A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Oral Intubation of Adult Zebrafish: A Model for Evaluating Intestinal Uptake of Bioactive Compounds
In This Article
Summary
The protocol describes intubating adult zebrafish with a biologic; then dissecting and preparing the intestine for cytometry, confocal microscopy and qPCR. This method allows administration of bioactive compounds to monitor intestinal uptake and the local immune stimulus evoked. It is relevant for testing the intestinal dynamics of oral prophylactics.
Abstract
Most pathogens invade organisms through their mucosa. This is particularly true in fish as they are continuously exposed to a microbial-rich water environment. Developing effective methods for oral delivery of immunostimulants or vaccines, which activate the immune system against infectious diseases, is highly desirable. In devising prophylactic tools, good experimental models are needed to test their performance. Here, we show a method for oral intubation of adult zebrafish and a set of procedures to dissect and prepare the intestine for cytometry, confocal microscopy and quantitative polymerase chain reaction (qPCR) analysis. With this protocol, we can precisely administer volumes up to 50 µL to fish weighing approximately 1 g simply and quickly, without harming the animals. This method allows us to explore the direct in vivo uptake of fluorescently labelled compounds by the intestinal mucosa and the immunomodulatory capacity of such biologics at the local site after intubation. By combining downstream methods such as flow cytometry, histology, qPCR and confocal microscopy of the intestinal tissue, we can understand how immunostimulants or vaccines are able to cross the intestinal mucosal barriers, pass through the lamina propria, and reach the muscle, exerting an effect on the intestinal mucosal immune system. The model could be used to test candidate oral prophylactics and delivery systems or the local effect of any orally administered bioactive compound.
Introduction
The goal of this article is to describe in depth a straightforward method for oral intubation of zebrafish, along with useful associated downstream procedures. Oral intubation using zebrafish has become a practical model in the study of infectious disease dynamics, oral vaccine/immunostimulant, drug/nanoparticle uptake and efficacy, and intestinal mucosal immunity. For example, zebrafish oral intubation has been used in the study of Mycobacterium marinum and Mycobacterium peregrinum infection1. Lovmo et al. also successfully used this model to deliver nanoparticles and M. marinum to the gastro-intestinal tract of adult zebrafish2. In addition, Chen et al. used zebrafish oral intubation to show that drugs encapsulated by nanoparticles, when administered via the gastro-intestinal tract, were transported across the blood brain barrier3. These authors performed intubation based on the gauvage method described by Collymore et al.4 with some modifications. However, they did not provide a highly detailed protocol describing the oral intubation procedure. Here, we present a method for oral intubation of adult zebrafish building on Collymore et al.4 We further include the preparation of the intestine for relevant downstream analysis by cytometry, confocal microscopy and qPCR.
The intestine and particularly its mucosa is the first-line of defense against infection and the primary site of nutrient uptake5. When the epithelial cells and antigen-presenting cells within mucosal barriers perceive danger signals, an immediate innate immune response is triggered. Next, the highly specific adaptive immune response is established by T and B lymphocytes6,7. Development of oral vaccines is a current focus area in vaccinology. Such vaccines would be an effective tool to protect the organism at exposed sites due to the specific response of immune cells in the mucosa-associated lymphoid tissues (MALT)8,9. In aquaculture, mucosal vaccines have obvious advantages compared to injectable vaccines. They are practical for mass vaccination, less labor-intensive, are less stressful to the fish, and can be administered to young fish. Nevertheless, mucosal vaccine candidates must reach the second gut segment without being denatured in the oral environment. They also must cross mucosal barriers in order to gain access to antigen presenting cells (APCs) to induce local and/or systemic responses10. Hence, testing of the mucosal uptake achieved by candidate oral antigens and their delivery systems, as well as the immune response evoked, is essential in the development of oral vaccines.
In a biomedical context, developing a model to test biological effects of compounds after oral intubation is of growing interest. Many of the anatomical and physiological features of the intestine are conserved between bilaterian lineages, with mammals and bony fishes11. This oral intubation model connected to downstream analysis can be a tool to provide insights into human biology, as well as a testing ground for biologics or other compounds in vivo.
The oral intubation protocol can be performed by one operator, e.g., successfully administrating up to 50 µL of the protein nanoparticle suspension to fish weighing 1 g, with a high survival rate. The procedure is simple to set up and quick; 30 fish can be intubated in 1 h. The protocol for intestine preparation is key to providing quality cell and tissue samples for subsequent analysis. Examples of downstream results are given which show the protocol's usefulness in obtaining data related to intestinal uptake and in isolating quality RNA for qPCR. The protocol would be of great use to those needing a suitable model to test the dynamics of oral prophylactics or other compounds in the intestine.
Protocol
All experimental procedures involving zebrafish (Danio rerio) were authorized by the Ethics Committee of the Universitat Autònoma de Barcelona (CEEH number 1582) in agreement with the International Guiding Principles for Research Involving Animals (EU 2010/63). All experiments with live zebrafish were performed at 26–28 °C.
1. Preparing the Equipment for Oral Intubation
- Place approximately 1 cm of a fine silicone tube on a 31 G Luer lock needle to cover the needle tip.
- Cut a 10 µL sterile filter pipette tip (around 2 cm), take the finer end and place it over the silicone tube as a sheath. Make sure the pipette extends beyond the tip of the needle to avoid injuring the animal.
- Attach the needle to a 100 µL Luer lock syringe.
NOTE: Always rinse with ethanol and then phosphate buffered saline (PBS, see Materials) thoroughly between treatments.
2. Solutions Required
- Prepare 150 mg/L (for anesthesia) or 300 mg/L (for euthanasia) of ethyl 3-aminobenzoate methanesulfonate (MS-222) solution with water from the aquarium where the zebrafish are maintained. Fill a small tank with 1 L of anesthetic solution and keep it aerated.
- Fill another small tank with 1 L of aquarium water without MS-222 for fish recovery and keep it aerated.
- Make 50 mL of 1x PBS from a 10x sterile stock solution.
- For cytometry analysis/intestinal cell isolation, prepare enough fresh 0.15% collagenase Type IV solution for 1 mL per fish from a stock solution or from powder in Dulbecco's modified eagle medium (DMEM) with 1% v/v penicillin and streptomycin (see materials). Make aliquots (1 per fish) of 1 mL in 2 mL centrifuge tubes. Keep at 4 °C until 30 min before the dissection step.
- For confocal microscopy/sample fixation, prepare 50 mL of fresh 4% paraformaldehyde (PFA) solution in PBS or thaw a stock solution from -20 °C freezer in a fume hood.
CAUTION: PFA is toxic. Please read the material safety data sheet before working with it. Gloves and safety glasses should be worn, and always leave solutions inside a fume hood.
3. Preparing the Fluorescent Nanoparticle Suspension
- Label the protein nanoparticle with Atto-488 NHS ester (see Table of Materials) or an appropriate fluorescent dye according to the manufacturer's instructions.
- Resuspend the nanoparticles in 0.1 M sodium bicarbonate buffer at the concentration of 2 mg/mL.
- Dissolve the Atto 488 NHS ester in amine-free dimethyl sulfoxide (DMSO) at 2 mg/mL. Keep an aliquot of 10 µL to check labeling efficiency (step 3.7–3.8).
- Mix the nanoparticles and the Atto 488 NHS ester at a molar ratio of 1:2 (protein:dye) by stirring in the dark.
- Spin down the labeled nanoparticles by centrifugation at 8,000 x g for 10 min at room temperature, remove the supernatant and keep it to check labeling efficiency (step 3.7–3.8).
- Wash the labeled nanoparticles by resuspending in 1 mL of 0.1 M sodium bicarbonate buffer by vortexing and pipetting up and down. Then discard the supernatant by centrifugation at 8,000 x g for 10 min at room temperature. Repeat step 3.6 for 5 times.
- Resuspend the pellet in 5 mL of 0.1 M sodium bicarbonate buffer in a 15 mL centrifuge tube and make aliquots of the fluorescent nanoparticle in 1.5 mL centrifuge tubes (30 aliquots). Spin down at 8,000 x g for 10 min at room temperature, discard the supernatant and store at -80 °C protected from the light.
- Measure the labeling efficiency using a microvolume spectrophotometer.
- Take 1 µL of the original Atto 488 solution kept from step 3.3 and further dilute it in DMSO (e.g., 1:20 according to the volume ratio). This is the volume used to get the molar ratio of the protein nanoparticle and Atto 488 mix at step 3.4.
- Take 1 µL of the saved supernatant from the labelling reaction in step 3.5. Measure the absorption (abs) at = 501 nm. The percentage of labelling is:
(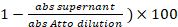
- Before the experiment, prepare the nanoparticle suspension at the desired concentration using 1x PBS solution.
4. Zebrafish Anesthetization and Oral Intubation
- Fast the fish (>0.5 g) at least 48 h before the experiment to empty the intestine.
- Move the fish (12 fish) to the experimental tanks (6 L) one night before the experiment to allow acclimatization12.
- Vortex the nanoparticle solution well (e.g., 2,500 rpm and 30 s) and draw up the desired volume of nanoparticle suspension (e.g., 20–50 µL) into the syringe attached to the protected needle.
- Place the fish in the aerated 150 mg/L MS-222 solution (see section 2) until they sink to the bottom of the tank and do not respond to a tail fin pinch; this process takes less than 5 min.
- Quickly transfer the anesthetized fish with a net to a wet plastic tray, orientate the animal horizontally to face the needle and immediately start the oral intubation.
- Carefully support the fish with one hand and open the mouth with the other hand using the protected needle. Gently insert the needle down the esophagus to about 1 cm from the mouth opening.
NOTE: The operator may feel a slight resistance when the end of the pipette tip has passed the gill. Take care not to angle the needle entry too much which may perforate the gill. - Slowly inject the nanoparticle suspension to the fish. Make sure the suspension does not flow outward through the gills or mouth.
- Gently remove the needle and place the fish into the recovery tank (see section 2). Recovery usually takes within 1 min.
- Check the fish carefully for any abnormality (e.g., bleeding at the gills is a sign of perforation).
- Once the fish have recovered, return them to the experimental tanks.
5. Zebrafish Intestine Dissection
- After a specified period of time post intubation (e.g., 5 h and/or 24 h), place the fish using a net into 300 mg/L MS-222 solution for euthanasia (see section 2). Make sure the operculum stops moving and there is no tail pinch reflex. Five minutes is normally sufficient.
- Pick up the euthanized animal with a net and place it on a filter paper.
NOTE: The filter paper is very useful for removing the adhesive tissue along the intestine. - Using sharp dissection scissors, make a semi-circular incision from the anus to the operculum and open incision using fine tweezers. Cut both ends of the intestine, take out all the internal organs, and place them on the filter paper.
CAUTION: Work quickly to reduce cell metabolism and death.
NOTE: Alternatively, remove the adhesive tissue in PBS and on ice. - Separate the intestine from internal organs making sure to keep its orientation (anterior to posterior intestinal segment) and stretch it out. Usually, the anterior segment of intestine is wider than posterior segment. Take care to obtain all of the intestine when dissecting.
NOTE: The posterior end is quite fine and fragile in small fish and may break off, particularly in animals <0.7 g. - Roll the intestine on the filter paper with tweezers in order to detach the adhesive tissue from the intestine.
- Proceed to prepare the intestine for various downstream analyses (sections 6, 7 and 8).
6. Preparing Intestinal Cells for Cytometry
- Prepare in advance aliquots of 0.15% collagenase solution (see section 2.4).
NOTE: Aliquots should be at room temperature before proceeding. - Optional: Continuing from step 5.5, slit open the intestine longitudinally and wash with 1x PBS.
- Using tweezers, place the intestine in the 2 mL centrifuge tube filled with 0.15% collagenase solution.
- Place the tubes on a vertical laboratory rotator for 1 h at room temperature in the dark.
- Place the intestine on a 100 µm cell strainer supported over a 50 mL centrifuge tube. Break up the intestine with a 5 mL syringe plunger, washing 3 times with 1x PBS, collecting the flow through sample in the 50 mL centrifuge tube.
- Centrifuge the 50 mL centrifuge tube at 400 x g for 10 min, at 4 °C.
- Carefully pipette off most of the supernatant while not losing the cells, some of which may be associated to the mucus.
- Resuspend the intestinal cells at the bottom of the centrifuge tube with 500 µL of 1x PBS and keep on ice until cytometry analysis
- Filter samples through a 30 µm cell filter into 5 mL round bottom tubes for cytometry.
- Set the parameters (e.g., the number of cells for analysis, the region of interest, voltage and compensation, selection of detectors) on a cytometer equipment (see materials).
- Immediately analyze the cells on a cytometer, following the instructions of use13.
7. Preparing Intestine Cryosections for Confocal Microscopy
- Fill the plastic mold (see Table of Materials) to half volume with optimum cutting temperature (O.C.T.) compound.
- Continuing from step 5.5 and immediately after the dissection, carefully place the intestine in the plastic mold. Make sure the intestine is totally embedded in the O.C.T. compound. If necessary, add more O.C.T. compound to the plastic mold.
NOTE: It is recommended to place the intestine with a "Z" shape in O.C.T. compound to easily follow its natural orientation. - Place the plastic mold on dry ice until it goes opaque (less than a minute).
- Store the plastic mold at -80 °C for long-term use or process immediately using the following procedure.
NOTE: The protocol can be paused here. - Slice the frozen intestine into 10 µm sections or appropriate thickness using a cryostat at -20 °C.
- Collect the intestine section with a fine brush onto a slide.
- Immerse the slide in 4% PFA for 15 min at room temperature to fix the sample.
CAUTION: PFA is toxic. Please read the material safety data sheet before working with it. Gloves and safety glasses should be worn, and always leave solutions inside a fume hood. - Wash the slide 3 times with 1x PBS, 10 min each.
- Add a drop of mounting medium and place a coverslip over the specimen.
NOTE: The protocol can be paused here. - Observe the sample under a confocal microscope at appropriate magnification.
8. Preparing the Intestine for Real Time qPCR (RT-qPCR)
- Continuing from step 5.5, put the intestine in a cryogenic vial and rapidly freeze the intestine in liquid nitrogen and store at -80 °C until use.
NOTE: The protocol can be paused here. - For homogenization, add 200 µL of 2% (v/v) chilled 1-Thioglycerol/Homogenization solution (see Table of Materials) or alternative homogenization solution to the intestine sample.
- Work quickly, homogenize the intestine sample on ice with a laboratory homogenizer at high speed (set at 25–30,000 rpm) until no visible tissue fragments remain. 3 times for 5 s is usually sufficient.
- Isolate RNA using a commercial kit (see Table of Materials) according to the manufacturer's instructions14 or a suitable alternative method. As needed, store the RNA at -80 °C for long-term use.
NOTE: The protocol can be paused here. - Quantify the RNA concentration using a spectrophotometer15 and evaluate the quality using a RNA analyzer16.
- Prepare 1 µg or an appropriate amount of cDNA using a cDNA synthesis kit according to the manufacturer's instructions.
NOTE: The protocol can be paused here. For qPCR analysis, please follow the MIQE guidelines17. - Design appropriate primer pairs for the gene/s of interest.
- Select a suitable reference gene and analyze the expression of each gene by a RT-qPCR detection system using a commercial kit (see materials).
NOTE: For example, add 5 µL of SYBR green supermix, 0.5 µM primers, 2.5 µL of diluted cDNA and 1.5 µL of water in a final volume of 10 µL for each well of qPCR plate.
Results
Zebrafish (average weight: 1.03 ± 0.16 g) of mixed sex were successfully intubated with different recombinant protein nanoparticles (bacterial inclusion bodies) using our home-made oral intubation device (Figure 1). We have successfully performed the oral intubation and achieved a low average percentage mortality (6.8%) (Table 1). Zebrafish were either intubated with 30 µL or 50 µL of nanoparticle suspensions and the mortality ...
Discussion
This protocol is an improvement of the previously described technique for oral intubation by Collymore et al.4 Our protocol describes in detail the oral intubation method and includes the preparation of the intestine for downstream analyses. Our method improves fish manipulation speed allowing one person to perform the whole protocol rapidly, without much variation between operators. A main difference of our protocol with the previous one is that we evaluate the success of an oral intubat...
Disclosures
The authors declare that no competing interests exist.
Acknowledgements
This work was supported by grants from the Spanish Ministry of Science, European commission and AGAUR funds to NR (AGL2015-65129-R MINECO/FEDER and 2014SGR-345 AGAUR). RT holds a pre-doctoral scholarship from AGAUR (Spain), JJ was supported by a PhD fellowship from the China Scholarship Council (China) and NR is supported by the Ramón y Cajal program (RYC-2010-06210, 2010, MINECO). We thank Dr. Torrealba for expert advice in protein production, N. Barba from the "Servei de Microscopia" and Dr. M. Costa from the "Servei de Citometria" of the Universitat Autònoma de Barcelona for helpful technical assistance.
Materials
| Name | Company | Catalog Number | Comments |
| Silicone tube | Dow Corning | 508-001 | 0.30 mm inner diameter and 0.64 mm outer diameter |
| Luer lock needle | Hamilton | 7750-22 | 31 G, Kel-F Hub |
| Luer lock syringe | Hamilton | 81020/01 | 100 μL, Kel-F Hub |
| Filtered pipette tip | Nerbe Plus | 07-613-8300 | 10 μL |
| MS-222 | Sigma Aldrich | E10521 | powder |
| 10x PBS | Sigma Aldrich | P5493 | |
| Filter paper | Filter-Lab | RM14034252 | |
| Collagenase | Gibco | 17104019 | |
| DMEM | Gibco | 31966 | Dulbecco's modified eagle medium |
| Penicillin and streptomycin | Gibco | 15240 | |
| Cell strainer | Falcon | 352360 | |
| CellTrics filters | Sysmex Partec | 04-004-2326 (Wolflabs) | 30 µm mesh size filters with 2 mL reservoir |
| Tissue-Tek O.C.T. compound | SAKURA | 4583 | |
| Plastic molds for cryosections | SAKURA | 4557 | Disposable Vinyl molds. 25 mm x 20 mm x 5 mm |
| Slide | Thermo Scientific | 10149870 | SuperFrost Plus slide |
| Cover glasses | Labbox | COVN-024-200 | 24´24 mm |
| Paraformaldehyde (PFA) | Sigma-Aldrich | 158127 | |
| Atto-488 NHS ester | Sigma-Aldrich | 41698 | |
| Sodium bicarbonate | Sigma-Aldrich | S5761 | |
| DMSO | Sigma-Aldrich | D8418 | |
| Maxwell RSC simplyRNA Tissue Kit | Promega | AS1340 | |
| 1-Thioglycerol/Homogenization solution | Promega | Inside of Maxwell RSC simplyRNA Tissue Kit | adding 20 μl 1-Thioglycerol to 1 mL homogenization solution (2%) |
| vertical laboratory rotator | Suministros Grupo Esper | 10000-01062 | |
| Cryostat | Leica | CM3050S | |
| Homogenizer | KINEMATICA | Polytron PT1600E | |
| Flow cytometer | Becton Dickinson | FACS Canto | |
| 5 mL round bottom tube | Falcon | 352058 | |
| Confocal microscope | Leica | SP5 | |
| Fume Hood | Kottermann | 2-447 BST | |
| Nanodrop 1000 | Thermo Fisher Scientific | ND-1000 | Spectrophotometer |
| Agilent 2100 Bioanalyzer System | Agilent | G2939A | RNA bioanalyzer |
| Maxwell Instrument | Promega | AS4500 | |
| iScript cDNA synthesis kit | Bio-rad | 1708891 | |
| CFX384 Real-Time PCR Detection System | Bio-Rad | 1855485 | |
| iTaq universal SYBR Green Supermix kit | Bio-rad | 172-5120 | |
| Water | Sigma-Aldrich | W4502 | |
| Cryogenic vial | Thermo Fisher Scientific | 375418 | CryoTube vial |
| Mounting medium | Sigma-Aldrich | F6057 | Fluoroshield with DAPI |
References
- Harriff, M. J., Bermudez, L. E., Kent, M. L. Experimental exposure of zebrafish, Danio rerio (Hamilton), to Mycobacterium marinum and Mycobacterium peregrinum reveals the gastrointestinal tract as the primary route of infection: A potential model for environmental mycobacterial infection. Journal of Fish Diseases. 30 (10), 587-600 (2007).
- Lovmo, S. D., et al. Translocation of nanoparticles and Mycobacterium marinum across the intestinal epithelium in zebrafish and the role of the mucosal immune system. Developmental and Comparative Immunology. 67, 508-518 (2017).
- Chen, T., et al. Small-Sized mPEG-PLGA Nanoparticles of Schisantherin A with Sustained Release for Enhanced Brain Uptake and Anti-Parkinsonian Activity. ACS Applied Materials and Interfaces. 9 (11), 9516-9527 (2017).
- Collymore, C., Rasmussen, S., Tolwani, R. J. Gavaging Adult Zebrafish. Journal of Visualized Experiments. (78), e50691-e50691 (2013).
- Kim, S. H., Jang, Y. S. Antigen targeting to M cells for enhancing the efficacy of mucosal vaccines. Experimental and Molecular Medicine. 46 (3), 85 (2014).
- Iwasaki, A., Medzhitov, R. Regulation of adaptive immunity by the innate immune system. Science. 327 (5963), 291-295 (2010).
- Kunisawa, J., Kiyono, H. A marvel of mucosal T cells and secretory antibodies for the creation of first lines of defense. Cellular and Molecular Life Sciences. 62 (12), 1308-1321 (2005).
- Rombout, J. H., Yang, G., Kiron, V. Adaptive immune responses at mucosal surfaces of teleost fish. Fish Shellfish Immunology. 40 (2), 634-643 (2014).
- Salinas, I. The Mucosal Immune System of Teleost Fish. Biology. 4, 525-539 (2015).
- Munang'andu, H. M., Mutoloki, S., Evensen, O. &. #. 2. 4. 8. ;. An overview of challenges limiting the design of protective mucosal vaccines for finfish. Frontiers in Immunology. 6, 542 (2015).
- Lickwar, C. R., et al. Genomic dissection of conserved transcriptional regulation in intestinal epithelial cells. PLoS Biology. 15 (8), 2002054 (2017).
- Brand, M., Granato, M., Nüsslein-Volhard, C. Keeping and raising zebrafish. Zebrafish. 261, 7-37 (2002).
- Rességuier, J., et al. Specific and efficient uptake of surfactant-free poly(lactic acid) nanovaccine vehicles by mucosal dendritic cells in adult zebrafish after bath immersion. Frontiers in Immunology. 8, 190 (2017).
- Kephart, D., Terry, G., Krueger, S., Hoffmann, K., Shenoi, H. High-Performance RNA Isolation Using the Maxwell 16 Total RNA Purification Kit. Promega Notes. , (2006).
- . Thermo Fisher Scientific NanoDrop 1000 spectrophotometer V3.8 user's manual. Thermo Fisher Scientific Incorporation. , (2010).
- Lightfoot, S. Quantitation comparison of total RNA using the Agilent 2100 bioanalyzer, ribogreen analysis, and UV spectrometry. Agilent Application Note. , (2002).
- Huggett, J. F., et al. The digital MIQE guidelines: Minimum information for publication of quantitative digital PCR experiments. Clinical Chemistry. 59 (6), 892-902 (2013).
- Matthews, M., Varga, Z. M. Anesthesia and euthanasia in zebrafish. ILAR Journal. 53 (2), 192-204 (2012).
- Renshaw, S., Loynes, C. A transgenic zebrafish model of neutrophilic inflammation. Blood. 108 (13), 3976-3978 (2006).
- Ellett, F., Pase, L., Hayman, J. W., Andrianopoulos, A., Lieschke, G. J. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood. 117 (4), (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved