A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Rat Carotid Artery Pressure-Controlled Segmental Balloon Injury with Periadventitial Therapeutic Application
In This Article
Summary
The rat carotid artery balloon injury mimics the clinical angioplasty procedure performed to restore blood flow in atherosclerotic vessels. This model induces the arterial injury response by distending the arterial wall, and denuding the intimal layer of endothelial cells, ultimately causing remodeling and an intimal hyperplastic response.
Abstract
Cardiovascular disease remains the leading cause of death and disability worldwide, in part due to atherosclerosis. Atherosclerotic plaque narrows the luminal surface area in arteries thereby reducing adequate blood flow to organs and distal tissues. Clinically, revascularization procedures such as balloon angioplasty with or without stent placement aim to restore blood flow. Although these procedures reestablish blood flow by reducing plaque burden, they damage the vessel wall, which initiates the arterial healing response. The prolonged healing response causes arterial restenosis, or re-narrowing, ultimately limiting the long-term success of these revascularization procedures. Therefore, preclinical animal models are integral for analyzing the pathophysiological mechanisms driving restenosis, and provide the opportunity to test novel therapeutic strategies. Murine models are cheaper and easier to operate on than large animal models. Balloon or wire injury are the two commonly accepted injury modalities used in murine models. Balloon injury models in particular mimic the clinical angioplasty procedure and cause adequate damage to the artery for the development of restenosis. Herein we describe the surgical details for performing and histologically analyzing the modified, pressure-controlled rat carotid artery balloon injury model. Additionally, this protocol highlights how local periadventitial application of therapeutics can be used to inhibit neointimal hyperplasia. Lastly, we present light sheet fluorescence microscopy as a novel approach for imaging and visualizing the arterial injury in three-dimensions.
Introduction
Cardiovascular disease (CVD) remains the leading cause of death worldwide1. Atherosclerosis is the underlying cause of most CVD-related morbidity and mortality. Atherosclerosis is the build-up of plaque inside arteries that results in a narrowed lumen, hindering proper blood perfusion to organs and distal tissues2. Clinical interventions for treating severe atherosclerosis include balloon angioplasty with or without stent placement. This intervention involves advancing a balloon catheter to the site of plaque, and inflating the balloon to compress the plaque to the arterial wall, widening the luminal area. This procedure damages the artery, however, initiating the arterial injury response3. Prolonged activation of this injury response leads to arterial restenosis, or re-narrowing, secondary to neointimal hyperplasia and vessel remodeling. During angioplasty the intimal layer is denuded of endothelial cells leading to immediate platelet recruitment and local inflammation. Local signaling induces phenotypic changes in vascular smooth muscle cells (VSMC) and adventitial fibroblasts. This leads to the migration and proliferation of VSMC and fibroblasts inwards to the lumen, leading to neointimal hyperplasia4,5. Circulating progenitor cells and immune cells also contribute to the overall volume of restenosis6. Where applicable, drug-eluting stents (DES) are the current standard for inhibiting restenosis7. DES inhibit arterial re-endothelialization, however, thus creating a pro-thrombotic environment that can result in late in-stent thrombosis8. Therefore, animal models are integral for both understanding the pathophysiology of restenosis, and for developing better therapeutic strategies to prolong the efficacy of revascularization procedures.
Several large and small animal models9 are utilized for studying this pathology. These include balloon-injury3,10 or wire-injury11 of the luminal side of an artery, as well as partial ligation12 or cuff placement13 around the artery. The balloon and wire injury both denude the endothelial layer of the artery, mimicking what occurs clinically after angioplasty. In particular, balloon-injury models utilize similar tools as in the clinical setting (i.e., balloon catheter). The balloon injury is best performed in rat models, as rat arteries are an appropriate size for commercially available balloon catheters. Herein we describe a pressure-controlled segmental arterial injury, a well-established, modified version of the rat carotid artery balloon injury. This pressure-controlled approach closely mimics the clinical angioplasty procedure, and allows for reproducible neointimal hyperplasia formation two weeks after injury14,15. Additionally, this pressure-controlled arterial injury results in complete endothelial layer restoration by 2 weeks after surgery16. This directly contrasts the original balloon injury model, described by Clowes, where the endothelial layer never returns to full coverage3.
After surgery, therapeutics may be applied to or directed towards the injured artery through several approaches. The method described herein uses periadventitial application of a small molecule embedded in a Pluronic gel solution. Specifically, we apply a solution of 100 μM cinnamic aldehyde in 25% Pluronic-F127 gel to the artery immediately after injury to inhibit neointimal hyperplasia formation15. Pluronic-F127 is a non-toxic, thermo-reversible gel able to deliver drugs locally in a controlled manner17. Meanwhile, arterial injury is local, hence local administration allows for testing an active principle while minimizing off-target effects. Nevertheless, effective delivery of a therapeutic using this method will depend on the chemistry of the small molecule or biologic used.
Protocol
All methods described here have been approved by the Institutional Animal Care and Use Committee (IACUC) of the University of North Carolina at Chapel Hill.
1. Preoperative procedures
- Sterilize surgical instruments. Autoclave all surgical instruments before surgery. If performing multiple surgeries on the same day, sterilize instruments between surgeries using a dry bead sterilizer.
- Prepare therapeutic in 25% Pluronic-127 gel (diluted in sterile distilled water).
- Set up a 2F Fogarty balloon catheter to the insufflator and place the balloon end of the catheter in a 1 mL syringe filled with saline solution.
- Induce anesthesia by placing the rat in a chamber with 5% isoflurane.
- Remove the rat from the chamber and record the rat’s weight. Use hair clippers to shave fur on the ventral neck region.
- Place the rat back into the chamber with 5% isoflurane to ensure induction of anesthesia.
- Place the rat supine on a surgical platform, inserting the face into the nose cone so that the rat face is toward the surgeon.
- Reduce inhalational anesthesia to 1.5% isoflurane. Verify the depth of anesthesia by a toe-pinch reflex on all four feet.
- Tape all four legs down to the surgical platform.
- Turn on the heat lamp.
- Inject Atropine (0.01 mg/kg) subcutaneously to reduce airway secretions.
- Inject Carprofen (5 mg/kg) subcutaneously for prophylactic pain management. If anti-inflammatory drugs are not acceptable for the experiment refer to steps 3.2.2 and 3.2.3.
- Apply lubricant eye ointment to both eyes using a sterile cotton swab to prevent corneas from drying during surgery.
- Swab the neck three times in a circular motion alternating between 70% ethyl alcohol followed by Betadine from the center of the shaved region outwards to sterilize the incision site.
- Infiltrate the incision line s.c. with 0.25% bupivacaine.
- Put on sterile surgical gloves before handling sterile surgical instruments and supplies.
- Lay out all autoclaved surgical instruments on a sterile surgical sheet.
- Cut three independent 1 inch strands of sterile 7-0 Prolene suture.
- Place cotton swabs and gauze on surgical sheet.
- Drape the rat with a sterile surgical sheet that only exposes the sterilized neck region.
- Cut an additional small opening in sheet that exposes part of the nose cone. This will be the site for taping down the balloon catheter during injury.
2. Operative procedures
- During the entire surgical procedure, assess depth of anesthesia by monitoring the respiratory rate (rate should be consistent and deemed normal) as well by toe pinch every 15 min. If respiratory rate increases or there is a response to the toe pinch, then pause surgical manipulation and increase isoflurane up to 2.5%.
- Expose the common carotid artery (CCA).
- Make a superficial, straight, longitudinal neckline incision between the jaw bones of the rat. Incision will be approximately 1.5-2 cm in length.
- Make an incision through the connective tissue under the skin until the muscle layer is exposed. Displace the salivary glands underneath the skin to access the muscle tissue.
- Bluntly separate the connective tissue from the muscle by inserting closed scissors between the muscle layer and connective tissue and gently opening the scissor while pulling the skin upward.
- Dissect the two visible muscles (sternohyoid and sternomastoid) longitudinally along the left side of the trachea until a third muscle (omohyoid) that runs perpendicular to the two superficial muscles is observed.
- Use forceps to create a window separating this perpendicular muscle (omohyoid) from the longitudinal muscle (sternohyoid) running atop the trachea. Gently perform this separation to prevent blunt trauma to the trachea.
- Reach forceps underneath the perpendicular muscle and cut to separate the two longitudinal muscles and expose the CCA.
- Dissect the CCA.
- Dissect the CCA near the bifurcation until the internal carotid artery (ICA) and external carotid artery (ECA) are exposed.
- Dissect the superior thyroid artery (STA), which branches from the ECA.
- Using the pre-cut Prolene sutures, ligate the STA and the ECA near their respective bifurcation. Leave the majority of the suture to one side of the knot and grab each suture with a curved hemostat.
- Finish dissecting around the ICA, reach forceps underneath and around the ICA, and use a non-crushing vascular clamp to achieve distal control. Clamp the occipital artery together with the ICA.
- Dissect the CCA proximal to the bifurcation, ensuring to separate the vagus nerve from the CCA.
- Reach forceps underneath and around the CCA and use a non-crushing vascular clamp to achieve proximal control. Place clamp at least 5 mm from the bifurcation.
- Perform balloon injury.
- Maneuver the curved hemostats holding each ligated artery branch to expose the bifurcation between the ECA and superior branch.
- Gently dissect tissue at the bifurcation and then make an arteriotomy incision between the ECA and superior branch using microdissection scissors.
- Use a cotton swab to push all blood out of the CCA and clean up the arteriotomy site.
- Insert the uninflated balloon catheter through the arteriotomy and advance into the CCA until the proximal end of the balloon is past the bifurcation.
- Tape catheter to the nose cone so the balloon does not slip out of the artery during inflation.
- Slowly inflate the balloon to 5 atmospheres of pressure and leave in the CCA for 5 min to induce arterial injury. Ensure that the pressure stays constant for the entire 5 min.
- After 5 min, deflate balloon and gently remove from the CCA through the arteriotomy.
- Flush the CCA by gently squeezing on the clamp at the CCA. Do not remove the clamp.
- Ligate the ECA proximal to the arteriotomy and then remove the clamps from the CCA and ICA to restore blood flow through the CCA to the ICA. Ensure there is no visible bleeding around the arteriotomy and that the CCA is pulsating.
- Apply 100 µL of therapeutic or Pluronic gel vehicle alone periadventitially along the injured CCA. Do so by applying 50 µL to the left side of the CCA and then 50 µL to the right side of the CCA to ensure even coating of the injured artery.
- Close the wound site.
- Cut excess Prolene sutures.
- Close the wound using interrupted 4-0 or 6-0 vicryl layers along the connective tissue.
- Finish closing the wound using running 4-0 nylon suture along the skin.
3. Postoperative procedures
- Place the rat alone in a clean cage with half the cage under a heating lamp and monitor until rat regains sufficient consciousness to maintain sternal recumbency. Keep the rat in a separate cage until the animal is fully alert and mobile before transferring back to their original cage.
- Monitor the rat daily for the next three days and then three times per week until euthanasia. Euthanize using isoflurane overdose followed by bilateral thoracotomy as described below.
- Utilize the National Centre for the Replacement Refinement & Reduction of Animals in Research (NC3Rs) grimace scale to identify postoperative pain levels. If any animal appears to be experiencing pain or develop any neurologic compromise, sacrifice immediately.
- For animals that do not receive carprofen, administer acetaminophen 6 mg/mL in their drinking water 24 h prior to surgery through 48 h post-surgery. Acetaminophen provides analgesia with minimal anti-inflammatory effects.
- Alternatively, other analgesia strategies with minimal anti-inflammatory effects can be used, such as buprenorphine or buprenorphine extended release. Consult the veterinary team at your institution.
4. Tissue harvest and imaging
- Two weeks after surgery, euthanize the rat by overdose of anesthesia (5% isoflurane). Alternatively, euthanize rats at an earlier time point to analyze the various aspects of the arterial injury response.
- Once breathing stops perform bilateral thoracotomy as a secondary method of euthanasia.
- Make a lateral incision through the abdomen, and then cut upwards, through the diaphragm and ribs, exposing the thoracic cavity.
- Perfuse and fix the arteries.
- Insert an 18 G cannula attached to a gravitational perfusion-fixation system through the left ventricle. Maintain equivalent pressure between rats by marking the height of the perfusion system relative to the benchtop (120 cm elevation, equivalent to 91 ± 3 mmHg).
- Clamp the cannula together with the ventricle using a curved hemostat.
- Make a cut in the right atrium, opening the vascular circuit, and begin perfusion with PBS followed by 2-4% paraformaldehyde (about 250 mL each).
- Prepare paraformaldehyde diluted in PBS the day of sacrifice, or at most the night before sacrifice. If preparing on the day of sacrifice, ensure paraformaldehyde has cooled to room temperature before beginning the perfusion. Store paraformaldehyde at 4 °C.
- After fixation, extract the left and right carotid arteries and store at 4 °C for 2 h in 2-4% paraformaldehyde.
- Transfer arteries to 30% sucrose and store overnight at 4 °C.
- After 16-24 h, embed the arteries in optimal cutting temperature (OCT) compound and freeze OCT-embedded artery blocks.
- Condition arteries in OCT at room temperature for 10 min. Place the artery parallel to the plane of the cryomold filled with OCT, marking the side of the cryomold to which the arterial bifurcation is facing. Snap-freeze in liquid nitrogen.
- Store frozen blocks long-term at -80 °C.
- Section frozen blocks using a cryostat.
- Collect six 5 µm thick arterial cross sections per slide, with slide 1 starting at the bifurcation.
- Section frozen blocks until hyperplasia no longer visible (around 100 slides).
- Hematoxylin & eosin (H&E) stain slides18
- Find the area of injury by staining one in every ten slides along the entire artery starting from the bifurcation (e.g., slides 1, 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100).
- Stain additional slides around the site of injury to find the slide with peak occlusion (e.g., slides 20, 30, and 40 had visible hyperplasia, thus stain slides 15, 25, 35, and 45).
- Stain and quantify the slide with peak occlusion and equidistant slides before and after the peak occlusion slide (e.g., peak occlusion found at slide 35, then stain and quantify slides 25, 45, etc.) for a total of 3-10 slides per rat.
- For light sheet fluorescence microscopy imaging, store arteries overnight at 4 °C after fixation in step 4.4.
- Probe artery with 1:500 dilution of rabbit anti-CD31 primary antibody in diluent (pH 7.4) for 3 days. Then counterstain artery with 1:500 dilution of anti-rabbit Alexa Fluor 647 secondary antibody for 2 days19.
- Clear the artery using iDISCO+20.
- Image the artery using a light sheet fluorescence microscope21. Render images using software (e.g., Imaris)19.
- Quantify neointimal hyperplasia. Perform quantification in a blinded manner if possible.
- Use ImageJ software to trace the perimeter of the intima, internal elastic lamina (IEL), and external elastic lamina (EEL) of an artery on each of the 3-10 slides determined above (step 4.8.3).
- Quantify the area of each traced region in ImageJ and export these values. The intima trace yields the lumen area, the IEL trace yields the IEL area, and the EEL trace yields the EEL area.
- Average the values obtained from the 3-10 slides to get the average injury (% occlusion, intima:media (I:M) ratio, neointimal hyperplasia) per rat carotid artery.
Neointimal Hyperplasia = IEL area - Lumen area

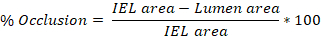
Results
Figure 1 shows all of the materials and surgical tools used to perform this surgery. Hematoxylin & eosin (H&E) staining of two-week injured arterial cross sections allows for clear visualization of neointimal hyperplasia. Figure 2 shows representative images of H&E-stained arterial cross-sections of a healthy, injured, and treated artery. Figure 2 also outlines how to quantify the level of neointimal hyperplasia in an in...
Discussion
The rat carotid artery balloon injury is one of the most extensively used and studied restenosis animal models. Both the original balloon injury model3 and the modified pressure-controlled segmental injury variation10 have informed many aspects of the arterial injury response that also occurs in humans, with the few limitations being that fibrin-rich thrombus rarely develops and local inflammation is minimal compared to other injury models such as in hypercholesterolemic ra...
Disclosures
The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
Acknowledgements
N.E.B. was supported by a training grant from the National Institute of Environmental Health Sciences (5T32ES007126-35, 2018), and an American Heart Association pre-doctoral fellowship (20PRE35120321). E.S.M.B. was a KL2 scholar partially supported by the UNC Clinical and Translational Science Award-K12 Scholars Program (KL2TR002490, 2018), and the National Heart, Lung, and Blood Institute (K01HL145354). The authors thank Dr. Pablo Ariel of the UNC Microscopy Services Laboratory for assisting with LSFM. Light Sheet Fluorescence Microscopy was performed at the Microscopy Services Laboratory. The Microscopy Services Laboratory, Department of Pathology and Laboratory Medicine, is supported in part by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center.
Materials
| Name | Company | Catalog Number | Comments |
| 1 mL Syringe | Fisher | 14955450 | |
| 1 mL Syringe with needle | BD | 309626 | |
| 2 French Fogarty Balloon Embolectomy Catheter | Edwards LifeSciences | 120602F | |
| 4-0 Ethilon (Nylon) Suture | Ethicon Inc | 662H | |
| 4-0 Vicryl Suture | Ethicon Inc | J214H | |
| 7-0 Prolene Suture | Ethicon Inc | 8800H | |
| 70% ethyl alcohol | |||
| Anti-Rabbit Alexa Fluor 647 | Thermo Fisher Scientific | A21245 | |
| Atropine Sulfate | Vedco Inc | for veterinary use | |
| Cotton Swabs | Puritan | 806-WC | |
| Curved Hemostats | Fine Science Tools | 13009-12 | |
| Fine Curved Forceps | Fine Science Tools | 11203-25 | |
| Fine Scissors | Fine Science Tools | 14090-11 | |
| Gauze | Covidien | 2252 | |
| IHC-Tek Diluent (pH 7.4) | IHC World | IW-1000 | |
| Insufflator | Merit Medical | IN4130 | |
| Iodine solution | |||
| Lubricating Eye Ointment | Dechra | for veterinary use | |
| Mayo Scissors | Fine Science Tools | 14010-15 | |
| Micro Serrefines | Fine Science Tools | 18055-05 | |
| Microdissection Scissors | Fine Science Tools | 15004-08 | |
| Micro-Serrefine Clamp Applying Forceps | Fine Science Tools | 18057-14 | |
| Needle Holder | Fine Science Tools | 12003-15 | |
| Pluronic-127 (diluted in sterile water) | Sigma-Aldrich | P2443 | 25% prepared |
| Rabbit Anti-CD31 | Abcam | ab28364 | |
| Retractor | Bent paper clips work well | ||
| Rimadyl (Carprofen) | Zoetis Inc | for veterinary use | |
| Saline solution | |||
| Standard Forceps | Fine Science Tools | 11006-12 | |
| Sterile Drape | Dynarex | 4410 | |
| T-Pins |
References
- American Heart Association. Cardiovascular Disease: A Costly Burden for America, Projections Through 2035. American Heart Association CVD Burden Report. , (2017).
- Singh, R. B., Mengi, S. A., Xu, Y. J., Arneja, A. S., Dhalla, N. S. Pathogenesis of atherosclerosis: A multifactorial process. Experimental and Clinical Cardiology. 7 (1), 40-53 (2002).
- Clowes, A. W., Reidy, M. A., Clowes, M. M. Mechanisms of stenosis after arterial injury. Laboratory Investigation. 49 (2), 208-215 (1983).
- Clowes, A. W., Reidy, M. A., Clowes, M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Laboratory Investigation. 49 (3), 327-333 (1983).
- Sartore, S., et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circulation Research. 89 (12), 1111-1121 (2001).
- Tanaka, K., et al. Circulating progenitor cells contribute to neointimal formation in nonirradiated chimeric mice. The FASEB Journal. 22 (2), 428-436 (2008).
- Henry, M., et al. Carotid angioplasty and stenting under protection. Techniques, results and limitations. The Journal of Cardiovascular Surgery. 47 (5), 519-546 (2006).
- Kounis, N. G., et al. Thrombotic responses to coronary stents, bioresorbable scaffolds and the Kounis hypersensitivity-associated acute thrombotic syndrome. Journal of Thoracic Disease. 9 (4), 1155-1164 (2017).
- Jackson, C. L. Animal models of restenosis. Trends in Cardiovascular Medicine. 4 (3), 122-130 (1994).
- Shears, L. L., et al. Efficient inhibition of intimal hyperplasia by adenovirus-mediated inducible nitric oxide synthase gene transfer to rats and pigs in vivo. Journal of the American College of Surgeons. 187 (3), 295-306 (1998).
- Takayama, T., et al. A murine model of arterial restenosis: technical aspects of femoral wire injury. Journal of Visualized Experiments. (97), (2015).
- Zhang, L. N., Parkinson, J. F., Haskell, C., Wang, Y. X. Mechanisms of intimal hyperplasia learned from a murine carotid artery ligation model. Current Vascular Pharmacology. 6 (1), 37-43 (2008).
- Jahnke, T., et al. Characterization of a new double-injury restenosis model in the rat aorta. Journal of Endovascular Therapy. 12 (3), 318-331 (2005).
- Gregory, E. K., et al. Periadventitial atRA citrate-based polyester membranes reduce neointimal hyperplasia and restenosis after carotid injury in rats. American Journal of Physiology-Heart and Circulatory Physiology. 307 (10), 1419-1429 (2014).
- Buglak, N. E., Jiang, W., Bahnson, E. S. M. Cinnamic aldehyde inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia in Zucker Diabetic Fatty rats. Redox Biology. 19, 166-178 (2018).
- Bahnson, E. S., et al. Long-term effect of PROLI/NO on cellular proliferation and phenotype after arterial injury. Free Radical Biology and Medicine. 90, 272-286 (2016).
- Gilbert, J. C. W., Davies, M. C., Hadgraft, J. The behaviour of Pluronic F127 in aqueous solution studied using fluorescent probes. International Journal of Pharmaceutics. 40 (1-2), 93-99 (1987).
- Tulis, D. A. Histological and morphometric analyses for rat carotid balloon injury model. Methods in Molecular Medicine. 139, 31-66 (2007).
- Buglak, N. E., et al. Light Sheet Fluorescence Microscopy as a New Method for Unbiased Three-Dimensional Analysis of Vascular Injury. Cardiovascular Research. , (2020).
- Renier, N., et al. iDISCO: a simple, rapid method to immunolabel large tissue samples for volume imaging. Cell. 159 (4), 896-910 (2014).
- Ariel, P. . UltraMicroscope II - A User Guide. , (2018).
- Touchard, A. G., Schwartz, R. S. Preclinical restenosis models: challenges and successes. Toxicologic Pathology. 34 (1), 11-18 (2006).
- Xiangdong, L., et al. Animal models for the atherosclerosis research: a review. Protein Cell. 2 (3), 189-201 (2011).
- Chen, H., Li, D., Liu, M. Novel Rat Models for Atherosclerosis. Journal of Cardiology and Cardiovascular Sceinces. 2 (2), 29-33 (2018).
- Xing, D., Nozell, S., Chen, Y. F., Hage, F., Oparil, S. Estrogen and mechanisms of vascular protection. Arteriosclerosis, Thrombosis, and Vascular Biology. 29 (3), 289-295 (2009).
- Tulis, D. A. Rat carotid artery balloon injury model. Methods in Molecular Medicine. 139, 1-30 (2007).
- Pellet-Many, C., et al. Neuropilins 1 and 2 mediate neointimal hyperplasia and re-endothelialization following arterial injury. Cardiovascular Research. 108 (2), 288-298 (2015).
- Wu, B., et al. Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rat model of arterial injury. Journal of Vascular Surgery. 65 (1), 207-217 (2017).
- Tan, J., Yang, L., Liu, C., Yan, Z. MicroRNA-26a targets MAPK6 to inhibit smooth muscle cell proliferation and vein graft neointimal hyperplasia. Scientific Reports. 7, 46602 (2017).
- Pearce, C. G., et al. Beneficial effect of a short-acting NO donor for the prevention of neointimal hyperplasia. Free Radical Biology and Medicine. 44 (1), 73-81 (2008).
- Cao, T., et al. S100B promotes injury-induced vascular remodeling through modulating smooth muscle phenotype. Biochimica et Biophysica Acta - Molecular Basis of Disease. 1863 (11), 2772-2782 (2017).
- Madigan, M., Entabi, F., Zuckerbraun, B., Loughran, P., Tzeng, E. Delayed inhaled carbon monoxide mediates the regression of established neointimal lesions. Journal of Vascular Surgery. 61 (4), 1026-1033 (2015).
- Khurana, R., et al. Angiogenesis-dependent and independent phases of intimal hyperplasia. Circulation. 110 (16), 2436-2443 (2004).
- Tsihlis, N. D., Vavra, A. K., Martinez, J., Lee, V. R., Kibbe, M. R. Nitric oxide is less effective at inhibiting neointimal hyperplasia in spontaneously hypertensive rats. Nitric Oxide. 35, 165-174 (2013).
- Chen, J., et al. Inhibition of neointimal hyperplasia in the rat carotid artery injury model by a HMGB1 inhibitor. Atherosclerosis. 224 (2), 332-339 (2012).
- Mano, T., Luo, Z., Malendowicz, S. L., Evans, T., Walsh, K. Reversal of GATA-6 downregulation promotes smooth muscle differentiation and inhibits intimal hyperplasia in balloon-injured rat carotid artery. Circulation Research. 84 (6), 647-654 (1999).
- Becher, T., et al. Three-Dimensional Imaging Provides Detailed Atherosclerotic Plaque Morphology and Reveals Angiogenesis after Carotid Artery Ligation. Circulation Research. 126 (5), 619-632 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved