A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Detergent-assisted Reconstitution of Recombinant Drosophila Atlastin into Liposomes for Lipid-mixing Assays
In This Article
Summary
Biological membrane fusion is catalyzed by specialized fusion proteins. Measuring the fusogenic properties of proteins can be achieved by lipid mixing assays. We present a method for purifying recombinant Drosophila atlastin, a protein that mediates homotypic fusion of the ER, reconstituting it to preformed liposomes, and testing for fusion capacity.
Abstract
Membrane fusion is a crucial process in the eukaryotic cell. Specialized proteins are necessary to catalyze fusion. Atlastins are endoplasmic reticulum (ER) resident proteins implicated in homotypic fusion of the ER. We detail here a method for purifying a glutathione S-transferase (GST) and poly-histidine tagged Drosophila atlastin by two rounds of affinity chromatography. Studying fusion reactions in vitro requires purified fusion proteins to be inserted into a lipid bilayer. Liposomes are ideal model membranes, as lipid composition and size may be adjusted. To this end, we describe a reconstitution method by detergent removal for Drosophila atlastin into preformed liposomes. While several reconstitution methods are available, reconstitution by detergent removal has several advantages that make it suitable for atlastins and other similar proteins. The advantage of this method includes a high reconstitution yield and correct orientation of the reconstituted protein. This method can be extended to other membrane proteins and for other applications that require proteoliposomes. Additionally, we describe a FRET based lipid mixing assay of proteoliposomes used as a measurement of membrane fusion.
Introduction
Membrane fusion is a critical process in many biological reactions. Under biological conditions, membrane fusion is not spontaneous and requires specialized fusion proteins to catalyze such reactions1. ER homotypic membrane fusion is mediated in animals by the dynamin related GTPase atlastin2. Atlastin’s role in homotypic fusion is fundamental for three-way junctions in peripheral ER, which constitutes a large interconnected network of tubules that extend throughout the cell. Atlastins have a conserved domain morphology consisting of a large GTPase, a three helix bundle middle domain, a hydrophobic membrane anchor, and a short cytoplasmic C-terminal tail3. In vitro studies with recombinant Drosophila atlastin have shown that when reconstituted to liposomes, it maintains its fusogenic properties. Other atlastins, including human homologs have not been able to recapitulate fusion in vitro. We describe here a methodology for purifying a GST and poly-histidine tagged recombinant Drosophila atlastin, reconstituting it to liposomes, and assaying fusion.
Studying membrane fusion in vitro presents a challenge as fusogenic proteins usually have a membrane anchor. In order to study them, it is necessary to reconstitute them into model lipid bilayers. Large unilamellar vesicles (LUV) are a useful tool to study lipid protein interactions. We present here a system to make LUVs of different lipid compositions for protein reconstitution and fusion assays. Reconstitution of integral proteins into LUVs can be achieved by a variety of methods including, organic solvent-mediated reconstitution, mechanical mechanisms, or detergent assisted reconstitution4. We present here a method for reconstituting Drosophila atlastin into preformed liposomes by detergent removal. Advantages of this reconstitution method include high reconstitution yields and proper orientation of atlastin in the lipid bilayer. Additionally, through this method, the protein is not dried or exposed to organic solvents thereby maintaining structure and function. Among its disadvantages, the presence of detergents may not be ideal for all proteins and the final proteoliposomes may have some incorporated detergent in the lipid bilayer. Further dialysis may be used to eliminate more of the detergent. However, dialysis may take a long time and can therefore lead to loss of protein activity.
Assessing atlastin’s fusion activity can be determined by lipid mixing assays as previously described2. Here, we delineate a method for measuring atlastin mediated fusion through N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD)/Lissamine rhodamine-B sulfonyl (rhodamine) labeled lipids. This assay requires fusion of donor (labeled) proteoliposomes and acceptor (unlabeled) proteoliposomes. A FRET release can be measured during the reaction as the dilution of a donor–acceptor pair from “labeled” liposomes to “unlabeled” liposomes as a result of lipid mixing during membrane fusion (Figure 1)5. While this assay serves as a proxy for membrane fusion, it is limited in distinguishing between membrane fusion and hemifusion, a state where only the outer leaflets mix. To address this issue, an alternative is outer leaflet quenching of NBD by dithionite. Following the same methodology as NBD/rhodamine lipid mixing assays, upon quenching the outer leaflet any NBD FRET release by fusion will be due to inner leaflet mixing8.
Alternative fusion assays by inner aqueous content mixing address full fusion only5. Examples of this are terbium (Tb)/dipicolinic acid (DPA) assays and aminonaphthalene trisulfonic acid (ANTS)/p-xylene bis(pyridinium) bromide (DPX) assays. In Tb/DPA assays, a pool of liposomes with encapsulated Tb are mixed and fused with liposomes with encapsulated DPA; upon fusion, fluorescence is increased via internal energy transfer from DPA to Tb within the [Tb(DPA)3]3- chelation complex6. In contrast, for ANTS/DPX assays, ANTS fluorescence is quenched by DPX7. While these systems address inner content mixing, more in-depth preparation of liposomes is required for removal of non-encapsulated reagents, as well as unintended interaction of the fluorophores.
Protocol
1. Purification of GST-DAtl-His8
- Protein expression and lysate preparation
- Transform BL21 (DE3) E. coli with the GST-DAtl-His8 construct in pGEX4-T32 and select on an ampicillin plate.
- Select a single transformant and inoculate 5 mL of LB + ampicillin (5 µL of 100 mg/mL ampicillin) in a 14 mL culture tube and incubate at 25 °C with shaking at 200 rpm for 6-8 h.
NOTE: Due to leaky expression, it is not recommended to incubate at higher temperatures. Growth at 25 °C reduces aggregation of protein during this growth period. - Inoculate 200 mL of LB + ampicillin with 1 mL of the 5 mL culture and incubate overnight (~15–18 h) at 25 °C.
- The next morning, harvest the cells by centrifugation (2,000 x g for 10 min) and resuspend in 5 mL of LB.
- Inoculate 4 L of LB + ampicillin and measure OD600 (0.05–0.15). Incubate at 25 °C with shaking.
NOTE: Reserve some media to serve as a blank for the OD600 measurements before adding bacteria. - Measure OD600 every hour until it reaches an OD between 0.4–0.5. At this point, reduce the temperature to 16 °C.
- Induce with 0.2 mM IPTG (800 µL of 1 M stock), 10 min after the incubator reaches 16 °C. Incubate overnight (~15–18 h) at 16 °C.
NOTE: The lower temperature improves the yield of functional protein by reducing aggregation of atlastin. - The next morning, harvest the cells by centrifuging at 7,500 x g at 4 °C.
- Resuspend the cells in 200 mL of A200 (25 mM HEPES (pH 7.4) and 200 mM KCl).
- Centrifuge the cells at 11,000 x g for 5 min at 4 °C.
- Resuspend the pellet in 40 mL of breaking buffer (A200 plus 10% glycerol, 2 mM 2-mercaptoethanol, 4% Triton X-100 (TX100), 40mM imidazole, and one EDTA-free protease inhibitor cocktail tablet).
NOTE: Add TX-100 after resuspending to avoid generating bubbles and foam. - Pass through an 18 G needle and run the cells through a cell disrupter three times at ~10,000 psi.
- Centrifuge the extract at 125,000 x g for 1 h.
NOTE: Optionally dissolve the pellet 1:2 (w:v) in 8 M Urea and nutate at room temperature overnight. Urea as a denaturant will dissolve slowly any insoluble pelleted protein for SDS-PAGE analysis. Save 1 μL for SDS-PAGE and Coomassie stain analysis. - Filter the extract through a 0.45 µm cellulose nitrate sterile membrane filter to remove bacteria and large bacterial debris.
NOTE: Optionally, save 1 μL for SDS PAGE and Coomassie stain analysis.
- Protein purification by affinity chromatography
- Load the filtered lysate onto an immobilized metal affinity chromatography (IMAC) resin column charged with Ni2+, pre-equilibrated with low imidazole buffer (A100 plus 10% glycerol, 2 mM 2-mercaptoethanol, 1% TX-100, 40 mM imidazole) at a rate of 1 mL/min at 4 °C.
- Wash the column with 25 mL of A100 plus 10% glycerol, 2 mM 2-mercaptoethanol, 0.1% Anapoe X-100, 40 mM imidazole with a rate of 1 mL/min at 4°C. Elute the protein with a 30 mL linear gradient of imidazole from 40 mM to 500 mM, and a final 5 mL wash at 500 mM.
- Pool together peak fractions and incubate for 1 h at 4 °C with swollen GSH-Agarose beads, previously swollen in water and equilibrated in A100 with 10% glycerol, 2 mM 2-mercaptoethanol, 0.1% Anapoe X-100, and 1 mM EDTA.
NOTE: GSH-Agarose beads can be swollen in 50 mL of water the day before and incubated overnight at 4 °C, or for 1 h at RT. To remove water and equilibration buffer, centrifuge in a swinging bucket rotor at 500 x g for 1 min without brake. Aspirate the supernatant with a 26 G needle. - Pellet GSH-Agarose beads by centrifuging in a swinging bucket rotor at 500 x g without brake and remove the lysate supernatant by aspiration with a 26 G needle.
- Transfer the beads to a 10 mL polyprep column and wash five times with 5 mL of equilibration buffer (A100 with 10% glycerol, 2 mM 2-mercaptoethanol, 0.1% Anapoe X-100, and 1 mM EDTA) by centrifuging at 500 x g.
- Elute the protein with 1–1.5 mL of equilibration buffer supplemented with 10 mM reduced glutathione. Aliquot eluted protein and flash freeze in liquid nitrogen. Protein can be stored at -80 °C indefinitely.
NOTE: Adjust pH of elution buffer to 7.4. - Quantify protein concentration by amido black protein assay9 and assess purity by SDS-PAGE and Coomassie staining.
2. Reconstitution of Recombinant Atlastin into Liposomes
- Liposome production by extrusion method10
- Make lipid mix stocks in chloroform (10 mM total lipid). The necessary lipids are 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC), 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (DOPS), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (NBD-DPPE), and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (Rh-DPPE). Acceptor liposomes consist of POPC: DOPS (85:15 molar ratio) and donor liposomes of POPC:DOPS:Rh-PE:NBD-PE (83:15:1.5:1.5 molar ratio).
NOTE: While POPC:DOPS lipid mixes have been traditionally used for in vitro lipid mixing assays, alternative lipid compositions may be adapted for different experimental purposes. POPC:DOPS liposomes are very stable and harder to fuse, therefore is a very stringent system for fusion. - Add 1 μCi/mL of L-α-dipalmitoyl-phosphatidylcholine (choline methyl-3H) to lipid mixes in order to determine lipid concentrations at later steps by liquid scintillation counting. Reserve at least 8 μL of this stock.
- Transfer desired amount of lipid mixes to flint glass tubes.
- Dry the lipid mixes under a gentle stream of N2 gas for ~10 min until no more chloroform is visible.
- Dry the lipid film further in a desiccator by vacuuming for 30 min.
- Add enough aqueous A100 with 10% glycerol, 2 mM 2-mercaptoethanol, and 1 mM EDTA to the lipid film and bring back the concentration to 10 mM. Resuspend the lipid film by vortexing lightly for 15 min at room temperature. A vortexer that can accommodate flint glass tubes is recommended.
- Freeze-thaw the hydrated lipids in liquid nitrogen ten times. After freezing in liquid nitrogen, thaw the liposomes by letting the solution sit at room temperature for 30 s, then transfer to water for faster thawing. This freeze-thaw cycling will minimize multilamellar vesicles.
CAUTION: Tubes may crack if they contain volumes larger than 0.5 mL and if they are transferred directly form liquid nitrogen to water. - Pass the lipid through polycarbonate filters with 100 nm pore size 19 times using mini-extruder.
- Determining total lipid concentration of liposomes by scintillation counting
NOTE: Some of the lipid may be left behind in glass tubes and in mini-extruder.- Add 4 μL of lipid stock and liposomes in 3 mL of scintillation cocktail.
- Measure average counts per minute (CPMA) for stock and liposomes. And calculate liposomes concentration with the following formula:
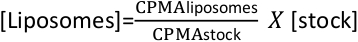
- Store the liposomes at 4 °C for up to a week. Longer storage is not recommended as liposomes might aggregate with time, decreasing reconstitution efficiencies.
- Make lipid mix stocks in chloroform (10 mM total lipid). The necessary lipids are 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC), 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (DOPS), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (NBD-DPPE), and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (Rh-DPPE). Acceptor liposomes consist of POPC: DOPS (85:15 molar ratio) and donor liposomes of POPC:DOPS:Rh-PE:NBD-PE (83:15:1.5:1.5 molar ratio).
- Reconstitution by detergent assisted incorporation into preformed liposomes
- Calculate the amount of buffer, protein, liposomes and extra detergent to be mixed together:
- Determine the desired total volume. This volume is made up of buffer A100 with 10% glycerol, 2 mM 2-mercaptoethanol, and 1 mM EDTA. Volumes less than 250 μL do not mix well in 0.5 mL microcentrifuge tubes.
- Calculate the volume of liposomes needed to give a final lipid concentration of about 1 mM. Subtract the volume from the buffer volume.
- Calculate the volume of protein needed to give the desired protein to lipid molar ratio (usually 1:400). Decrease the buffer volume accordingly.
- Determine how much extra detergent needs to be added to saturate the liposomes aiming for an effective detergent to lipid ratio (Reff) between 0.64–0.8. Remember to consider the detergent that is added with the protein. The (Reff) is determined by the equation
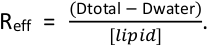 Dtotal is the total detergent concentration and Dwater is the monomeric detergent concentration (0.18 mM for TX-100 and Anapoe X-100 in the presence of detergent)4,11.
Dtotal is the total detergent concentration and Dwater is the monomeric detergent concentration (0.18 mM for TX-100 and Anapoe X-100 in the presence of detergent)4,11.
- Mix the solutions together in a 0.5 mL tube in the following order: buffer, detergent, protein, and liposomes. Add the liposomes rapidly and vortex immediately for 5 s to homogenize the mixture.
- Incubate the reaction in a nutator for 1 h at 4 °C.
- Make 0.2 g/mL nonpolar polystyrene adsorbent bead “slurry” in water.
NOTE: Make the polystyrene adsorbent bead “slurry” during previous the 1 h incubation in step 2.2.3. - Weigh out 0.2 g of polystyrene adsorbent beads and transfer to a microcentrifuge tube.
- Degas the beads by adding 1 mL of methanol to the tube and mix for 1 min. Degassed beads will sink.
- Aspirate the methanol and add water to the beads. Let the beads mix with water for 5 min, then aspirate the water. Repeat four times, then bring the polystyrene adsorbent bead “slurry” to 1 mL volume with water and a final concentration of 0.2 g/mL.
NOTE: Beads should still settle to the bottom of the tube. If they do not, degas again. Use a 21 G or higher needle to aspirate methanol and water from beads. - Calculate the amount of polystyrene adsorbent beads needed to absorb all the detergent in each sample. 1 g of polystyrene adsorbent beads absorbs 70 mg of TX-100. To calculate the volume of polystyrene adsorbent bead slurry needed for each reaction, divide the total detergent in the reaction (step 2.2.1.4) by 70 mg, and then by the concentration of the bead slurry (0.2 g/mL (step 2.2.7).
- Transfer calculated amount of polystyrene adsorbent bead slurry to a 0.5 mL tube and aspirate the water.
NOTE: Cut the end of a 20–200 µL tip to transfer the beads, vortex the tube just prior to pipetting to resuspend settled beads. - Add the samples to the 0.5 mL tube containing polystyrene adsorbent beads and incubate the sample nutating for 1 h at 4 °C.
- Repeat twice leaving old beads behind and transferring the sample to fresh beads.
- Add the sample to a fourth tube with fresh beads and incubate overnight (~15–18 h) at 4 °C.
- Calculate the amount of buffer, protein, liposomes and extra detergent to be mixed together:
- In the morning, remove the sample from polystyrene adsorbent beads and pellet insoluble protein aggregates by centrifuging for 10 min at 16,000 x g at 4 °C.
- Recover the supernatant and determine the final lipid concentration by liquid scintillation counting (see step 2.1.9.2). Optionally, protein concentration may be determined by amido black protein assay9.
NOTE: For atlastin proteoliposomes, enzymatic assays should be performed with fresh liposomes. Long storage at 4 °C or freezing leads to significant loss in atlastin activity.
3. Lipid Mixing Assays
- Bring the donor and acceptor proteoliposomes to a concentration of 0.15 mM each in A100 with 10% glycerol, 2 mM β-mercaptoethanol, 1 mM EDTA and 5 mM MgCl2. Each reaction (50 μL) should be added to a well in a flat white 96 well plate suitable for fluorescence readings. Prepare at least 4 reactions including a triplicate, and a no-GTP negative control.
- Place the plate in a preheated plate reader at 37 °C. Measure NBD fluorescence (excitation 460 nm and emission 535 nm) for 5 min every min.
- Induce fusion by adding 5 mM GTP (5 μL of 50 mM GTP).
- Measure NBD fluorescence every minute for 1 h.
- Add 5 μL of 2.5% w/v n-Dodecyl β-D-maltoside to dissolve the proteoliposomes and measure the maximum NBD fluorescence. Read NBD fluorescence for 15 min every min.
4. Liposome Floatation on Iohexol Discontinuous Gradient12
- (optional) Analyze the efficiency of reconstitution by floatation assays13.
- Prepare an 80% and a 30% w/v iohexol in A100 with 10% glycerol, 2 mM 2-mercaptoethanol, and 1 mM EDTA. Iohexol does not dissolve readily, so this must be prepared a day before by nutating overnight at 4 °C.
- Thoroughly mix 150 μL of 80% iohexol stock with 150 μL of proteoliposomes sample to bring it to a 40% iohexol. Add this to a 5 x 41 mm2 Ultra-clear tube avoiding bubbles.
- Layer 250 μL of 30% iohexol stock slowly on top of the sample to make a middle layer. Avoid any bubbles and disturbing the bottom layer. On top of the middle layer, slowly add 50 μL of A100 with 2 mM 2-mercaptoethanol and 1 mM EDTA.
- Centrifuge the gradient in a swinging bucket rotor at 220,000 x g for 4 h at 4 °C with slow acceleration and no break.
- Harvest the layers of the gradient and analyze by SDS-PAGE and Coomassie stain, quantification may be done by densitometry. Reconstituted protein should float to the top layer, while protein and lipid aggregates will sediment at the bottom or in the middle layer.
5. Analysis of Orientation of Reconstituted Protein by Thrombin Proteolysis
NOTE: The atlastin construct reported here has a thrombin cut site between the end of the N-terminal GST tag and the beginning of atlastin. Atlastin in the correct orientation will have this cut site accessible to the protease, while protein in the wrong orientation will be protected by the lipid bilayer.
- To assay atlastin proteoliposome orientation, reserve at least 8 μL of fresh reconstituted proteoliposomes.
- Add 8 μL of proteoliposomes and 1 μL of 1 U/μL thrombin. Incubate at 37 °C for 1 h.
- Quench the protease with 1 μL of 5 mg/mL of EDTA-free protease inhibitor cocktail and incubate for 30 min at 37 °C.
- Analyze the sample by SDS-PAGE and Coomassie stain. Proportion of cleaved and uncleaved protein may be determined by densitometry.
Results
The efficiency of atlastin reconstitution is presented in Figure 2. Reconstituted proteoliposomes were floated in an iohexol discontinuous gradient. Unincorporated protein was sedimented in the bottom layer (B) or in the middle layer (M). Reconstituted protein would float to the top layer (T). Samples of the gradient were harvested and analyzed by SDS-PAGE and Coomassie staining. The quantification of the gel by densitometry shows a very high efficiency of reconstitution with negligible lose...
Discussion
The methods here delineate an efficient method for purifying, reconstituting, and measuring fusion activity of recombinant atlastin. To ensure high yields of functional atlastin some critical steps must be considered. Expression of atlastin must be done at low temperatures (16 °C) to avoid aggregation and one should aim for a final concentration between 0.4–1.5 mg/mL. Very dilute protein will not be reconstituted optimally at a 1:400 protein to lipid ratio. Reconstitution efficiency can be optionally analyzed ...
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Michael Stern and his lab for their insights and feedback on atlastin related projects. This work was supported by the National Institute of General Medical Sciences [R01GM101377] and the National Institute of Neurological Disorders and Stroke [R01NS102676].
Materials
| Name | Company | Catalog Number | Comments |
| 10 mL poly-prep chromatography columns | Biored | 731-1550 | |
| 10 x 75 mm Flint glass tubes | VWR | 608225-402 | |
| 47 mm diameter, 0.45um pore whatman sterile membrane filters | Whatman | 7141 104 | |
| 96 well white plate | NUNC | 437796 | |
| Anapoe X-100 | Anatrace | 9002-931-1 | |
| Cell disrupter | Avestin | Avestin Emulsiflex C3 | |
| DOPS (1,2-dioleoyl-sn-glycero-3-phospho-L-serine (sodium salt)) | Avanti | 840035P-10mg | DOPS |
| EDTA | Research organics inc. | 6381-92-6 | Ethylenediaminetetraacetic acid |
| EDTA-free protease inhibitor cocktail | Roche | 11873580001 | Complete protease inhibitor |
| Extruder | Sigma Aldrich | Z373400 | Liposofast Basic Extruder |
| GE Akta Prime liquid chromatography system | GE Pharmacia | 8149-30-0006 | |
| Glutathione agarose beads | Sigma aldrich | G4510-50ml | |
| Glycerol | EMD | GX0185-5 | |
| GTP | Sigma Aldrich | 36051-31-7 | Guanosine 5' triphosphate sodium salt hydrate |
| HEPES, acid free | Omnipur | 5330 | |
| Imidazole | fluka | 5670 | |
| Immobilized metal affinity chromatography (IMAC) resin column | GE Healthcare | 17040801 | 1 mL HiTrap Chelating HP immobilized metal affinity chromatography columns |
| Iohexol | Accurate chemical and scientific corporation | AN 7050 BLK | Accudenz/Nycodenz |
| IPTG | Research products international corp. | I56000-100.0 | IPTG, dioxane free |
| L-Glutathione reduced | Sigma-Aldrich | G4251-5g | |
| Magnesium chloride | Fisher | 7791-18-6 | |
| Methanol | Omnisolv | MX0488-1 | |
| NBD-DPPE (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) (ammonium salt)) | Avanti | 236495 | NBD-DPPE |
| n-Dodecyl β-D-maltoside | Chem-Impex International | 21950 | |
| Nonpolar polystyrene adsorbent beads | BioRad | 152-3920 | SM2 Biobeads |
| Nuclepore track-etch polycarbonate 19 mm 0.1 um pore membrane | Whatman | 800309 | |
| Optima LE80K Ultra centrifuge | Beckman Coulter | ||
| Phosphatidylcholine, L-α-dipalmitoyl [choline methyl-3H] | ARC | ART0284 | Titriated lipids |
| Plate reader | TECAN | TECAN infinite M200 plate reader | |
| POPC (1-palmitoyl-2-oleoyl-glycero-3-phosphocholine) | Avanti | 850457C-25mg | POPC |
| Potassium chloride | MP | 151944 | |
| Rh-DPPE (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (ammonium salt)) | Avanti | 236495 | Rh-DPPE |
| Scintillation Cocktail | National Diagnostics | LS-272 | Ecoscint XR Scintillation solution for aqueous or non-aqueous samples |
| Scintillation vials | Beckman | 592928 | Fast turn cap Mini Poly-Q Vial |
| Thrombin | Sigma | T1063-1kU | Thrombin from human plasma |
| Triton X-100 | Fisher | BP151-500 | |
| Ultra-clear centrifuge tubes 5 x 41 mm | Beckman | 344090 | |
| Vortex 9 to 13mm Tube Holder | VWR | 58816-138 | Insert for vortexing flint glass tubes |
| Vortex Insert Retainer | VWR | 58816-132 | Retainer needed for vortex tube holder |
| Vortexer | VWR | 2.235074 | Vortex Genie 2 model G560 |
| β-mercaptoethanol molecular biology grade | Calbiochem | 444203 |
References
- Chernomordik, L. V., Kozlov, M. M. Mechanics of membrane fusion. Nature Structural and Molecular Biology. 15, 675-683 (2008).
- Orso, G., et al. Homotypic fusion of ER membranes requires the dynamin-like GTPase Atlastin. Nature. 460, 978-983 (2009).
- McNew, J. A., Sondermann, H., Lee, T., Stern, M., Brandizzi, F. GTP-dependent membrane fusion. Annual Review of Cell and Developmental Biology. 29, 529-550 (2013).
- Rigaud, J. L., Pitard, B., Levy, D. Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1231, 223-246 (1995).
- Düzgüneş, N. Fluorescence Assays for Liposome Fusion. Methods in Enzymology. 372, 260-274 (2003).
- Wilschut, J., Duzgunes, N., Fraley, R., Papahadjopoulos, D. Studies on the mechanism of membrane fusion: kinetics of calcium ion induced fusion of phosphatidylserine vesicles followed by a new assay for mixing of aqueous vesicle contents. Biochemistry. 19, 6011-6021 (1980).
- Ellens, H., Bentz, J., Szoka, F. C. Proton- and calcium-induced fusion and destabilization of liposomes. Biochemistry. 24, 3099-3106 (1985).
- Meers, P., Ali, S., Erukulla, R., Janoff, A. S. Novel inner monolayer fusion assays reveal differential monolayer mixing associated with cation-dependent membrane fusion. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1467, 277-243 (2000).
- Schaffner, W., Weissmann, C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Analytical Biochemistry. 56, 502-514 (1973).
- MacDonald, R. C., et al. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochimica Et Biophysica Acta. 1061, 297-303 (1991).
- Paternostre, M. T., Roux, M., Rigaud, J. L. Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 1. Solubilization of large unilamellar liposomes (prepared by reverse-phase evaporation) by Triton X-100, octyl glucoside, and sodium cholate. Biochemistry. 27, 2668-2677 (1988).
- Scott, B. L., et al. Liposome Fusion Assay to Monitor Intracellular Membrane Fusion Machines. Methods in Enzymology. 372, 274-300 (2003).
- Zhang, W., et al. Crystal structure of an orthomyxovirus matrix protein reveals mechanisms for self-polymerization and membrane association. PNAS. 114, 8550-8555 (2017).
- Parlati, F., et al. Rapid and efficient fusion of phospholipid vesicles by the α-helical core of a SNARE complex in the absence of an N-terminal regulatory domain. PNAS. 96, 12565-12570 (1999).
- Sugiura, S., Mima, J. Physiological lipid composition is vital for homotypic ER membrane fusion mediated by the dynamin-related GTPase Sey1p. Scientific Reports. 6, 20407 (2016).
- Powers, R. E., Wang, S., Liu, T. Y., Rapoport, T. A. Reconstitution of the tubular endoplasmic reticulum network with purified components. Nature. 543, 257-260 (2017).
- Betancourt-Solis, M. A., Desai, T., McNew, J. A. The atlastin membrane anchor forms an intramembrane hairpin that does not span the phospholipid bilayer. Journal of Biological Chemistry. 293, 18514-18524 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved