A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Scratch Migration Assay and Dorsal Skinfold Chamber for In Vitro and In Vivo Analysis of Wound Healing
In This Article
Summary
Here, we present a protocol for an in vitro scratch assay using primary fibroblasts and for an in vivo skin wound healing assay in mice. Both assays are straightforward methods to assess in vitro and in vivo wound healing.
Abstract
Impaired cutaneous wound healing is a major concern for patients suffering from diabetes and for elderly people, and there is a need for an effective treatment. Appropriate in vitro and in vivo approaches are essential for the identification of new target molecules for drug treatments to improve the skin wound healing process. We identified the β3 subunit of voltage-gated calcium channels (Cavβ3) as a potential target molecule to influence the wound healing in two independent assays, i.e., the in vitro scratch migration assay and the in vivo dorsal skinfold chamber model. Primary mouse embryonic fibroblasts (MEFs) acutely isolated from wild-type (WT) and Cavβ3-deficient mice (Cavβ3 KO) or fibroblasts acutely isolated from WT mice treated with siRNA to down-regulate the expression of the Cacnb3 gene, encoding Cavβ3, were used. A scratch was applied on a confluent cell monolayer and the gap closure was followed by taking microscopic images at defined time points until complete repopulation of the gap by the migrating cells. These images were analyzed, and the cell migration rate was determined for each condition. In an in vivo assay, we implanted a dorsal skinfold chamber on WT and Cavβ3 KO mice, applied a defined circular wound of 2 mm diameter, covered the wound with a glass coverslip to protect it from infections and desiccation, and monitored the macroscopic wound closure over time. Wound closure was significantly faster in Cacnb3-gene-deficient mice. Because the results of the in vivo and the in vitro assays correlate well, the in vitro assay may be useful for the high-throughput screening before validating the in vitro hits by the in vivo wound healing model. What we have shown here for wild-type and Cavβ3-deficient mice or cells might also be applicable for specific molecules other than Cavβ3.
Introduction
Skin wound healing starts immediately after the skin injury in order to restore the skin's integrity and to protect the organism from infections. The wound healing process goes through four overlapping phases; coagulation, inflammation, new tissue formation, and tissue remodeling1. Cell migration is crucial during these phases. Inflammatory cells, immune cells, keratinocytes, endothelial cells, and fibroblasts are activated at different time points and invade the wound area2. Methods to investigate wound healing in vitro and in vivo are of great interest not only to understand the underlying mechanisms but also to test new drugs and to develop new strategies aiming to ameliorate and accelerate skin wound healing.
To monitor and analyze cell migration, the scratch migration assay can be used. It is often referred to as in vitro wound healing assay. This method requires a cell culture facility3. It is a simple procedure, there is no need of high-end equipment and the assay can be performed in most cell biology laboratories. In this assay, a cell-free area is created by the mechanical disruption of a confluent cell monolayer, preferably epithelial- or endothelial-like cells or fibroblasts. Cells on the edge of the scratch will migrate in order to repopulate the created gap. Quantification of the decreasing cell-free area over time resembles the migration rate and indicates the time, which the cells need to close the gap. For this purpose, investigators can use either acutely isolated cells from WT mice or mice lacking a gene of interest4, or immortalized cells available from reliable cell repositories. The scratch assay allows studying the influence of pharmacologically active compounds or the effect of transfected cDNAs or siRNAs on cell migration.
In vivo, wound healing is a complex physiological process, requiring different cell types including keratinocytes, inflammatory cells, fibroblasts, immune cells and endothelial cells in order to restore the skin’s physical integrity as fast as possible1. Different methods to study in vivo wound healing have been developed and used in the past5,6,7,8. The dorsal skinfold chamber described in this article was previously used for wound healing assays9. It is used as a modified dorsal skinfold chamber preparation for mice. The modified skinfold chamber model has several advantages. 1) It minimizes skin contraction, which prevents observing the wound healing process and might influence wound repair in mice. 2) This chamber makes use of covering the wound with a glass coverslip, reducing tissue infections and desiccation, which could delay the healing process. 3) Blood flow and vascularization can be directly monitored. 4) It allows repetitive topical application of pharmacologically active compounds and reagents in order to treat the wound and accelerate healing9,10.
We identified the β3 subunit of high voltage-gated calcium channels (Cavβ3) as a potential target molecule to influence skin wound healing using two independent protocols, i.e., the in vitro scratch migration assay and the in vivo dorsal skinfold chamber model. For the in vitro assay, we used primary fibroblasts, these cells do express the Cacnb3 gene encoding the Cavβ3 protein but lack depolarization-induced Ca2+ influx or voltage-dependent Ca2+ currents. We described a novel function of Cavβ3 in these fibroblasts: Cavβ3 binds to the inositol 1,4,5-trisphosphate receptor (IP3R) and constraints calcium release from the endoplasmic reticulum. Deletion of the Cacnb3 gene in mice leads to increased sensitivity of the IP3R for IP3, enhanced cell migration and increased skin wound repair4.
Protocol
All experimental procedures were approved and performed in accordance with the ethics regulations and the animal welfare committees of Saarland and Saarland University.
1 Primary cell culture and siRNA transfection
NOTE: In the described method, primary fibroblasts are used. These cells play a crucial role in wound healing and tissue remodeling11. In this experiment, the Cacnb3 gene, encoding the Cavβ3 subunit of high voltage-gated calcium channels12 was down-regulated, thereby showing its role in cell migration in vitro and skin wound repair in vivo4.
- Preparation of siRNA: Before reconstituting the siRNAs, briefly centrifuge the tubes to ensure that the content is at the bottom. Reconstitute the siRNAs in 100 µL RNase-free buffer (100 mM potassium acetate, 30 mM HEPES, pH 7.5) provided by the manufacturer at a concentration of 20 µM. This is a stock solution of siRNAs.
- Aliquot this stock solution at 10 µL per tube (20 µM concentration) and store at -20 °C until use.
- Using an ultrafine permanent marker, mark a 6-well plate with a horizontal line at the bottom of each well in order to be able to always identify the same scratch region of interest and to follow its closure.
NOTE: 6-well culture plates were used in this assay because they are large enough, to provide enough space and flexibility to apply a consistent, reproducible and vertical scratch using a 200 µL pipette tip across the cell monolayer. If a limited number of cells are available, an alternative and probably the cost-efficient way would be to use 12- or 24-well culture plates. - Plate primary fibroblasts, isolated from the wild-type and β3-deficient mice4, in a 6-well plate at a density of 5 x 105 cells/well in the presence of 2 mL Dulbecco’s modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FCS).
NOTE: 5 x 105 cells per well has been established for 6-well culture plate and for primary mouse fibroblasts. Tests may be needed if using 12- or 24-well cell culture plates or other cell types, which could be different in size. Cells should be handled in a sterile environment such as biological safety cabinets class II. - Label the 6-well plate with the cell type, genotype, and the date.
- Move the 6-well plate into the cell culture incubator and maintain cells at 37 °C and 5% CO2 for 24 h.
- The next day, take the plate out of the incubator, aspirate the cell culture medium out of the well, discard it and replace it with 2.25 mL fresh culture medium by adding it carefully against the wall of the well.
- In order to transfect fibroblasts with siRNAs, use a lipid-based transfection reagent as recommended by the manufacturer.
- For each transfection, label two microcentrifuge tubes. In the first one, add 9 µL of the transfection reagent and dilute it with 150 µL reduced serum medium. In the second tube, add 1.5 µL siRNA (Cacnb3 siRNA-1, Cacnb3 siRNA-2 or scrambled siRNA as a negative control) and dilute it with 150 µL reduced serum medium.
- Add the diluted siRNA into the tube containing diluted transfection reagent and vortex for 2 s. Incubate the mixture for 5 min at 21 °C.
- Label wells with either Cacnb3 siRNA-1, Cacnb3 siRNA-2 or scrambled siRNA. Add 250 µL of the siRNA-transfection reagent mixture dropwise to the cells.
- Place the 6-well culture plate back into the incubator and keep cells at 37 °C and 5% CO2 for 72 h.
- In order to check the efficiency of Cacnb3 gene silencing, collect transfected cells and perform immunoblot analysis as described previously4.
2. In vitro wound healing assay (scratch migration assay)
- Take the cell culture plate out of the incubator and examine the cells under the microscope using the 10x objective. Start with the scratch assay only when they have reached 100% confluency.
NOTE: For the accuracy and reproducibility, 100% confluency is a mandatory factor for starting the scratch migration assay. Therefore, it is important to seed the same number of cells into the culture wells, to examine each well for confluency and to apply the scratch at the same time point (day 0 confluency). Waiting for longer after the cells reach 100% confluency can evoke different responses. - Once the cell reaches 100% confluency, aspirate the culture medium out of the well and discard it.
- Use a pipette tip (200 µL) to manually create a scratch vertical to the horizontal line marked at the bottom of the well, across the confluent cell monolayer in the middle of the well.
- Rinse each well twice with 2 mL phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8.1 mM Na2HPO4, pH 7.4) to remove the released factors from damaged cells, loose cells, and debris from the scratched area. Add 2 mL of PBS carefully against the wall of the well to avoid detaching cells from the cell culture well.
- Add 2 mL of cell culture medium containing either 10% serum or 1% serum carefully to each well.
NOTE: It is recommended to perform the scratch assay under 10% serum and under 1% serum to confirm that the observed effect is caused by the cell proliferation and migration or by cell migration only. - Move the plate to the microscope stage and capture bright field images of the cell-free area (two areas per well) immediately after scratching (t=0h) at a 10x magnification using a light microscope. To image always the same region of the scratch, use the horizontal line, which was prepared in step (1.3), and take one image above this line and one image below this line. Save images as TIFF or JPEG.
- Because the microscope stage does not maintain the cell growth condition, move the plate back to the cell culture incubator and keep the cells at 37 °C and 5% CO2.
- After 6, 10 and 30 h, move the plate to the microscope stage again and capture images the same way as described in step 2.6.
NOTE: These time points have been established for the described procedure and for primary fibroblasts. During the first pilot experiment, more time points were tested to see how fast fibroblasts repopulate the gap. Although 0, 6, 10 and 30 h are reasonable starting time points, the investigators should optimize and establish the appropriate time points for each application and for each cell type. The more accurate alternative, if available, would be to use time-lapse microscopy. - Using ImageJ13, quantify the initial cell-free area (100%) and the remaining area after 6, 10 and 30 h (Figure 1). The percentage of scratch area repopulated by migrating cells is then calculated relative to the initial scratch area.
3. Analysis of the scratch area
- Open ImageJ software13.
- Upload the first image as JPEG (e.g., 24-bit RGB images 1360x1024) by dropping the image into the ImageJ menu bar.
- Select the Freehand selections button and mark the cell-free area
- Click on Analyze and select Measure. A window with the results will appear containing the area value.
- Transfer this value into an analysis spreadsheet.
- Repeat steps 3.2-3.5 for each image from time point 0 h and then start again for the next time points 6, 10 and 30 h.
- Calculate the percentage of scratch area repopulated by migrating cells after 6, 10 and 30 h for each scratch using the following equation:
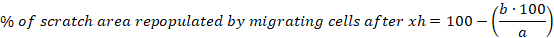
a = cell free area of the initial scratch, b = cell free area after 6 h - Calculate the mean and the standard error of the mean (S.E.M.) for the percentage of scratch area repopulated by migrating cells after 6 h. Show data as a column bar graph or a scatter plot.
4. In vivo skin wound healing assay
NOTE: C57BL/6 wild-type males (8-12 weeks old with 22-26 g body weight) and Cavβ3-deficient mice as a control are used for this study.
- One day before starting the experiment, autoclave all the surgical instruments, screws, nuts and titanium frames to be used for the skinfold chamber preparation.
NOTE: The titanium frame is composed of two symmetrical complementary halves and it has a circular observation window where the wound will be applied and followed by microscopy (see Figure 2a). - Anesthetize a wild-type or β3-deficient mouse (22-26 g body weight) by intraperitoneal (i.p.) injection of 0.1 mL saline/10 g body weight containing a mixture of ketamine (75 mg/kg body weight) and xylazine (25 mg/kg body weight). Check the depth of anesthesia by the lack of response to a toe pinch.
NOTE: This injection gives around 30 min surgical anesthesia and the depth of anesthesia must be controlled through the surgical procedure, by checking the reflexes of the mouse. - To avoid dryness or damage of the eyes, apply ophthalmic ointment to both eyes and repeat the application if necessary.
- Carefully shave the mouse dorsum, using an electric shaver followed by the application of a depilation cream to the shaved area to remove any remaining hair. Take care not to injure the mouse skin. Leave the depilation cream for around 10 min to completely remove all hair.
- Prepare the titanium chamber by taking one part of the symmetrical titanium chamber frame and fix the connecting screws with nuts on one side. These nuts will serve as a spacer to keep 400-500 µm between the two symmetrical parts of the chamber to avoid compression of blood vessels in the skin.
- Remove the cream from the back of the mouse and clean the hair-free region with warm (35-37 °C) tap water.
- Make sure that the place to perform surgery is clean, warm (37 °C) and humidified.
- Disinfect the hair-free area of the mouse with skin disinfectant. Take a fold of the back skin of the mouse in front of a light source and position the middle line of the double layer of the skin where the titanium chamber will be implanted. After that, fix the skinfold with a polypropylene suture cranially and caudally and tighten the other side of the suture on a metal rack to lift the mouse folded skin. Adjust the height of the rack to allow the mouse to sit comfortably.
- Implant the titanium chamber into the fold of the back skin of the mouse in a way to sandwich the folded dorsal skin layer between the two symmetrical halves of the titanium frame. Attach the first half of the titanium frame by polypropylene sutures on its superior edge to the back of the dorsal skinfold.
NOTE: On the titanium frame, there are 8 holes on the superior edge (Figure 2a) and the folded skin should be well fixed by polypropylene sutures on each of the eight holes. - Before moving to the next step, check the reflexes of the mouse to make sure that the depth of anesthesia is maintained.
- At the base of the skinfold, pass the two connecting screws, attached to the first half of the titanium chamber, to penetrate the skinfold from back to the front side. Make small incisions on the skin (using fine scissors) to help smooth penetration of the connecting screws.
- Detach the mouse from the rack and place it on a lateral position. Put the second complementary half of the titanium chamber on top of the 3 connecting screws (see Figure 2a) and apply slight pressure with fingers in order to pass these screws through the second half of the titanium frame. Then, fix both symmetrical parts with stainless steel nuts.
- Pay careful attention to the tightness of the screws at this step, since it might detach, if it is too loose. In contrast, if it is too tight, it will squeeze the skinfold, reduce blood flow and can lead to tissue impairment and necrosis.
NOTE: Nuts prepared in step 4.5 serve as a spacer to keep a distance of 400-500 µm between the two symmetrical halves of the titanium chamber. The nuts should be tightened until a slight resistance is felt. - Cut the remaining part of the screws using pliers.
NOTE: It is necessary at this step to use laboratory safety glasses for eye protection in case the screw comes off the wrong way. - Mark the wound area by a standardized biopsy punch (2 mm in diameter), in the center of the skin within the observation window (see Figure 2a) of the skinfold chamber in order to ensure reproducible wound sizes.
- By using fine forceps and scissors, create a circular wound within the marked area by removing the complete skin with epidermis and dermis. The final wound area will be around 3.5-4.5 mm2, see Figure 2b. Clean the wound with 0.5 mL sterile saline (0.9 % NaCl, 37 °C).
- Cover the wound with a glass coverslip and fix this glass coverslip with a snap ring using the snap ring plier on the titanium chamber.
- Immediately after finishing the surgical procedure, place the mouse on the imaging stage of a stereomicroscope equipped with a camera and take images (day 0) under illumination. Use the 40X magnification and save the images for future off-line analysis.
NOTE: The investigator should examine the images immediately after capture to ensure that quality is sufficient for future off-line analyses. The preparation of the skinfold chamber and performance of the skin wound takes around 30 minutes. - Keep the mouse at a warm place during recovery from anesthesia for at least 2 h. Thereafter, transfer mice in individual cages back to the animal facility (12 h light/dark cycle) and make sure that mice have access to food and water.
- Three days post-wounding place the mouse in a mouse-restrainer and fix the restrainer on top of the imaging stage.
- Place the stage under a stereomicroscope equipped with a camera. Take images under illumination with 40x magnification, record all pictures and save them for future off-line analyses
- Repeat steps 4.20 and 4.21 again at day 6, 10 and day 14 post-wounding.
- Use the wound images, for off-line analysis in ImageJ13. The wound area at day 0 is considered as 100 % and the wound closure over time is plotted relative to the initial wound area. Representative results are shown in Figure 2c,d.
- Calculate the percentage (%) of the wound area at each time point using the following equation:
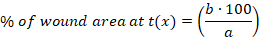
x: time point (day 0, 3, 10 or 14), a: wound area at day 0, b: wound area at time point x
Results
The scratch assay was performed on a confluent cell monolayer of wild-type and β3-deficient MEFs (Figure 1c). After performing the "scratch" using a 200 μL pipette tip, cells from both genotypes migrate into the scratch area and close the gap. Images were taken after 6, 10 and 30 h (Figure 1a). Cell migration was quantified as the percentage (%) of scratch area repopulated by migrating cells 6 hours after ...
Discussion
In this manuscript, we describe an in vitro and in vivo wound healing assay and correlate the results obtained. For the in vitro assay, we used primary mouse fibroblasts4,14,15 which play an important role in wound healing and tissue remodeling11. Other adherent cell types growing as monolayers (e.g., epithelial cells, endothelial cells, keratinocytes) can be used as well. Plating the same number of viabl...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Petra Weissgerber and the Transgene Unit of the SPF animal facility (project P2 of SFB 894) of the Medical Faculty and the animal facility at the Institute of Clinical and Experimental Surgery at the Medical Faculty of Saarland University, Homburg. We thank Dr. Andreas Beck for critical reading of the manuscript. This study was funded by the Deutsche Forschungsgemeinschaft (DFG) Sonderforschungsbereich (SFB) 894, project A3 to A.B. and V.F.).
Materials
| Name | Company | Catalog Number | Comments |
| 0.9 % NaCl | |||
| 1 ml syringes | BD Plastipak | 303172 | |
| 6 well plate | Corning | 3516 | |
| Biopsy punch | Kai Industries | 48201 | 2 mm |
| Cacnb3 Mouse siRNA Oligo Duplex (Locus ID 12297) | Origene | SR415626 | |
| Depilation cream | any depilation cream | ||
| Dexpanthenol 5% (BEPANTHEN) | Bayer | 3400935940179.00 | (BEPANTHEN) |
| Dihydroxylidinothiazine hydrochloride (Xylazine) | Bayer Health Care | Rompun 2% | |
| Dulbecco's Modified Eagle Medium (DMEM) | Gibco by life technologies | 41966-029 | |
| Fetal bovine serum | Gibco by life technologies | 10270-106 | |
| Hexagon full nut | |||
| Ketamine hydrochloride | Zoetis | KETASET | |
| Light microscope | Keyence, Osaka, Japan | BZ-8000 | Similar microscopes might be used |
| Lipofectamine RNAiMAX Transfection Reagent | Thermo Fisher Scientific | 13778075 | |
| Micro-forceps | |||
| Micro-Scissors | |||
| Mouse restrainer | Home-made | ||
| Normal scissors | |||
| Objective | Nikon | plan apo 10x/0.45 | |
| Opti-MEM | Gibco by life technologies | 51985-026 | |
| Polypropylene sutures | |||
| Screwdriver | |||
| Skin disinfectant (octeniderm) | Schülke & Mayr GmbH | 118212 | |
| Slotted cheese head screw | |||
| Snap ring | |||
| Snap ring plier | |||
| Surgical microscope with camera | Leica | Leica M651 | |
| Titanium frames for the skinfold chamber | IROLA | 160001 | Halteblech M |
| Wire piler |
References
- Gurtner, G. C., Werner, S., Barrandon, Y., Longaker, M. T. Wound repair and regeneration. Nature. 453, 314-321 (2008).
- Martin, P. Wound healing--aiming for perfect skin regeneration. Science. 276, 75-81 (1997).
- Gabbiani, G., Gabbiani, F., Heimark, R. L., Schwartz, S. M. Organization of actin cytoskeleton during early endothelial regeneration in vitro. Journal of Cell Science. 66, 39-50 (1984).
- Belkacemi, A., et al. IP3 Receptor-Dependent Cytoplasmic Ca(2+) Signals Are Tightly Controlled by Cavbeta3. Cell Reports. 22, 1339-1349 (2018).
- Breuing, K., Eriksson, E., Liu, P., Miller, D. R. Healing of partial thickness porcine skin wounds in a liquid environment. Journal of Surgical Research. 52, 50-58 (1992).
- Colwell, A. S., Krummel, T. M., Kong, W., Longaker, M. T., Lorenz, H. P. Skin wounds in the MRL/MPJ mouse heal with scar. Wound Repair and Regeneration. 14, 81-90 (2006).
- Vagesjo, E., et al. Accelerated wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria. Proceedings National Academy of Science. 115, 1895-1900 (2018).
- Eming, S. A., et al. Accelerated wound closure in mice deficient for interleukin-10. American Journal of Pathology. 170, 188-202 (2007).
- Sorg, H., Krueger, C., Vollmar, B. Intravital insights in skin wound healing using the mouse dorsal skin fold chamber. Journal of Anatomy. 211, 810-818 (2007).
- Laschke, M. W., Vollmar, B., Menger, M. D. The dorsal skinfold chamber: window into the dynamic interaction of biomaterials with their surrounding host tissue. European Cell and Materials. 22, 147-164 (2011).
- Trepat, X., Chen, Z., Jacobson, K. Cell migration. Comprehensive Physiology. 2, 2369-2392 (2012).
- Hofmann, F., Belkacemi, A., Flockerzi, V. Emerging Alternative Functions for the Auxiliary Subunits of the Voltage-Gated Calcium Channels. Current Molecular Pharmacology. 8, 162-168 (2015).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9, 676-682 (2012).
- Chen, L., et al. Protein 4.1G Regulates Cell Adhesion, Spreading, and Migration of Mouse Embryonic Fibroblasts through the beta1 Integrin Pathway. Journal of Biological Chemistry. 291, 2170-2180 (2016).
- Dewor, M., et al. Macrophage migration inhibitory factor (MIF) promotes fibroblast migration in scratch-wounded monolayers in vitro. FEBS Letters. 581, 4734-4742 (2007).
- Handly, L. N., Wollman, R. Wound-induced Ca(2+) wave propagates through a simple release and diffusion mechanism. Molecular Biology of the Cell. 28, 1457-1466 (2017).
- Cappiello, F., Casciaro, B., Mangoni, M. L. A Novel In Vitro Wound Healing Assay to Evaluate Cell Migration. Journal of Visualized Experiments. (133), 56825 (2018).
- Hernandez Vera, R., Schwan, E., Fatsis-Kavalopoulos, N., Kreuger, J. A Modular and Affordable Time-Lapse Imaging and Incubation System Based on 3D-Printed Parts, a Smartphone, and Off-The-Shelf Electronics. PLoS One. 11, 0167583 (2016).
- Reinhart-King, C. A. Endothelial cell adhesion and migration. Methods in Enzymology. 443, 45-64 (2008).
- Treloar, K. K., Simpson, M. J. Sensitivity of edge detection methods for quantifying cell migration assays. PLoS One. 8, 67389 (2013).
- Pirkmajer, S., Chibalin, A. V. Serum starvation: caveat emptor. American Journal of Physiology-Cell Physioliology. 301, 272-279 (2011).
- Papenfuss, H. D., Gross, J. F., Intaglietta, M., Treese, F. A. A transparent access chamber for the rat dorsal skin fold. Microvascular Research. 18, 311-318 (1979).
- Deoliveira, D., et al. An ear punch model for studying the effect of radiation on wound healing. International Journal of Radiation Biology. 87, 869-877 (2011).
- Chen, L., Mirza, R., Kwon, Y., DiPietro, L. A., Koh, T. J. The murine excisional wound model: Contraction revisited. Wound Repair and Regeneration. 23, 874-877 (2015).
- Dunn, L., et al. Murine model of wound healing. Journal of Visualized Experiment. (75), e50265 (2013).
- Wahedi, H. M., Park, Y. U., Moon, E. Y., Kim, S. Y. Juglone ameliorates skin wound healing by promoting skin cell migration through Rac1/Cdc42/PAK pathway. Wound Repair and Regeneration. 24, 786-794 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved