A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Analysis of Iophenoxic Acid Analogues in Small Indian Mongoose (Herpestes Auropunctatus) Sera for Use as an Oral Rabies Vaccination Biological Marker
In This Article
Summary
We offered captive mongooses placebo oral rabies vaccine baits with ethyl or methyl iophenoxic acid as a biomarker and verified bait uptake using a novel liquid chromatography with tandem mass spectrometry (LC-MS/MS) method.
Abstract
The small Indian mongoose (Herpestes auropunctatus) is a reservoir of rabies virus (RABV) in Puerto Rico and comprises over 70% of animal rabies cases reported annually. The control of RABV circulation in wildlife reservoirs is typically accomplished by a strategy of oral rabies vaccination (ORV). Currently no wildlife ORV program exists in Puerto Rico. Research into oral rabies vaccines and various bait types for mongooses has been conducted with promising results. Monitoring the success of ORV relies on estimating bait uptake by target species, which typically involves evaluating a change in RABV neutralizing antibodies (RVNA) post vaccination. This strategy may be difficult to interpret in areas with an active wildlife ORV program or in areas where RABV is enzootic and background levels of RVNA are present in reservoir species. In such situations, a biomarker incorporated with the vaccine or the bait matrix may be useful. We offered 16 captive mongooses placebo ORV baits containing ethyl-iophenoxic acid (et-IPA) in concentrations of 0.4% and 1% inside the bait and 0.14% in the external bait matrix. We also offered 12 captive mongooses ORV baits containing methyl-iophenoxic acid (me-IPA) in concentrations of 0.035%, 0.07% and 0.14% in the external bait matrix. We collected a serum sample prior to bait offering and then weekly for up to eight weeks post offering. We extracted Iophenoxic acids from sera into acetonitrile and quantified using liquid chromatography/mass spectrometry. We analyzed sera for et-IPA or me-IPA by liquid chromatography-mass spectrometry. We found adequate marking ability for at least eight and four weeks for et- and me-IPA, respectively. Both IPA derivatives could be suitable for field evaluation of ORV bait uptake in mongooses. Due to the longevity of the marker in mongoose sera, care must be taken to not confound results by using the same IPA derivative during consecutive evaluations.
Introduction
Rabies virus (RABV) is a negative sense single stranded lyssavirus, and circulates among diverse wildlife reservoir species within the orders Carnivora and Chiroptera. Multiple species of mongoose are reservoirs of RABV, and the small Indian mongoose (Herpestes auropunctatus) is the only reservoir in Puerto Rico and other Caribbean islands in the Western Hemisphere1,2,3. The control of RABV circulation in wildlife reservoirs is typically accomplished through a strategy of oral rabies vaccination (ORV). In the United States (US), this management activity is coordinated by the USDA/APHIS/Wildlife Services National Rabies Management Program (NRMP)4. Currently no wildlife ORV program exists in Puerto Rico. Research into rabies vaccines and various bait types for mongooses has been conducted with promising results suggesting an ORV program for mongooses is possible5,6,7,8.
Monitoring the impact of ORV relies on estimating bait uptake by target species, which typically involves evaluating a change in RV antibody seroprevalence. However, this strategy may be challenging in areas with an active wildlife ORV programs or in areas where RV is enzootic and background levels of RABV neutralizing antibodies (RVNA) are present in reservoir species. In such situations, a biomarker included in the bait or the external bait matrix may be useful.
Various biological markers have been used to monitor bait uptake in numerous species, including raccoons (Procyon lotor)9,10,stoats (Mustela ermine)11,12, European badgers (Meles meles)13, wild boars (Sus scrofa)14, small Indian mongooses15 and prairie dogs (Cynomysludovicianus)16,17, among others. In the US, operational ORV baits often include a 1% tetracycline biomarker in the bait matrix to monitor bait uptake18,19. However, drawbacks to the use of tetracycline include a growing concern over the distribution of antibiotics into the environment and that detection of tetracycline is typically invasive, requiring tooth extraction or destruction of the animal to obtain bone samples20. Rhodamine B has been evaluated as a marker in a variety of tissues and can be detected using ultraviolet (UV) light and fluorescence in hair and whiskers10,21.
Iophenoxic acid (IPA) is a white, crystalline powder that has been used to evaluate bait consumption in coyotes (Canis latrans)22, arctic fox (Vulpes lagopus)23, red fox (Vulpes vulpes)24, raccoons9,25, wild boar14, red deer (Cervus elaphus scoticus)26, European badgers12 and ferrets (M. furo)27, among several other mammalian species. Retention times of IPA varies by species from less than two weeks in some marsupials28,29, to at least 26 weeks in ungulates26 and over 52 weeks in domestic dogs (Canis lupus familiaris)30. Retention times may also be dose-dependent31. Iophenoxic acid binds strongly to serum albumin and was historically detected by measuring blood iodine levels32. This indirect approach was supplanted by high-performance liquid chromatography (HPLC) methods to directly measure iophenoxic acid concentrations with UV detection33, and eventually with liquid chromatography and mass spectrometry (LCMS)34,35. For this study, a highly sensitive and selective liquid chromatography with tandem mass spectrometry (LC-MS/MS) method was developed that utilizes multiple reaction monitoring (MRM) to quantify two analogues of iophenoxic acid. Our objective was to use this LC-MS/MS method to evaluate the marking ability of 2-(3-hydroxy-2,4,6-triiodobenzyl)propanoic acid (methyl-IPA or me-IPA) and 2-(3-hydroxy-2,4,6-triiodobenzyl)butanoic acid (ethyl-IPA or et-IPA) and when delivered in an ORV bait to captive mongooses.
Mongooses were live captured in cage traps baited with commercially available smoked sausages and fish oil. Mongooses were housed in individual 60 cm x 60 cm x 40 cm stainless steel cages and fed a daily ration of ~50 g commercial dry cat food, supplemented twice per week with a commercially available chicken thigh. Water was available ad libitum. We delivered two derivatives of IPA, ethyl-IPA and methyl-IPA, to captive mongooses in placebo ORV baits. All baits were composed of a 28 mm x 20 mm x 9 mm foil blister pack with an external coating (hereafter "bait matrix") containing powdered chicken egg and gelatin (Table of Materials). Baits contained 0.7 mL of water or IPA derivative and weighed approximately 3 g, of which ~2 g was the external bait matrix.
We offered 16 captive mongooses et-IPA in three concentrations: 0.14% (2.8 mg et-IPA in ~2 g bait matrix; 3 males [m], 3 females [f]), 0.4% (2.8 mg et-IPA in 0.7 mL blister pack volume; 3m, 3f), and 1.0% (7.0 mg ethyl-IPA in 0.7 mL blister pack volume; 2m, 2f). The overall dose of 2.8 mg corresponds to a dose rate of 5 mg/kg27,36 and is based on an average mongoose weight of 560 g in Puerto Rico. We selected 1% as the highest concentration as research suggests taste aversion to some biomarkers may occur at concentrations >1% in some species37. We only offered the 1% dose in the blister pack as flocculation prevented the solute from dissolving in the solvent sufficiently to be evenly incorporated into the bait matrix. One control group (2m, 1f) received baits filled with sterile water and no IPA. We offered baits to mongooses in the morning (~8 a.m.) during or prior to feeding of their daily maintenance ration. Bait remains were removed after approximately 24 hours. We collected blood samples prior to treatment, one day post-treatment and then weekly up to 8 weeks post-treatment. We anesthetized mongooses by inhalation of isoflurane gas and collected up to 1.0 mL of whole blood by venipuncture of the cranial vena cava as described for ferrets38. We centrifuged whole blood samples, transferred sera to cryovials and stored them at -80 °C until analysis. Not all animals were sampled during all time periods to minimize the impacts of repeated blood draws on the health of the animals. Control animals were sampled on day 0, then weekly for up to 8 weeks post-treatment.
We delivered me-IPA in three concentrations: 0.035% (0.7 mg), 0.07% (1.4 mg) and 0.14% (2.8 mg), all incorporated into the bait matrix, with 2 males and 2 females per treatment group. Two males and two females received baits filled with sterile water and no IPA. Bait offering times and mongoose anesthesia are described above. We collected blood samples prior to treatment on day 1, and then weekly up to 4 weeks post-treatment.
We tested serum concentration data for normality and estimated means for serum IPA concentrations of different treatment groups. We used a linear mixed model to compare mean serum et-IPA concentrations pooled across individuals. Bait type (matrix/blister pack) was a fixed effect in addition to experimental day, whereas animal ID was a random effect. All procedures were run using common statistical software (Table of Materials) and significance was evaluated at α = 0.05.
Protocol
All procedures were approved by the USDA National Wildlife Research Center's institutional Animal Care and Use Committee under approved research protocol QA-2597.
NOTE: The following protocol describes the analysis procedure to detect methyl-iophenoxic acid in mongoose serum. This method is the final version of an iterative process that began with analysis of ethyl-iophenoxic acid in mongoose serum. During the initial evaluation of ethyl-iophenoxic acid minor modifications were made to the methods, resulting in the final protocol presented below. Representative results include those obtained during both iterations.
1. Preparation of solutions and standards
- Purchase me-IPA and et-IPA.
- For mobile phase A, prepare 1 L of 0.1% (v/v) formic acid in water by combining 1 mL of formic acid with 1 L of ultrapure water (≥ 18 MΩ). For mobile phase B, prepare 1 L of 0.1% (v/v) formic acid in acetonitrile (ACN) by combining 1 mL of formic acid with 1 L of ACN.
- For diluent, prepare 200 mL of 0.5% (v/v) trifluoroacetic acid (TFA) in ACN by combining 1 mL of TFA with 200 mL of ACN.
- Prepare concentrated IPA stock solutions of me-IPA and et-IPA in ACN at concentrations of approximately 1,000 µg/mL.
- Weigh approximately 10 mg of me-IPA on a microbalance and record the mass to ± 0.0001 mg. Quantitatively transfer the me-IPA to a 10 mL Class A volumetric flask using 45 mL ACN. Sonicate 1 min to dissolve all solids, and then bring to volume with ACN.
- Transfer ~8 mL of each stock to amber 8 mL glass vials with poly-tetrafluoroethylene (PTFE)-lined caps. Store at room temperature (RT). Transfer the remaining stock to hazardous waste.
- For the 25x-7 me-IPA stock (Table 1), prepare a stock of me-IPA in ACN at approximately 200 µg/mL. Example: Transfer 1 mL of the me-IPA concentrated stock from step 1.4.2 to a 5 mL Class A volumetric flask using a 1,000 µL glass syringe. Dilute to volume with ACN. Transfer the stock to an amber 8 mL glass vial with PTFE-lined cap. Store at RT.
- Prepare the six additional 25x me-IPA Stocks described in Table 1. For each stock, combine the volumes indicated using a repeat pipettor in an amber 8 mL amber glass vial with PTFE-lined cap. Store each stock at RT.
- For the 25x surrogate stock, prepare a surrogate stock of me-IPA in ACN at approximately 10 µg/mL from the concentrated stock prepared in step 1.4.2. Transfer 0.100 mL of the concentrated me-IPA stock to a 10 mL Class A volumetric flask using a 100 µL glass syringe, and then dilute to volume with ACN.
- Transfer ~8 mL to an amber 8-mL glass vial with PTFE-lined cap. Store at RT. Transfer the remaining stock to hazardous waste.
- Prepare 4x stocks containing both analytes in 2 mL screw-top glass autosampler vials as described in Table 2.
- For example, to prepare stock 4x-7, to a 2 mL vial, add 0.20 mL of the 25x-7 me-IPA stock from step 1.5 using a repeat pipettor with 0.5 mL capacity tip. Add 0.20 mL of the 25x surrogate et-IPA stock from step 1.7 using a repeat pipettor with 0.5 mL capacity tip.
- Add 0.85 mL of ACN using a repeat pipettor with 1 mL capacity tip. Cap the vial securely and invert 5x to mix.
- Prepare the standard curve in 2 mL screw-top autosampler vials as described in Table 3.
- For example, to prepare standard 7 (Std 7), to a 2-mL vial, add 0.20 mL of the 4x-7 Stock from step 1.8.2 using a repeat pipettor with 0.5 mL capacity tip. Add 0.60 mL of ultrapure DI water using a repeat pipettor with 1 mL capacity tip. Cap the vial securely and invert 5x to mix.
2. Sample preparation
CAUTION: Personnel performing this procedure must have received the full series of rabies pre-exposure prophylaxis and have a documented rabies antibody titer above 0.5 IU from a Federal Occupational Health designated medical facility. Personnel must wear lab coats and eye protection at all times while performing the extraction. CAUTION: Perform steps 2.3−2.6 in a class II biosafety cabinet.
- For each sample, prepare a 1.5 mL microcentrifuge tube containing 200−300 mg of NaCl.Arrange the tubes in an 80-position plastic rack. Set aside for use in step 2.6.
NOTE: A micro scoop (or other small measuring device) is recommended for large numbers of samples. - For each sample, label two 1.5 mL microcentrifuge tubes: one as "A" and the other as "B". Arrange the tubes in an 80-position plastic rack.
- Place the following materials and equipment needed for serum extraction in a class II biosafety cabinet: microcentrifuge tubes (in racks) prepared in steps 2.1 and 2.2, a vortex mixer, repeat pipettor with 0.5 mL and 5 mL capacity tips, 100−1,000 µL air displacement pipette with 1,000 µL tips, containers with approximately 100 mL each of diluent and ultrapure DI water, and a biohazard waste container.
- Remove serum samples from frozen storage and warm to RT in the biosafety cabinet. Vortex mix each serum sample prior to sampling.
- Using a repeat pipettor with 0.5 mL capacity tip, dispense 0.050 mL of mongoose serum into tube "A" and add 0.050 mL of 25x surrogate stock. Then add 0.950 mL of diluent to tube "A" using a repeat pipettor with 5 mL capacity tip. Cap securely and vortex mix for 10−15 s.
- Dispense the pre-weighed NaCl from step 2.1 into tube "A" and vortex mix 3x for 8−12 s. Wipe down the outside surfaces of the vial rack containing tube "A" using 70% (v/v) isopropanol.
NOTE: The rack of samples may now be removed from the class II biosafety cabinet. - Centrifuge tube "A" at 12,000 x g for 1 min to separate the aqueous and ACN phases. Pipette 0.80 mL of the upper ACN phase to tube "B" using a 100−1,000 µL air displacement pipette. Transfer the remaining solution in tube "A" to hazardous waste and discard the empty tube in a biohazardous waste container.
- Remove ACN and TFA from tube "B" with a gentle flow of N2 gas in a 45 °C water bath.
- Add 0.250 mL of ACN to tube "B" using a repeat pipettor, vortex mix for 4−5 s, and then centrifuge briefly (2−4 s) at 12,000 x g to collect the liquid in the bottom of the tube.
- Add 0.750 mL of ultrapure DI water to tube "B" using a repeat pipettor with 5 mL capacity tip, vortex mix for 4−5 s, and then centrifuge for 1 min at 12,000 x g to clarify the sample.
- Transfer 0.75 mL of the supernatant to an autosampler vial using a 1,000 µL air displacement pipette. Discard pipette tips in biohazard waste container.
- Cap autosampler vials securely and analyze by LC-MS/MS (section 4). Transfer the remaining solution in tube "B" to hazardous waste and discard the empty tube to a biohazardous waste container. Dispose of all biohazardous waste by autoclaving or incineration.
3. Quality control samples
CAUTION: Follow the cautionary statements described in section 2.
NOTE:The following procedure describes the minimum number of quality control (QC) samples required for an analysis. Replicates at each level are recommended if sufficient control mongoose serum is available.
- Prepare four 1.5 mL microcentrifuge tubes containing 200−300 mg of NaCl. Arrange the tubes in an 80-position plastic rack.
- For each QC sample, label two 1.5 mL microcentrifuge tubes: one as "A" and the other as "B". Arrange the tubes in an 80-position plastic rack.
- Repeat step 2.3.
- Remove control mongoose serum from frozen storage and warm to RT in the biosafety cabinet. Vortex mix the control serum prior to sampling.
- Dispense 0.050 mL of control mongoose serum into the four 1.5-mL "A" tubes using a repeat pipettor with 0.5 mL capacity tip.
- Fortify each of the four QC samples as specified in Table 4 using a repeat pipettor with 0.5 mL capacity tip. Cap each QC sample securely and vortex mix for 10−15 s.
- Perform the extraction procedure as described in steps 2.6−2.12.
4. LC-MS/MS analysis
- Configure the LC-MS/MS with all parameters described in Table 5. Power on the LC-MS/MS and allow the column to reach 70 °C before setting the flow rate to 0.800 mL/min.
- Set up a sequence in the data acquisition software (Table of Materials) to inject the standard curve before and after each batch consisting of quality control samples and unknown samples.
- Inject all standards and samples and acquire MRM ion chromatograms using parameters listed in Table 5.
- After sequence completion, turn off the LC-MS/MS and dispose of all autosampler vials as hazardous waste.
5. Quantification
- Use the data analysis software to generate a calibration curve of relative responses versus relative concentrations for me-IPA using et-IPA as the internal standard. Calculate the relative responses from the quantifier MRM transition for me-IPA (556.6 → 428.7) divided by the MRM transition for et-IPA (570.7 → 442.7). Construct a 7-level calibration curve using a second order quadratic function that is weighted 1/x and ignores the origin.
- Calculate the serum concentration (Cserum) of me-IPA using the following equation:
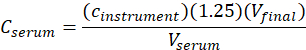
where cinstrument is the concentration determined by the instrument from the calibration curve in units of µg/mL, 1.25 is the dilution factor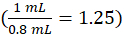 , Vfinal is the final sample volume (1.0 mL), and Vserum is the serum volume in mL (0.050 mL nominal).
, Vfinal is the final sample volume (1.0 mL), and Vserum is the serum volume in mL (0.050 mL nominal).
Results
Representative ion chromatograms from a me-IPA analysis are presented in Figure 1. The control mongoose serum (Figure 1A) illustrates the retention time of et-IPA (surrogate analyte) and the absence of me-IPA at the indicated retention time. The quality control sample (Figure 1B) illustrates the baseline separation of me-IPA from et-IPA as well as the quantifier and qualifier transitions for me-IPA. ...
Discussion
The LC-MS/MS method developed for the studies utilized the selectivity of multiple reaction monitoring to accurately quantify me-IPA and et-IPA in mongoose serum. The selectivity of MS/MS detection also allowed for a simple clean-up protocol relying solely on acetonitrile to precipitate proteins from serum prior to analysis.
Iophenoxic acids are soluble in ACN but are practically insoluble in water. To exclude water from the ACN extraction, sodium chloride was added to force a clear water:ACN ...
Disclosures
Authors AV and SO are fulltime employees of an oral rabies vaccine bait manufacturer.
Acknowledgements
This research was supported in part by the intramural research program of the US Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, National Rabies Management Program and IDT Biologika (Dessau-Rosslau, Germany).
Materials
| Name | Company | Catalog Number | Comments |
| Acetonitrile, Optima grade | Fisher | A996 | |
| Analytical balance | Mettler Toledo | XS204 | |
| C18 column, 2.1 x 50 mm, 2.5-µm particle size | Waters Corp. | 186003085 | |
| ESI Source | Agilent | G1958-65138 | |
| Ethyl-iophenoxic acid, 97 % | Sigma Aldrich | N/A | Lot MKBP5399V |
| Formic acid, LC/MS grade | Fisher | A117 | |
| LCMS software | Agilent | MassHunter Data Acquisition and Quantitative Analysis | |
| Methyl-iophenoxic acid, 97 % (w/w) | PR EuroChem Ltd. | N/A | Lot PR0709514717 |
| Microanalytical balance | Mettler Toledo | XP6U | |
| Microcentrifuge | Eppendorf | 5415C | |
| MS/MS | Agilent | G6470A | |
| N-Evap | Organomation | 115 | |
| Oral Rabies Vaccine Baits | IDT Biologika, Dessau Rossleau, Germany | N/A | |
| Propyl-iophenoxic acid, 99 % (w/w) | PR EuroChem Ltd. | N/A | Lot PR100612108RR |
| Repeat pipettor | Eppendorf | M4 | |
| Screw-top autosampler vial caps, PTFE-lined | Agilent | 5190-7024 | |
| Sodium chloride, Certified ACS grade | Fisher | S271 | |
| Statistical Software Package | SAS Institute, Cary, North Carolina, USA | N/A | |
| Trifluoroacetic acid, 99 % | Alfa Aesar | L06374 | |
| UPLC | Agilent | 1290 Series | |
| Vortex Mixer | Glas-Col | 099A PV6 | |
| 0.2-mL pipettor tips | Eppendorf | 30089.413 | |
| 0.5-mL pipettor tips | Eppendorf | 30089.421 | |
| 1.5-mL microcentrifuge tubes | Fisher | 14-666-325 | |
| 1250-µL capacity pipette tips | GeneMate | P-1233-1250 | |
| 1-mL pipettor tips | Eppendorf | 30089.43 | |
| 2-mL amber screw-top autosampler vials | Agilent | 5182-0716 | |
| 5-mL pipettor tips | Eppendorf | 30089.456 | |
| 80-position microcentrifuge tube rack | Fisher | 05-541-2 | |
| 8-mL amber vials with PTFE-lined caps | Wheaton | 224754 | |
| 70 % (v/v) isopropanol | Fisher | A459 | |
| 100-1000 µL air displacement pipette | Eppendorf | ES-100 |
References
- Nel, L. H., et al. Mongoose rabies in southern Africa: a re-evaluation based on molecular epidemiology. Virus Research. 109 (2), 165-173 (2005).
- Zieger, Z., et al. The phylogeography of rabies in Grenada, West Indies, and implications for control. PLOS Neglected Tropical Diseases. 8 (10), e3251 (2004).
- Monroe, B. P., et al. Rabies surveillance in the United States during 2014. Journal of the American Veterinary Medical Association. 248 (7), 777-788 (2015).
- Slate, D., et al. Status of oral rabies vaccination in wild carnivores in the United States. Virus Research. 111, 68-76 (2005).
- Blanton, J. D., et al. Vaccination of small Asian mongoose (Herpestes javanicus) against rabies. Journal of Wildlife Diseases. 42 (3), 663-666 (2006).
- Vos, A., et al. Oral vaccination of captive small Indian mongoose (Herpestes auropunctatus) against rabies. Journal of Wildlife Diseases. 49 (4), 1033-1036 (2013).
- Berentsen, A. R., Johnson, S. R., VerCauteren, K. C. Evaluation of ONRAB® bait matrix flavor preference by mongoose (Herpestes auropunctatus) in Puerto Rico: Implications for Oral Rabies Vaccination. Caribbean Journal of Science. 48 (1), 52-58 (2014).
- Ortmann, S., et al. Safety studies with the oral rabies virus vaccine strain SPBN GASGAS in the small Indian mongoose (Herpestes auropunctatus). BMC Veterinary Research. 14 (1), 90 (2018).
- Linhart, S. B., et al. A field evaluation of baits for delivering oral rabies vaccines to raccoons (Procyon lotor). Journal of Wildlife Diseases. 30 (2), 185-194 (1994).
- Fry, T. L., Atwood, T., Dunbar, M. R. Evaluation of rhodamine B as a biomarker for raccoons. Human Wildlife Interactions. 4 (2), 275-280 (2010).
- Jones, C., Moller, H., Hamilton, W. A review of potential techniques for identifying individual stoats (Mustela erminea) visiting control or monitoring stations. New Zealand Journal of Zoology. 31 (3), 193-203 (2004).
- de Leeuw, A. N. S., Smith, G. C., Woods, J. A. Use of iophenoxic acid to assess bait uptake by European badgers. Advances in Vertebrate Pest Management. 4, 243-254 (2006).
- Southey, A. K., Sleeman, D. P., Gormley, E. Sulfadimethoxine and rhodamine B as oral biomarkers for European badgers (Meles meles). Journal of Wildlife Diseases. 38 (2), 378-384 (2002).
- Massei, G., Jones, A., Platt, T., Cowan, D. P. Iophenoxic Acid as a Long-Term Marker for Wild Boar. Journal of Wildlife Management. 73 (3), 458-461 (2009).
- Creekmore, T. E., et al. Field evaluation of baits and baiting strategies for delivering oral vaccine to mongooses in Antigua, West Indies. Journal of Wildlife Diseases. 30 (4), 497-505 (1994).
- Creekmore, T. E., Rock, R. E., Hurley, J. A baiting system for delivery of an oral plague vaccine to black-tailed prairie dogs. Journal of Wildlife Diseases. 38 (1), 32-39 (2002).
- Fernandez, J. R. R., Rocke, R. E. Use of Rhodamine B as a biomarker for oral plague vaccination of prairie dogs. Journal of Wildlife Diseases. 47 (3), 765-768 (2011).
- Johnston, J. J., et al. Evaluation and significance of tetracycline stability in rabies vaccine baits. Journal of Wildlife Diseases. 41 (3), 549-558 (2005).
- Algeo, T. P., et al. Oral rabies vaccination variation in tetracycline biomarking among Ohio Raccoons. Journal of Wildlife Diseases. 49 (2), 332-337 (2013).
- Crier, J. K. Tetracyclines as a fluorescent marker in bones and teeth of rodents. Journal of Wildlife Management. 34 (4), 829-834 (1970).
- Fisher, P. Review of using Rhodamine B as a marker for wildlife studies. Wildlife Society Bulletin. 27 (2), 318-329 (1999).
- Knowlton, F. K., Savarie, P. J., Wahlgren, C. E., Hayes, D. J., Shumake, S. A., Bullard, R. W. Physiological marks by coyotes ingesting baits containing iophenoxic acid, Mirex and Rhodamine B. Vertebrate Pest Control and Management Materials. , 141-147 (1987).
- Follmann, E. H., Savarie, P. J., Ritter, D. G., Baer, G. M. Plasma marking of arctic foxes with iophenoxic acid. Journal of Wildlife Diseases. 23 (4), 709-712 (1987).
- Saunders, G., Harris, S., Eason, C. T. Iophenoxic acid as a quantitative bait marker for foxes. Wildlife Research. 20, 297-302 (1993).
- Hadidian, J., et al. Acceptance of simulated oral rabies vaccine baits by urban raccoons. Journal of Wildlife Diseases. 25 (1), 1-9 (1989).
- Sweetapple, P. J., Nugent, G. Iophenoxic acid as a serum marker for red deer (Cervus elaphus scoticus). Wildlife Research. 25, 649-654 (1998).
- Ogilvie, S. C., Eason, C. T. Evaluation of iophenoxic acid and rhodamine B for marking feral ferrets (Mustela furo). New Zealand Journal of Zoology. 25 (2), 105-108 (1998).
- Eason, C. T., Batcheler, D., Frampton, C. M. Comparative pharmacokinetics of iophenoxic acid in cats and brushtail possums. Wildlife Research. 21, 377-380 (1994).
- Fisher, P. M., Marks, C. A. Evaluation of iophenoxic acid as a biomarker for swamp wallabies (Wallabia bicolor). Wildlife Research. 24, 97-103 (1997).
- Baer, G. M., Shaddock, J. H., Hayes, D. J., Savarie, P. Iophenoxic acid as a serum marker in carnivores. Journal of Wildlife Management. 49 (1), 49-51 (1985).
- Spurr, E. B. Iophenoxic acid as a systemic blood marker for assessment of bait acceptance by stoats (Mustela ermine) and weasels (Mustela nivalis). New Zealand Journal of Zoology. 29 (2), 135-142 (2002).
- Mudge, G. H., Strewler, G. J., Desbiens, N., Berndt, W. O., Wade, D. N. Excretion and distribution of iophenoxic acid. Journal of Pharmacology and Experimental Therapeutics. 178 (1), 159-172 (1971).
- Jones, A. High-performance liquid chromatographic determination of iophenoxic acid in serum. Journal of Chromatography B: Biomedical Sciences and Applications. 654 (2), 293-296 (1994).
- Wiles, M. C., Campbell, T. A. Liquid chromatography-electrospray ionization mass spectrometry for direct identification and quantification of iophenoxic acid in serum. Journal of Chromatography B. 832 (1), 144-157 (2006).
- Ballesteros, C., et al. Analysis by LC/ESI-MS of iophenoxic acid derivatives and evaluation as markers of oral baits to deliver pharmaceuticals to wildlife. Journal of Chromatography B. 878 (22), 1997-2002 (2010).
- Purdey, D. C., Petcu, M., King, C. M. A simplified protocol for detecting two systemic bait markers (Rhodamine B and iophenoxic acid) in small mammals. New Zealand Journal of Zoology. 30 (3), 175-184 (2003).
- Tobin, M. E., Koehler, A. E., Sugihara, R. Tetracyclines as a fluorescent marker in bones and teeth of rodents. Journal of Wildlife Management. 60 (1), 202-207 (1996).
- Briscoe, J. A., Syring, R. Techniques for emergency airway and vascular access in special species. Seminars in Avian and Exotic Pet Medicine. 13 (3), 118-131 (2004).
- Eason, C. T., Frampton, C. M. The plasma pharmacokinetics of iophenoxic and iopanoic acids in goat. Xenobiotica. 2 (2), 185-189 (1992).
- Hilton, H. E., Dunn, A. M. S. . Mongooses: their natural history. , (1967).
- Stevens, C. E., Hume, I. D. . Comparative physiology of the vertebrate digestive system. Second edition. , (1995).
- National Rabies Management Program (NRMP). . Oral rabies vaccination draft operations manual. , (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved