:4ω6). When employing yeast as diet these are fungal markers such as linoleic acid (18:2ω6). A good choice is the so-called FAME mix, comprising 37 different fatty acids frequent in animal, fungal and plant material, and the bacterial acid methyl ester (BAME) mix (see Table of Materials).
- To run the samples on the GC-FID, set up a sequence with the respective software of the instrument. For instructions, refer to the manufacturer's manual. The sequence starts with the external standard mixtures (e.g. FAME and BAME mix), followed by the samples. Note that there is a retention time shift, i.e., a slight delay in elution time of the fatty acids from the GC column with each run, while running samples! Either include a standard every 10th run in the sample sequence or use retention time locking for palmitic acid (16:0).
- Adapt GC settings dependent on the instrument. The following program is suggested for a high performance (HP) capillary column (25 m x 0.2 mm i.d., film thickness 0.33 µm. Set injection volume to 1 µL in splitless mode and use hydrogen as carrier gas. Employ a temperature program beginning at 50 °C (held for 1 min) and increasing by 25 °C min-1 to 175 °C followed by 3 °C min-1 to 230 °C (held for 5.7 min).
- Calculate the nmol fatty acid per gram fresh (dry) weight of organisms using the response obtained by FID for each FAME applying the known amounts for the respective fatty acid using the following formula:
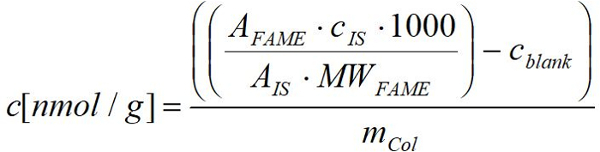
AFAME: Peak area of the respective FAME in the sample
MWFM: Molecular weight of the respective FAME in µg/µmol
CIS: Concentration of the internal standard in µg
AIS: Peak area of the internal standard
mCol: Fresh (dry) weight of respective Collembola sample in g
1000: Conversion factor from µmol to nmol
cBW: Concentration of the respective FAME in the mean of corresponding blank values in nmol
5. 13C Analysis by Isotopologue Profiling
- Use a GC system coupled to a Mass Selective Detector (MS) supplied with an electron ionization (EI) source for isotopologue determination.
- Use a polar capillary column (e.g., DB 23, CP-Sil 88) as this further allows the separation of unsaturated fatty acids even with the same numbers of double bonds. The choice of the GC column is critical for the results as it determines the good representation of the molecule ion in the fatty acids.
- For a DB 23 column (60 m x 0.25 mm i.d., film thickness 0.15 µm), start oven temperature at 130 °C and increase by 6.5 °C/min to 170 °C. Follow with an increase of 3 °C/min to 203 °C and hold for 1.9 min. Follow with an increase of 40 °C/min to 230 °C and hold for 8.3 min. Set transfer line temperature to 280 °C. Again, adjust GC method to the instrument.
- Use quantitative standards comprising known amounts of FAMEs for all fatty acids to be investigated for 13C incorporation. Put these standards at the start and end of each sample sequence run. Take the retention times of the fatty acids of interest from these standards.
- Measure samples from the experiments always starting with the unlabeled samples, and the labelled probes thereafter. Apply a split ratio appropriate to the sample concentrations, e.g. 1:12.5.If available for the instrument, apply a backflush with helium after each sample run to clear off column from remaining analytes.
- Apply retention time locking to the GC-MS method so that the SIM acquisition does not suffer from shifting and there is reproducible analyte retention times.
- Determine 13C incorporation in the molecular ion of fatty acids by the GC/EI-MS using the selected ion monitoring (SIM) mode of the instrument. Operation in SIM mode allows to detect specific analytes with increased sensitivity relative to full scan mode.
- Run an initial scan first to see what is present and then run SIM on appropriate ions. Acquire data at molecular masses of interest by selecting m/z scan windows (SIM groups) encompassing the chromatographic peak time of the respective fatty acid. Typically, monitor two to four ions per analyte and time window.
- In order to increase sensitivity, adjust the mass scan rate and dwell times (the time spent looking at each mass). The best quality data are obtained at the lowest possible speed, and a generally rule in SIM is 8 to 12 scans over the analyte peak. A proxy for instrument settings is an average dwell time per mass of 9 ms, a cycle time of 6 s and a scan time of 175 ms cyle-1.
- Detect the molecular ion (M+) of the respective fatty acid and all its isotopologues (M+1, M+2 and so forth). For examples, see Representative Results.
- Record the abundance of each ion fragment (isotopologue). Note that the abundance of the molecule ion and its isotopologues is relatively low and the quality of quantification depends greatly on the performance of the MS system. Run a tune before starting a large sample sequence (experiment) and clean the ion source if necessary.
NOTE: Firstly, these data yield the overall 13C enrichments of each fatty acids by the consumed precursor (here 16:0). - Use the proportion of the isotope composition of labelled fatty acids from their unmarked counterparts to assign the trophic carbon flux. Use the atom% formula in step 6.1 to calculate the percentage of the labelled carbon (atom percent, atom%) in the respective fatty acid. Compare the percentage of 13C in fatty acids of Collembola between labelled (day 1 and later) and unlabeled animals (day 0) as relative indication for the flow of 13C from diet into consumer.
- Assign the position of 13C incorporation into the fatty acid chain. Based on the distribution of the isotopologues disentangle the dietary routing of the entire marked tracer fatty acid (here 16:0) from chain elongation by lipid metabolism. While assimilation of the entire marker molecule increases the abundance of isotopologues farthest to the parent ion (M+), e.g., M+15, M+16, with chain elongation by the use of 13C labelled C2 fragments (13C acetyl-CoA) the isotopologues close to the molecular ion get more frequent.
6. Calculations of 13C Enrichment
- According to the distribution of the isotopologues, calculate the overall percentage of the labelled carbon (in atom%) in the respective fatty acid using the following relationship: atom% = (ratio 13C isotopologue incorporated) x (frequency respective isotopologue)
This is calculated after Kuppardt et al.31 as: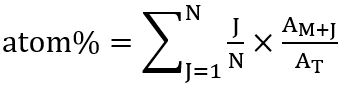
where N is the number of carbon atoms in the fatty acid, J is the number of 13C isotopes, AM+J is the abundance of the respective isotopologue, and AT the total abundance of all isotopologues. - For calculation, sum the peak area values of the molecular ion (M+) of the respective FA and all isotopologues (M+1, M+2, and so forth), detected by SIM-MS analysis, and set to 100% relative abundance. Calculate the portion of each detected isotopologue is easily by following the rule of three.
- Subtract the non-labelled day 0 control value (natural 13C background) from the experimental values to obtain final data traced only back to the performed external 13C-labeling.
Results
Fresh weight and lipid content of Collembola
In the course of the described experiment, the content in NLFAs and PLFAs did not change significantly over time, whereas the fresh weight of specimens increased slightly but not significantly24. Both parameters indicate a good level of physical fitness of the Collembola specimens. Be aware to investigate Collembola's fresh weight and lipid content throughout the experiment corresponding to the...
Discussion
Isotopologue profiling
A detailed analysis of the quantitative aspects in 13C distribution in FAs needs cutting-edge technology to assign carbon partitioning in food webs. The present work employed isotopologue profiling to assess the 13C/12C ratios in common FA biomarkers for tropic interactions. This method is well established for amino acid analysis by liquid chromatography (LC-MS) and was applied for investigations of carbon metabolism in p...
Acknowledgements
The financial support of R. Menzel and L. Ruess by the Deutsche Forschungsgemeinschaft (RU RU780/11-1) is gratefully acknowledged. R. Nehring was funded by RU 780/10-1. Finally, we are extremely thankful to Dr. Hazel Ruvimbo Maboreke for proofreading our manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| neoLab-Round jars | neoLab | 2-1506 | 69 x 40 mm, 10 pacs/pack |
| Charcoal activated | Carl Roth | X865.1 | p.a., powder, CAS No. 7440-44-0 |
| Alabaster Dental | RÖHRICH-GIPSE | --- | http://www.roehrich-gipse.de/dentalgipse.php |
| Chloroform | Carl Roth | 7331.1 | HPLC ≥ 99,9 % |
| Methanol | Carl Roth | P717.1 | HPLC ≥ 99,9 % |
| Hexan | Carl Roth | 7339.1 | HPLC ≥ 98 % |
| tert-Butyl methyl ether (MTBE) | Carl Roth | T175.1 | HPLC ≥ 99,5 % |
| Aceton | Carl Roth | 7328.2 | HPLC ≥ 99,9 % |
| NaOH | Carl Roth | 6771.1 | p.a. ≥99 %, in pellets |
| di-Natriumhydrogenphosphat | Carl Roth | P030.1 | p.a. ≥99 % , water free |
| Na-dihydrogenphosphat Dihydrat | Carl Roth | T879.1 | p.a. ≥99 % |
| Hypochloric acid (6 N) | VWR International | 26,115,000 | AVS TITRINORM vol. solution |
| Bond Elut (Columns) | Agilent Tech. | 14102037 | HF Bond Elut-SI, 500 mg, 3 mL, 50/PK |
| Präparatengläser Duran | Glasgerätebau Ochs | 135215 | Ø 16 x 100 mm, plus screw cap with handy knurl and integrated PTFE/silicone gasket |
| Supelco 37 Component FAME Mix | Sigma-Aldrich | 47885-U Supelco | 10 mg/mL in methylene chloride, analytical standard |
| FlowMesh | Carl Roth | 2796.1 | Polypropylene mesh, approximately 0.3 mm thick, with 1 mm strand spacing |
| Bacterial Acid Methyl Ester (BAME) Mix | Sigma-Aldrich | 47080-U Supelco | 10 mg/mL in methyl caproate, analytical standard |
| Methyl nonadecanoate | Sigma-Aldrich | 74208 | analytical standard ≥ 98.0 % |
| Hexadecanoic acid-1-13C (Palmitic) | Larodan Fine Chemicals | 78-1600 | GC ≥ 98.0 % (13C: 99.0 %) |
| RVC 2-25 CDplus | Martin Christ Gefrier-trocknungsanlagen | Compact benchtop midi concentrator | |
| Alpha 2-4 LDplus | Martin Christ Gefrier-trocknungsanlagen | Drying manifold | |
| MZ 2C NT | Vacuubrand GMBH | Vacuum pump | |
| Roto-Shake Genie | Scientific Industries | Combined rocking and rotating device | |
| XP64 Micro Comparator | Mettler Toledo | Super high precision balance | |
| GC-System 7890A | Agilent Tech. | Gas chromatograph | |
| 7000 GC/MS Triple Quad | Agilent Tech. | Triple Quad mass spectrometer | |
| 7683B Series Injector | Agilent Tech. | Sample injector | |
| Heraeus Multifuge 3SR+ | Thermo Scientific | Centrifuge with 10 ml tube rotor |
References
- Ruess, L., et al. Application of lipid analysis to understand trophic interactions in soil. Ecology. 86 (8), 2075-2082 (2005).
- Ruess, L., et al. Lipid composition of Collembola and their food resources in deciduous forest stands - Implications for feeding strategies. Soil Biology and Biochemistry. 39 (8), 1990-2000 (1990).
- Chamberlain, P. M., Bull, I. D., Black, H. I. J., Ineson, P., Evershed, R. P. Fatty acid composition and change in Collembola fed differing diets: identification of trophic biomarkers. Soil Biology and Biochemistry. 37 (9), 1608-1624 (2005).
- Stott, A. W., Davies, E., Evershed, R. P., Tuross, N. Monitoring the routing of dietary and biosynthesised lipids through compound-specific stable isotope (delta C-13) measurements at natural abundance. Naturwissenschaften. 84 (2), 82-86 (1997).
- Ruess, L., Haggblom, M. M., Langel, R., Scheu, S. Nitrogen isotope ratios and fatty acid composition as indicators of animal diets in belowground systems. Oecologia. 139 (3), 336-346 (2004).
- Pollierer, M. M., Scheu, S., Haubert, D. Taking it to the next level: Trophic transfer of marker fatty acids from basal resource to predators. Soil Biology and Biochemistry. 42 (6), 919-925 (2010).
- Ferlian, O., Scheu, S., Pollierer, M. M. Trophic interactions in centipedes (Chilopoda, Myriapoda) as indicated by fatty acid patterns: Variations with life stage, forest age and season. Soil Biology and Biochemistry. 52, 33-42 (2012).
- Ruess, L., Chamberlain, P. M. The fat that matters: Soil food web analysis using fatty acids and their carbon stable isotope signature. Soil Biology and Biochemistry. 42 (11), 1898-1910 (2010).
- Traugott, M., Kamenova, S., Ruess, L., Seeber, J., Plantegenest, M. Empirically characterising trophic networks: What emerging DNA-based methods, stable isotope and fatty acid analyses can offer. Adv Ecol Res. 49, 177-224 (2013).
- Hammer, B. T., Fogel, M. L., Hoering, T. C. Stable carbon isotope ratios of fatty acids in seagrass and redhead ducks. Chemical Geology. 152 (1-2), 29-41 (1998).
- Budge, S. M., Iverson, S. J., Koopman, H. N. Studying trophic ecology in marine ecosystems using fatty acids: A primer on analysis and interpretation. Marine Mammal Science. 22 (4), 759-801 (2006).
- Haubert, D., et al. Trophic structure and major trophic links in conventional versus organic farming systems as indicated by carbon stable isotope ratios of fatty acids. Oikos. 118 (10), 1579-1589 (2009).
- Ngosong, C., Raupp, J., Richnow, H. H., Ruess, L. Tracking Collembola feeding strategies by the natural 13C signal of fatty acids in an arable soil with different fertilizer regimes. Pedobiologia. 54 (4), 225-233 (2011).
- Bec, A., et al. Assessing the reliability of fatty acid-specific stable isotope analysis for trophic studies. Methods in Ecology and Evolution. 2 (6), 651-659 (2011).
- Gladyshev, M. I., Makhutova, O. N., Kravchuk, E. S., Anishchenko, O. V., Sushchik, N. N. Stable isotope fractionation of fatty acids of Daphnia fed laboratory cultures of microalgae. Limnologica. 56 (Supplement C. 56 (Supplement C), 23-29 (2016).
- Gladyshev, M. I., Sushchik, N. N., Kalachova, G. S., Makhutova, O. N. Stable isotope composition of fatty acids in organisms of different trophic levels in the Yenisei river. PLoS One. 7 (3), e34059 (2012).
- Eisenreich, W., Dandekar, T., Heesemann, J., Goebel, W. Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nature Reviews Microbiology. 8 (6), 401-412 (2010).
- Eylert, E., Bacher, A., Eisenreich, W. NMR-based isotopologue profiling of microbial carotenoids. Methods Mol Biol. 892, 315-333 (2012).
- Garton, N. J., O'Hare, H. M. Tuberculosis: feeding the enemy. Chemical Biology. 20 (8), 971-972 (2013).
- Rosenblatt, J., Chinkes, D., Wolfe, M., Wolfe, R. R. Stable isotope tracer analysis by GC-MS, including quantification of isotopomer effects. Am J Physiol. 263 (3), E584-E596 (1992).
- Fernandez, C. A., Des Rosiers, C., Previs, S. F., David, F., Brunengraber, H. Correction of 13C mass isotopomer distributions for natural stable isotope abundance. J Mass Spectrom. 31 (3), 255-262 (1996).
- Heuner, K., Eisenreich, W. The intracellular metabolism of legionella by isotopologue profiling. Methods Mol Biol. 954, 163-181 (2013).
- Willenborg, J., et al. Characterization of the pivotal carbon metabolism of Streptococcus suis serotype 2 under ex vivo and chemically defined in vitro conditions by isotopologue profiling. J Biol Chem. 290 (9), 5840-5854 (2015).
- Menzel, R., Ngosong, C., Ruess, L. Isotopologue profiling enables insights into dietary routing and metabolism of trophic biomarker fatty acids. Chemoecology. 27 (3), 101-114 (2017).
- Buse, T., Ruess, L., Filser, J. New trophic biomarkers for Collembola reared on algal diets. Pedobiologia. 56 (3), 153-159 (2013).
- Hutson, B. R. Effects of variations of the plaster-charcoal culture method on a Collembolan, Folsomia candida. Pedobiologia. 18, 138-144 (1978).
- Fountain, M. T., Hopkin, S. P. Folsomia candida (Collembola): a "standard" soil arthropod. Annu Rev Entomol. 50, 201-222 (2005).
- ISO, I. O. f. S. . Soil Quality-Inhibition of reproduction of Collembola (Folsomia candida) by soil pollutants. , (1999).
- Welch, D. F. Applications of cellular fatty acid analysis. Clin Microbiol Rev. 4 (4), 422-438 (1991).
- Dodds, E. D., McCoy, M. R., Rea, L. D., Kennish, J. M. Gas chromatographic quantification of fatty acid methyl esters: flame ionization detection vs. electron impact mass spectrometry. Lipids. 40 (4), 419-428 (2005).
- Kuppardt, S., Chatzinotas, A., Kastner, M. Development of a fatty acid and RNA stable isotope probing-based method for tracking protist grazing on bacteria in wastewater. Appl Environ Microbiol. 76 (24), 8222-8230 (2010).
- Zhang, X., He, H., Amelung, W. A GC/MS method for the assessment of 15N and 13C incorporation into soil amino acid enantiomers. Soil Biology and Biochemistry. 39 (11), 2785-2796 (2007).
- Vetter, W., Thurnhofer, S. Analysis of fatty acids by mass spectrometry in the selected ion monitoring mode. Lipid Technol. 19 (8), 184-186 (2007).
- Thurnhofer, S., Vetter, W. A gas chromatography/electron ionization-mass spectrometry-selected ion monitoring method for determining the fatty acid pattern in food after formation of fatty acid methyl esters. J Agric Food Chem. 53 (23), 8896-8903 (2005).
- Haubert, D., Haggblom, M. M., Scheu, S., Ruess, L. Effects of fungal food quality and starvation on the fatty acid composition of Protaphorura fimata (Collembola). Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology. 138 (1), 41-52 (2004).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved