A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Electrochemical Roughening of Thin-Film Platinum Macro and Microelectrodes
* These authors contributed equally
In This Article
Summary
This protocol demonstrates a method for electrochemical roughening of thin-film platinum electrodes without preferential dissolution at grain boundaries. The electrochemical techniques of cyclic voltammetry and impedance spectroscopy are demonstrated to characterize these electrode surfaces.
Abstract
This protocol demonstrates a method for electrochemical roughening of thin-film platinum electrodes without preferential dissolution at grain boundaries of the metal. Using this method, a crack free, thin-film macroelectrode surface with up to 40 times increase in active surface area was obtained. The roughening is easy to do in a standard electrochemical characterization laboratory and incudes the application of voltage pulses followed by extended application of a reductive voltage in a perchloric acid solution. The protocol includes the chemical and electrochemical preparation of both a macroscale (1.2 mm diameter) and microscale (20 µm diameter) platinum disc electrode surface, roughening the electrode surface and characterizing the effects of surface roughening on electrode active surface area. This electrochemical characterization includes cyclic voltammetry and impedance spectroscopy and is demonstrated for both the macroelectrodes and the microelectrodes. Roughening increases electrode active surface area, decreases electrode impedance, increases platinum charge injection limits to those of titanium nitride electrodes of same geometry and improves substrates for adhesion of electrochemically deposited films.
Introduction
Nearly five decades ago, the first observation of surface enhanced Raman spectroscopy (SERS) occurred on electrochemically roughened silver1. Electrochemical roughening of metal foils is still attractive today because of its simplicity over other roughening methods2,3 and its usefulness in many applications like improving aptamer sensors4, improving neural probes5, and improving adhesion to metal substrates6. Electrochemical roughening methods exist for many bulk metals1,5,7,8,9,10. Until recently, however, there was no report on the application of electrochemical roughening to thin (hundreds of nanometers thick) metal films6, despite the prevalence of microfabricated thin-film metal electrodes in a number of fields.
Established methods to roughen thick platinum (Pt) electrodes5,8 delaminate thin-film Pt electrodes6. By modulating the frequency of the roughening procedure and the electrolyte used for the for the roughening, Ivanovskaya et al. demonstrated Pt thin-film roughening without delamination. That publication focused on using this new approach to increase the surface area of platinum recording and stimulation electrodes on microfabricated neural probes. The roughened electrodes were demonstrated to improve recording and stimulation performance and improve adhesion of electrochemically deposited films and improve biosensor sensitivity6. But this approach also likely improves surface cleaning of microfabricated electrode arrays and enhances the capabilities of thin-film electrodes for other sensor applications (e.g., aptasensors) as well.
The approach to roughen thin-film macroelectrodes (1.2 mm diameter) and microelectrodes (20 µm diameter) is described in the following protocol. This includes preparation of the electrode surface for roughening and how to characterize the roughness of the electrode. These steps are presented along with tips on how to optimize the roughening procedure for other electrode geometries and the most important factors to ensure an electrode is roughened nondestructively.
Protocol
CAUTION: Please consult all relevant safety data sheets (SDS) before use. Several of the chemicals used in this protocol are acutely toxic, carcinogenic, oxidizing and explosive when used at high concentrations. Nanomaterials may have additional hazards compared to their bulk counterpart. Please use all appropriate safety practices when carrying out this protocol including the use of engineering controls (fume hood) and personal protective equipment (safety glasses, gloves, lab coat, full length pants, closed-toe shoes).
1. Cleaning the Pt electrode(s) before initial characterization and surface roughening
- Chemically clean the electrodes under ozone with a laboratory UV-ozone cleaner at 80 °C for 10 min.
- Soak the portion of the probe containing the electrode(s) in a solvent (e.g., a 30 min soak in acetone for the microelectrodes demonstrated in this protocol).
NOTE: Other methods may be more effective for removing organics from the electrodes depending on electrode housing and geometry, but this solvent soaking works well for the electrodes in the protocol. - Electrochemically clean the surface of all electrodes by repetitive potential cycling in an acidic solution of perchloric acid. The perchloric acid solution does not need purging to change the concentration of any gasses present.
- Load settings onto the potentiostat to apply cyclic voltammograms (CVs) to the electrodes. Scan from 0.22 V to 1.24 V vs Ag|AgCl (or -0.665 V to 0.80 V vs mercury sulfate reference electrode (MSE), the reference used for roughening) at a scan rate of 200 mV/s.
NOTE: Regardless of reference material used, all potentials in this paper are given with respect to Ag|AgCl (saturated with KCl) reference electrode. The potential offset between the MSE (containing 1.0 M H2SO4) used in this study and Ag|AgCl (saturated with KCl)is 0.44 V11.- In the EC-Lab Software, under the Experiment tab, press the + sign to add electrochemical technique. In the pop-up window, Insert techniques will appear.
- Click on Electrochemical techniques. When it expands, click on Voltamperometric techniques. When that expands, double click on Cyclic Voltammetry - CV. 1-CV line will appear in the Experiment window.
- In the Experiment window, fill in the following parameters:
Ei = 0 V vs Eoc
dE/dt = 200 mV/s
E1 = -0.665 V vs Ref
E2 = 0.8 V vs Ref
n = 200
Measure <I> over last 50% of the step duration
Record <I> averaged over N = 10 voltage steps
E Range = -2.5; 2.5 V
Irange = Auto
Bandwidth = 7
End scan Ef = 0 V vs Eoc
- Submerge the electrode tip of the device in a 500 mM perchloric acid (HClO4) solution that also contains a Pt wire counter electrode and MSE reference.
NOTE: To avoid alterations in the electrochemical processes from chloride ion contamination, a chloride-free reference electrode (e.g., leakless Ag|AgCl or MSE, etc.) must be used for all tests performed inside acidic electrolytes in this protocol. - Connect one electrode or short several electrodes of a multielectrode device together as the working electrode.
- Connect the working, counter, and reference electrodes to the potentiostat.
- In the EC-Lab Software, in the Experiment window, press Advanced settings on the left.
- Under Advanced settings, select Electrode configuration = CE to ground. Connect the working, counter and reference electrode to the instrument leads as shown on the Electrode connection diagram.
- Press the Run button (green triangle under Experiment window) to begin the experiment.
- Perform repetitive potential cycles until the voltammograms visually appear to overlap from one cycle to the next. This typically occurs after 50-200 CVs.
- Load settings onto the potentiostat to apply cyclic voltammograms (CVs) to the electrodes. Scan from 0.22 V to 1.24 V vs Ag|AgCl (or -0.665 V to 0.80 V vs mercury sulfate reference electrode (MSE), the reference used for roughening) at a scan rate of 200 mV/s.
2. Electrochemical characterization of the electrode surface before roughening
- Perform all electrochemical characterizations in the 3-electrode configuration described above in steps 1.3.2 - 1.3.4. All potentials in the following steps are given with respect to an Ag|AgCl reference electrode. Use a Pt wire as the counter electrode. Use a conventional Ag|AgCl electrode for characterization performed in phosphate buffered saline (PBS), but use a leakless Ag|AgCl or MSE as the reference for all tests performed in acidic solutions.
- Load settings on the potentiostat for the application of CVs from -0.22 to 1.24 V vs Ag|AgCl (or -0.665 V to 0.80 V vs MSE) at a scan rate of 50 mV/s. Submerge the electrode tip of the device in a beaker of deoxygenated 500 mM HClO4 (deoxygenated with N2 gas for ≥10 min) that also contains a Pt wire counter electrode and MSE reference.
- In the EC-Lab Software, under the Experiment tab, press the + sign to add electrochemical technique. In the pop-up window, Insert techniques will appear.
- Click on Electrochemical techniques. When it expands, click on Voltamperometric techniques. When that expands, double click on Cyclic Voltammetry - CV. 1-CV line will appear in the Experiment window.
- In the Experiment window, fill in the following parameters:
Ei = 0 V vs Eoc
dE/dt = 50 mV/s
E1 = -0.665 V vs Ref
E2 = 0.8 V vs Ref
n = 10
Measure <I> over last 50% of the step duration
Record <I> averaged over N = 10 voltage steps|
E Range = -2.5; 2.5 V
Irange = Auto
Bandwidth = 7
End scan Ef = 0 V vs Eoc
NOTE: The only differences between this setup and that described previously in step 1.3 are the use of deoxygenated 500 mM HClO4 and ensuring that only one electrode is used as the working electrode. In the EC-Lab Software, in the Experiment window, press Advanced settings on the left. - Under Advanced settings, select Electrode configuration = CE to ground. Connect the working, counter and reference electrode to the instrument leads as shown on the Electrode connection diagram.
- Press the Run button (green triangle under Experiment window) to begin the experiment.
- Perform repetitive potential cycles until the voltammograms visually appear to overlap from one cycle to the next.
- Calculate the electrode surface area from the hydrogen adsorption peaks of the highly reproducible (overlapping) CVs using the method of J. Rodríguez, et al.11.
- Determine the charge associated with adsorption of a hydrogen monolayer (Q) to the electrode surface by integrating the two cathodic peaks of a CV between the potentials where the cathodic current deviates from the double layer current (
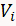 ) and the hydrogen evolution starts (
) and the hydrogen evolution starts (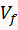 ) after subtracting the charge associated with monolayer charging (
) after subtracting the charge associated with monolayer charging (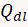 ). Scan rate (ν) also effects this adsorption. Use the equation below to determine Q.
). Scan rate (ν) also effects this adsorption. Use the equation below to determine Q.
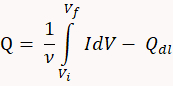
Graphical representation of integrated area can be found in J. Rodríguez, et al.11. - Calculate the effective surface area (A) of an electrode by dividing Q by the charge density of the formation of hydrogen monolayer (k). For an atomically flat polycrystalline Pt surface, k = 208 µC/cm2.
A = Q / k
- Determine the charge associated with adsorption of a hydrogen monolayer (Q) to the electrode surface by integrating the two cathodic peaks of a CV between the potentials where the cathodic current deviates from the double layer current (
- If the two cathodic peaks of a Pt CV are poorly resolved, estimate the electrode surface area from the double layer capacitance at the electrode-solution interface. Use of the approach described in step 2.1.1 when hydrogen peaks are poorly resolved will lead to inaccurate results.
- Measure the impedance spectra of a single electrode under open circuit conditions in PBS (pH 7.0, 30 mS/cm conductivity). Submerge the electrode tip of the device in PBS that also contains a Pt wire counter electrode and MSE reference. Connect one electrode at a time as the working electrode. Next, use a potentiostat to apply an impedance sign wave with an amplitude of 10 mV over the frequency range 1 Hz - 100 kHz.
- In the EC-Lab Software, under the Experiment tab, press the + sign to add electrochemical technique. In the pop-up window, Insert techniques will appear.
- Click on Electrochemical techniques. When it expands, click on Impedance Spectroscopy. When that expands, double click on Potentio Electrochemical Impedance Spectroscopy. 1-PEIS line will appear in the Experiment window.
- In the Experiment window, fill in the following parameters:
Ei = 0 V vs Eoc
fi = 1 Hz
ff = 100 kHz
Nd = 6 points per decade
In Logarithmic spacing
Va = 10 mV
Pw = 0.1
Na = 3
nc = 0
E Range = -2.5; 2.5 V
Irange = Auto
Bandwidth = 7 - In the EC-Lab Software, in the Experiment window, press Advanced settings on the left.
- Under Advanced settings, select Electrode configuration = CE to ground. Connect the working, counter and reference electrode to the instrument leads as shown on the Electrode connection diagram.
- Press the Run button (green triangle under Experiment window) to begin the experiment.
- Measure the impedance spectra of a single electrode under open circuit conditions in PBS (pH 7.0, 30 mS/cm conductivity). Submerge the electrode tip of the device in PBS that also contains a Pt wire counter electrode and MSE reference. Connect one electrode at a time as the working electrode. Next, use a potentiostat to apply an impedance sign wave with an amplitude of 10 mV over the frequency range 1 Hz - 100 kHz.
- Determine the double layer capacitance from the electrode's impedance spectra (collected in step 2.1.4.1) by fitting the spectra with an equivalent circuit model using impedance analysis software.
NOTE: Analysis in representative results and in Ivanovskaya, et al.6 was carried out with the impedance analysis fitting tool Z Fit.- In the EC-Lab Software, click Load data file under Experiment list menu.
- Select Nyquist Impedance plot type at the top menu bar.
- Click Analysis, then select Electrochemical Impedance Spectroscopy, and click Z Fit.
- When then Z-Fit Bio-Logics pop-up window appears, click the Edit button
- Select Display circuit with 2 elements and choose R1 + Q1 from the list of equivalent circuit models. Click OK.
- Expand the Fit section of the pop-up window and make sure that the settings are Randomize + Simplex, stop randomize at 5,000 iterations, and stop fit on 5,000 iterations.
- Press the Calculate button and observe initial fit spectra added to the plot. Press Minimize and observe finalized fit.
- Adjust fit boundaries (green circles) to exclude noisy or distorted data from the fit. Estimated fit parameters will appear under Results section.
- Ensure that the calculated equivalent circuit model fits a Nyquist plot of the data that includes ohmic resistance (R) in series with a constant phase angle (CPE).
- Take note of the double layer capacitance value (Q) that is part of CPE in the equivalent circuit model.
- Estimate the change in surface area as a ratio of Q measured before and after roughening since double layer capacitance (Q) increases linearly with active surface area12.
- Load settings on the potentiostat for the application of CVs from -0.22 to 1.24 V vs Ag|AgCl (or -0.665 V to 0.80 V vs MSE) at a scan rate of 50 mV/s. Submerge the electrode tip of the device in a beaker of deoxygenated 500 mM HClO4 (deoxygenated with N2 gas for ≥10 min) that also contains a Pt wire counter electrode and MSE reference.
3. Electrochemical roughening of a macroelectrode
NOTE: Electrochemical roughening is driven by series of oxidation/reduction pulses that result in oxide growth and dissolution. In the case of a weakly adsorbing anion (like HClO4), this dissolution is accompanied by Pt crystallite redeposition while in the case of strongly adsorbing anions (like H2SO4) this process results in preferential intergrain Pt dissolution that creates microcracks in the electrode surface6. Therefore, usage of high purity HClO4 electrolyte is essential to prevent microcracks in the electrode surface.
- Use a potentiostat able to apply voltage pulses with the 2 ms pulse width to roughen macroelectrodes. This procedure can be done with either potentiostat on the accompanying materials list.
- Program the following parameters into the potentiostat to roughen a 1.2 mm diameter Pt disk macroelectrode.
- Begin the roughening protocol with a series of oxidation/reduction pulses between -0.15 V (Vmin) and 1.9 - 2.1 V (Vmax) at 250 Hz with a duty cycle of 1:1 for 10 - 300 s. The duration of pulse application determines the extent of roughening, the longer the pulsing the more roughening occurs. Use Figure 1A and the discussion as a guide to help determine the specific parameters required to achieve a particular surface roughness.
- Open the VersaStudio program.
- Expand the Experiment menu and select New.
- In the Select Action pop-up window that appears, choose Fast potential pulses and enter the desired file name when prompted. Fast potential pulses line will then appear under Actions to be performed tab.
- Fill out the following under the Properties of Fast Potential Pulses/Pulse properties. Enter Number of pulses = 2, Potential (V) 1 = -0.39 vs Ref for 0.002 s, and Potential (V) 2 = 1.56 vs Ref for 0.002 s.
- Under Scan properties, fill out: Time per point = 1 s, number of cycles: 50,000 (for 200 s duration).
- Under Instrument properties, enter Current range = Auto.
- Program the potentiostat to immediately follow the series of pulses with a prolonged application of a constant reduction potential (-0.15 V (or -0.59 V vs MSE) for 180 s) to fully reduce any oxides produced and stabilize the electrode surface.
- In the VersaStudio Software, press the + button to insert a new step.
- Double click on Chronoamperometry.
- Enter Potential (V) = -0.59, Time per point (s) = 1, and Duration (s) = 180.
- Use the visual representation of the paradigm described in steps 3.2.1. and 3.2.2 ( Figure 2) to aid in programming the potentiostat.
NOTE: Specific parameters will vary for different electrode geometries but using the parameters above as a starting point and then varying Vmax and pulse duration is the recommended method to optimize roughening parameters for other geometries. Using a high purity HClO4 solution is essential for this step.
- Begin the roughening protocol with a series of oxidation/reduction pulses between -0.15 V (Vmin) and 1.9 - 2.1 V (Vmax) at 250 Hz with a duty cycle of 1:1 for 10 - 300 s. The duration of pulse application determines the extent of roughening, the longer the pulsing the more roughening occurs. Use Figure 1A and the discussion as a guide to help determine the specific parameters required to achieve a particular surface roughness.
- Submerge the electrode containing the tip of the device in 500 mM HClO4 that also contains a Pt wire counter electrode and MSE reference electrode. Then connect an individual electrode as the working electrode and apply the pulsing paradigm to roughen the electrode.
- In VersaStudio, press the Run button at the menu to start roughening.
4. Electrochemical roughening of a microelectrode
- Use a potentiostat that can apply voltage pulses with the 62.5 µs pulse width to roughen microelectrodes. The VMP-300 potentiostat on the materials list is not capable of applying these short pulses, while the VersaSTAT 4 potentiostat can apply the rapid pulses required to roughen thin-film microelectrodes.
- Program the following parameters into the potentiostat to roughen a 20 µm diameter Pt disk microelectrode fabricated flush with its insulating material. The roughening protocol can be applied to a single electrode or several electrodes shorted together (see additional explanation in step 4.3).
- Begin the roughening protocol with a series of oxidation/reduction pulses between -0.25 V (Vmin) and 1.2 - 1.4 V (Vmax) at 4,000 Hz with a duty cycle of 1:3 (oxidation:reduction pulse widths) for 100 s. Use guidance in the discussion to help determine the specific parameters required for other electrode geometries.
- Open the VersaStudio program.
- Expand the Experiment menu and select New.
- In the Select Action pop-up window that appears, choose Fast potential pulses and enter the desired file name when prompted. Fast potential pulses line will then appear under Actions to be performed tab.
- Fill out the following under the Properties of Fast Potential Pulses / Pulse properties, enter Number of pulses = 2, Potential (V) 1 = -0.49 vs Ref for 0.0625 ms, and Potential (V) 2 = 1.06 vs Ref for 0.1875 ms.
- Under Scan properties, fill out: Time per point = 1 s, and number of cycles: 400,000 (for 100 s duration).
- Under Instrument properties, enter Current range = Auto.
- Program the potentiostat to immediately follow the series of pulses with a prolonged reduction potential (-0.20 V for 180 s) to fully reduce any oxides produced and stabilize the chemistry of the electrode surface.
- In the VersaStudio Software, press the + button to insert a new step.
- Double click on Chronoamperometry.
- Enter Potential (V) = -0.64, Time per point (s) = 1, and Duration (s) = 180.
NOTE: Using a high purity HClO4 solution is essential for this step.
- Begin the roughening protocol with a series of oxidation/reduction pulses between -0.25 V (Vmin) and 1.2 - 1.4 V (Vmax) at 4,000 Hz with a duty cycle of 1:3 (oxidation:reduction pulse widths) for 100 s. Use guidance in the discussion to help determine the specific parameters required for other electrode geometries.
- Submerge the electrode containing tip of the device in 500 mM HClO4 that also contains a Pt wire counter electrode and MSE reference. Then connect an individual electrode or several shorted electrodes as the working electrode and apply the pulsing paradigm. In potentiostatic mode, electrodes can be shorted when trace resistance within the device is small. In that situation, ohmic drop through a device is negligible so all shorted electrodes will experience the applied potential.
- In VersaStudio, press the Run button at the menu on the top of the screen to start the roughening.
NOTE: Roughening of microelectrodes may require adjustment of the pulsing parameters depending on the electrode geometry, Pt composition, and topology (e.g., well depth for an electrode recessed in insulating material). Start with the parameters listed here and modify the Vmax value to begin optimization of roughening parameters for different electrode geometries. The different pulsing parameters for three different geometries are summarized in Table 1.
5. Characterization of electrode surface after roughening
- Determine the increase in effective surface area of macroelectrodes using steps 2.1.1-2.1.5.
- Determine the increase in effective surface area of microelectrodes using steps 2.1.1-2.1.5.
- Observe the changes in electrode appearance after roughening in optical microscopy as a loss of metal shininess (see Representative Results) and in scanning electron microscopy (SEM)6 as an obvious decrease in surface smoothness.
Results
A schematic showing the voltage application for roughening both macroelectrodes and microelectrodes is shown in Figure 2. Optical microscopy can be used to visualize the difference in the appearance of a roughened macroelectrode (Figure 3) or microelectrode (Figure 4). In addition, electrochemical characterization of the Pt surface using impedance spectroscopy and cyclic voltammetry can readily show ...
Discussion
The electrochemical roughening of thin-film macroelectrodes and microelectrodes is possible with oxidation-reduction pulsing. This simple approach does require several key elements to nondestructively roughen thin-film electrodes. Unlike foils, roughening of thin metal films may lead to sample destruction if parameters are not properly chosen. Critical parameters of the roughening procedure are pulse amplitude, duration and frequency. Additionally, ensuring electrode cleanliness and perchloric acid purity prior to the pr...
Disclosures
The authors declare no competing financial interests.
Acknowledgements
The authors would like to thank Lawrence Livermore National Laboratory's Center for Bioengineering for support during the preparation of this manuscript. Professor Loren Frank is kindly acknowledged for his collaborations with the group that have enabled fabrication and design of the thin-film Pt microarrays discussed in the above work. This work was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344 and funded by Lab Directed Research and Development Award 16-ERD-035. LLNL IM release LLNL-JRNL-762701.
Materials
| Name | Company | Catalog Number | Comments |
| Acetone | Fisher Scientific, Sigma Aldrich or similar | n/a | Laboratory grade |
| EC-Lab Software | Bio-Logic Science Instruments | n/a | For instrument control and data analysis |
| Leakless Silver/Silver Chloride Reference | eDAQ Company, Australia | ET069-1 | Free from chloride anion contamination (or other type of chloride free electrode e.g. Mercury sulfate electrode) |
| Mercury Sulfate & Acid Electrode Kit | Koslow, Scientific Testing Instruments | 5100A | glass, 9mm version |
| Milipore DI water | MilliporeSigma | n/a | Certified resistivity of 18.2 MΩ.cm (at 25°C) |
| Perchloric acid, 99.9985% | Sigma Aldrich | 311421 | High Purity |
| Phosphate-buffered saline | Teknova | P4007 | 10mM PBS with 100mM NaCl, pH 7 or similar product from elsewhere |
| Platinum Wire Auxiliary Electrode (7.5 cm) | BASi | MW-1032 | Counter electrode |
| Pt macroelectrodes | Lawrence Livermore National Laboratory | n/a | 1.2 mm diameter, 250 nm thick Pt disc electrodes insulated in polyimide. More information in Reference 9. |
| Pt microelectrode arrays | Lawrence Livermore National Laboratory | n/a | 20 µm diameter 250 nM thick Pt disc electrodes insulated in polyimide. More information in Reference 9. |
| Sulfuric acid, 99.999% | Sigma Aldrich | 339741 | High Purity |
| UV & Ozone Dry Stripper | Samco | UV-1 | for cleaning electrodes |
| VersaSTAT 4 Potentiostat | AMETEK, Inc. | n/a | Good time resolution for pulsing tests |
| VersaStudio Software | AMETEK, Inc. | n/a | For instrument control |
| VMP-200 Potentiostat | Bio-Logic Science Instruments | n/a | Low current resolution option is preferable for measurements with microelectrodes |
References
- Fleischmann, M., Hendra, P. J., McQuillan, A. J. Raman spectra of pyridine adsorbed at a silver electrode. Chemical Physics Letters. 26 (2), 163-166 (1974).
- Chung, T., et al. Electrode modifications to lower electrode impedance and improve neural signal recording sensitivity. Journal of Neural Engineering. 12 (5), 056018 (2015).
- Green, R. A., et al. Laser patterning of platinum electrodes for safe neurostimulation. Journal of Neural Engineering. 11 (5), 056017 (2014).
- Arroyo-Currás, N., Scida, K., Ploense, K. L., Kippin, T. E., Plaxco, K. W. High Surface Area Electrodes Generated via Electrochemical Roughening Improve the Signaling of Electrochemical Aptamer-Based Biosensors. Analytical Chemistry. 89 (22), 12185-12191 (2017).
- Weremfo, A., Carter, P., Hibbert, D. B., Zhao, C. Investigating the interfacial properties of electrochemically roughened platinum electrodes for neural stimulation. Langmuir. 31 (8), 2593-2599 (2015).
- Ivanovskaya, A. N., et al. Electrochemical Roughening of Thin-Film Platinum for Neural Probe Arrays and Biosensing Applications. Journal of The Electrochemical Society. 165 (12), G3125-G3132 (2018).
- Cai, W. B., et al. Investigation of surface-enhanced Raman scattering from platinum electrodes using a confocal Raman microscope: dependence of surface roughening pretreatment. Surface Science. 406 (1), 9-22 (1998).
- Tykocinski, M., Duan, Y., Tabor, B., Cowan, R. S. Chronic electrical stimulation of the auditory nerve using high surface area (HiQ) platinum electrodes. Hearing Research. 159 (1-2), 53-68 (2001).
- Liu, Y. C., Wang, C. C., Tsai, C. E. Effects of electrolytes used in roughening gold substrates by oxidation-reduction cycles on surface-enhanced Raman scattering. Electrochemistry Communications. 7 (12), 1345-1350 (2005).
- Liu, Z., Yang, Z. L., Cui, L., Ren, B., Tian, Z. Q. Electrochemically Roughened Palladium Electrodes for Surface-Enhanced Raman Spectroscopy: Methodology, Mechanism, and Application. The Journal of Physical Chemistry C. 111 (4), 1770-1775 (2007).
- Rodríguez, J. M. D., Melián, J. A. H., Peña, J. M. Determination of the Real Surface Area of Pt Electrodes. Journal of Chemical Education. 77 (9), 1195-1197 (2000).
- Lvovich, V. F. . Impedance Spectroscopy: Applications to Electrochemical and Dielectric Phenomena. , (2012).
- Tooker, A., et al. Towards a large-scale recording system: demonstration of polymer-based penetrating array for chronic neural recording. Conference proceedings - IEEE Engineering in Medicine and Biology Society. 2014, 6830-6833 (2014).
- Tooker, A., et al. Microfabricated polymer-based neural interface for electrical stimulation/recording, drug delivery, and chemical sensing development. Conference proceedings - IEEE Engineering in Medicine and Biology Society. 2013, 5159-5162 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved