A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Screening for Thermotoga maritima Membrane-Bound Pyrophosphatase Inhibitors
* These authors contributed equally
In This Article
Summary
Here we present a screening method for membrane-bound pyrophosphatase (from Thermotoga maritima) inhibitors based on the molybdenum blue reaction in a 96 well plate format.
Abstract
Membrane-bound pyrophosphatases (mPPases) are dimeric enzymes that occur in bacteria, archaea, plants, and protist parasites. These proteins cleave pyrophosphate into two orthophosphate molecules, which is coupled with proton and/or sodium ion pumping across the membrane. Since no homologous proteins occur in animals and humans, mPPases are good candidates in the design of potential drug targets. Here we present a detailed protocol to screen for mPPase inhibitors utilizing the molybdenum blue reaction in a 96 well plate system. We use mPPase from the thermophilic bacterium Thermotoga maritima (TmPPase) as a model enzyme. This protocol is simple and inexpensive, producing a consistent and robust result. It takes only about one hour to complete the activity assay protocol from the start of the assay until the absorbance measurement. Since the blue color produced in this assay is stable for a long period of time, subsequent assay(s) can be performed immediately after the previous batch, and the absorbance can be measured later for all batches at once. The drawback of this protocol is that it is done manually and thus can be exhausting as well as require good skills of pipetting and time keeping. Furthermore, the arsenite-citrate solution used in this assay contains sodium arsenite, which is toxic and should be handled with necessary precautions.
Introduction
Approximately 25% of the total cellular proteins are membrane proteins and about 60% of them are drug targets1,2. One of the potential drug targets3, membrane-bound pyrophosphatases (mPPases), are dimeric enzymes that pump proton and/or sodium ion across the membrane by hydrolysis of pyrophosphate into two orthophosphates4. mPPases can be found in various organisms5 such as bacteria, archaea, plants, and protist parasites, with the exception of humans and animals4. In protist parasites, for example Plasmodium falciparum, Toxoplasma gondii and Trypanosoma brucei, mPPases are essential for the parasite virulence6 and knockout of this expression in the parasites lead to failure in maintaining intracellular pH upon exposure to the external basic pH7. Due to their importance and lack of homologous protein present in vertebrates, mPPases can be considered as potential drug targets for protistal diseases3.
The in vitro screening of mPPase inhibitors in this work is based on a TmPPase model system. TmPPase is a sodium ion pumping and potassium ion dependent mPPase from T. maritima and has its optimum activity at 71 °C8. Benefits of this enzyme are for example its ease in production and purification, good thermal stability and high specific activity. TmPPase shows both high similarity in addition to the complete conservation of the position as well as identity of all catalytic residues to the protist mPPases3,9 and to the solved structure of Vigna radiata10 mPPase. The available structures of TmPPase in different conformations are also useful for structure-based drug design experiment (as virtual screening and de novo design).
Here we report a detailed protocol for screening of TmPPase inhibitors in a 96 well plate format (Figure 1). The protocol is based on the colorimetric method of the molybdenum blue reaction, which was first developed by Fiske and Subbarow11. This method involves the formation of 12-phosphomolybdic acid from orthophosphate and molybdate under acidic conditions, which is then reduced to give characteristic blue-colored phosphomolybdenum species12.
Protocol
1. Protein preparation
NOTE: The expression and purification of TmPPase has been described elsewhere13.
- Prepare 10 mL of the reactivation buffer solution containing 20 mM 2-(N-morpholino)ethanesulfonic acid (MES) pH 6.5, 3.5% (v/v) glycerol, 2 mM dithiothreitol (DTT), and 0.05% dodecyl maltoside (DDM).
- Prepare 10 mL of the reaction mixture containing 200 mM Tris-Cl pH 8.0, 8.0 mM MgCl2, 333 mM KCl, and 67 mM NaCl.
NOTE: Mg2+ is required to chelate the pyrophosphate as the substrate of mPPase, K+ is required to increase the enzyme activity as TmPPase is a potassium dependent mPPase, and Na+ is needed for the enzyme activity during sodium ion translocation by TmPPase. - Prepare 30 mg/mL liposomes for enzyme reactivation.
- Add 10 mL of 20 mM Tris-HCl pH 8.0 with 1 mM DTT to 0.3 g of L-α-phosphatidylcholine from soybean to 10 mL of 20 mM Tris-HCl pH 8.0 with 1 mM DTT.
- Put the liposome on ice, and sonicate with 1 second pulse interval for 1 minute, pause for 1 minute, and repeat until the solution becomes transparent yellow.
- Aliquot the liposomes, freeze in liquid nitrogen and store at -80 °C until used.
- Reactivate the enzyme.
- Mix 40 µL of the liposomes solution with 22.5 µL of 20% DDM.
- Heat the mixture at 55 °C for 15 min and allow it to cool to room temperature.
- Add 36.5 µL of the reactivation buffer solution, mix, and add 1 µL of concentrated protein (13 mg/mL) to make a total concentration of 0.13 mg/mL.
NOTE: Protein is usually frozen in 10 µL aliquots after purification and thawed on ice before use.
- Take 20 µL of the reactivated enzyme and add to 1,480 µL of the reaction mixture, then mix gently.
NOTE: The addition of the reactivated enzyme to the reaction mixture should be performed just before it is used.
2. Compound preparation
- Dissolve the compounds in dimethyl sulfoxide (DMSO) to make stock solutions of 25−100 mM in 50−200 µL, based on the availability of the compounds.
NOTE: All compounds used here (Figure 2A) have been published previously9. If the compound solubility is low, the stock concentration can be adjusted accordingly. - Prepare three different concentrations of each compound in water.
NOTE: The final concentrations in the reaction mixture will be 1, 5, and 50 micromolar or 1, 5, and 20 micromolar for soluble and sparingly soluble compounds, respectively.- Dilute the stock solution with water to 1 mL in microtubes to give 2 µM, 10 µM and 100 µM for soluble compounds, or alternatively 2 µM, 10 µM and 40 µM for sparingly soluble compounds.
- Vortex the compound solution instantly after dilution of the stock solution for proper mixing.
- Check for compound aggregation using a nephelometer.
NOTE: This was studied as triplicates in three concentrations (1 µM, 5 µM and 20 µM) and normalized to the blank in a 96 well plate.- Dispense 75 µL of the reaction mixture into each well using a multichannel pipette.
- Add 75 µL of each compound (for the blank, use 75 µL of water instead) and mix by pipetting up and down 5×.
- Measure each well at 300 V using a microplate nephelometer.
3. Reagents for the assay preparation
- Prepare the arsenite-citrate solution.
- Weigh 5 g of sodium arsenite and 5 g of trisodium citrate dihydrate.
CAUTION: Sodium arsenite is toxic, thus use proper protective equipment and handle with special care. As precaution, do not handle before all necessary safety precautions have been read and understood. Handle only in a fume hood in order not to inhale dust/vapors of the compound or its solution(s). If inhaled, move to fresh air and obtain medical attention. Wear appropriate chemical safety goggles, protective gloves and clothing to avoid ingestion and eye/skin contact. If swallowed, call immediately a poison center or doctor/physician. If it gets on the skin or in the eye(s), wash with plenty of water and obtain medical attention. - Dissolve into 100 mL of water.
- Add 5 mL of glacial acetic acid, mix, and add water to 250 mL.
- Store at room temperature protected from light.
NOTE: The solution is stable for more than a year.
- Weigh 5 g of sodium arsenite and 5 g of trisodium citrate dihydrate.
- Prepare solution A and solution B.
- For solution A, add 10 mL of ice cold 0.5 M HCl to 0.3 g of ascorbic acid. Dissolve the ascorbic acid by vortexing.
- For solution B, add 1 mL of ice cold water to 70 mg of ammonium heptamolybdate tetrahydrate and vortex to dissolve.
NOTE: Store both solutions on ice until use. For the consistency of the assay result, both solutions can be stored on ice for a maximum of one week.
- Prepare the phosphate (Pi) standard with the concentration of 0 µM, 62.5 µM, 250 µM and 500 µM for calibration.
- Add 0 µL, 25 µL, 50 µL, and 100 µL of 5 mM Na2HPO4 dihydrate to four microtubes containing 370 µL of the reaction mixture.
- Top up to 1 mL with water.
4. Activity assay for one 96 well plate
NOTE: See Figure 1 for the schematic workflow of the assay.
- Add 1 mL of solution B to 10 mL of solution A, mix by vortexing and store the solution on ice.
NOTE: This solution should be transparent and yellow. Keep solution A + B on ice for at least 30 min prior to use. However, use the solution within 3 h as it will go bad after long-term storage. - Add 40 µL of 0 µM, 62.5 µM, 250 µM and 500 µM Pi standard to the tube strips in triplicate using a multichannel pipette.
NOTE: The reaction mixture with no Pi added will be used as a blank. - Add 25 µL of compound solution to the tube strips using a multichannel pipette.
NOTE: Each compound has three different concentrations in triplicate which is enough for initial estimation of the half maximal inhibitory concentration (IC50). For a more accurate IC50 determination, eight different compound concentrations can be used. For the uninhibited enzyme the compound solution is replaced with equal amount of water. As positive controls 2.5 µM, 25 µM, and 250 µM of imidodiphosphate (IDP) sodium salt were used. - Add 15 µL of mPPase solution mixture to the tube strips (except to the tubes containing Pi standard) using a multichannel pipette.
- Seal the tube strips with an adhesive sealing sheet. Cut the sealing sheet to separate each tube strip.
- Pre-incubate the samples for 5 min at 71 °C. Place the samples on the heating block with 20 s interval between each strip in order to minimize the time consumption during the subsequent steps.
- For each strip, open the adhesive sealing. Add 10 µL of 2 mM sodium pyrophosphate dibasic using a multichannel pipette and mix by pipetting up and down for 5×. Seal the tube strip again using the same sealing.
NOTE: This step might initially be difficult to accomplish in 20 s; however, it will become easier after some assays. - Incubate at 71 °C for 5 min.
- Place the samples on the cooling apparatus with 20 s interval between each strip. Let them cool for 10 min but centrifuge each strip briefly after 5 min of cooling, to decant water drops under the sealing sheet, then put it back to the cooling apparatus and remove the sealing.
NOTE: The cooling apparatus can simply be made by placing a 96 well PCR plate on a polystyrene Petri dish (size 150 mm × 15 mm) filled with water and frozen for at least 1 h. The apparatus should be taken out from the freezer about 5 min prior to the beginning of the assay. Do not take out the cooling apparatus right before sample cooling as it will freeze the reaction mixture and hinder color development. - After 10 min of cooling, add 60 µL of solution A + B, mix by pipetting up and down for 5× and keep the tube strips on the cooling apparatus for 10 min.
- Add 90 µL of the arsenite-citrate solution and keep at room temperature for at least 30 min to produce a stable blue color.
CAUTION: Due to its toxicity all solutions containing sodium arsenite should be handled with extra care at all time. Thus, the addition of arsenite-citrate solution should be done in a fume hood. - Dispense 180 µL of each reaction mixture into a clear 96 well polystyrene microplate.
- Measure the absorbance of each well at 860 nm using a microplate spectrophotometer.
5. Result analysis
- Average the triplicates of each sample and the Pi standards. Then subtract with the blank to eliminate the background signal.
- Make a calibration curve by plotting the absorbance (A860) values against the amount of Pi standard (nmol) and perform a linear regression to obtain the trendline function using the following formula:
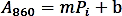
- Calculate the phosphate amount (nmol) released from the enzymatic reaction based on the linear regression formula above.
- Calculate the specific activity using the following formula:

where nPi is the amount of phosphate released from the reaction (nmol), t is the reaction time (min), and mTmPPase is the amount of the pure TmPPase used in the assay (mg). - Calculate the percent activity for each inhibitor concentration using the following formula:

where SAi is the specific activity of a sample with inhibitor and SAun is the specific activity of the uninhibited sample. - Calculate the logIC50 (estimate) and IC50 (estimate) with a nonlinear regression fit from the four-parameter dose-response curve using the following formula:
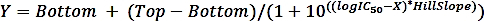
where X is log of concentration ( µM), Y is activity (%), Top and Bottom are plateaus in the same unit as Y (100% and 0%, respectively), logIC50 has the same log units as X, and HillSlope = slope factor or hill slope, which is unitless.
NOTE: Software (Table of Materials) is used for the fitting. Use the concentration of 0.01 µM (instead of 0.00 µM) for the sample without inhibitor as the logarithm of zero is not defined.
Results
In this protocol, eight compounds (1−8) were tested (Figure 2A) together with IDP, a common inhibitor of pyrophosphatases, as a positive control. Each compound was tested at three different concentrations (1 µM, 5 µM and 20 µM) in triplicate. The workflow of the screening is depicted in Figure 1, starting from sample and reagent preparation until the absorbance measurement at 860 nm.
At the end of this protocol, a...
Discussion
Here we report a detailed protocol for simple screening of inhibitors for membrane-bound pyrophosphatase from T. maritima in a 96 well plate format based on Vidilaseris et al.14. This protocol is inexpensive and based on 12-phosphomolybdic acid, which is formed from orthophosphate and molybdate under acidic conditions and reduced to phosphomolybdenum species with a distinct blue color12. This method is preferred over other protocols, such as the more sensitive mala...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the grants from the Jane and Aatos Erkko Foundation and the BBSRC (BB/M021610) to Adrian Goldman, the Academy of Finland (No. 308105) to Keni Vidilaseris, (No. 310297) to Henri Xhaard, and (No. 265481) to Jari Yli-Kauhaluoma, and the University of Helsinki Research Funds to Gustav Boije af Gennäs. The authors thank Bernadette Gehl for her technical help during the project.
Materials
| Name | Company | Catalog Number | Comments |
| Adhesive sealing sheet | Thermo Scientific | AB0558 | |
| Ammonium heptamolybdate tetrahydrate | Merck | F1412481 636 | |
| Ascorbic acid | Sigma-Aldrich | 95212-250G | |
| BioLite 96Well Multidish | Thermo Scientific | 130188 | |
| Dimethyl sulfoxide (DMSO) | Merck | 1167431000 | |
| 8-well PCR Tube Strips 0.2 ml without caps (120) | Nippon genetics | FG-028 | |
| Dodecyl maltoside (DDM) | Melford | B2010-100G | |
| Ethanol | Merck | 1009901001 | |
| Glacial acetic acid | Merck | 1000631011 | |
| Hydrochloric acid | Sigma-Aldrich | 258148-500ML | |
| Imidodiphosphate sodium salt | Sigma-Aldrich | I0631-1G | |
| L-α-Phosphatidyl choline from soybean lecithin | Sigma | 429415-100GM | |
| Magnesium chloride | Sigma-Aldrich | 8147330500 | |
| Multiplate 96-Well PCR Plates | Bio-Rad | MLL9651 | |
| MultiSkan Go | Thermo Scientific | 10680879 | |
| Nepheloskan Ascent (Type 750) | Labsystems | ||
| Polystyrene Petri dish (size 150 mm x 15 mm) | Sigma-Aldrich | P5981-100EA | |
| Potassium chloride | Merck | 104936 | |
| Prism 6 software | GraphPad | ||
| QBT2 Heating block | Grant Instruments | ||
| Sodium meta-arsenite | Fisher Chemical | 12897692 | |
| Sodium phosphate dibasic (Pi) | Sigma | S0876-1KG | |
| Sodium pyrophosphate dibasic | Fluka | 71501-100G | |
| Trisodium citrate dihydrate | Fluka | 71404-1KG |
References
- Terstappen, G. C., Reggiani, A. In silico research in drug discovery. Trends in Pharmacological Sciences. 22 (1), 23-26 (2001).
- Rask-Andersen, M., Almen, M. S., Schioth, H. B. Trends in the exploitation of novel drug targets. Nature Reviews Drug Discovery. 10 (8), 579-590 (2011).
- Shah, N. R., Vidilaseris, K., Xhaard, H., Goldman, A. Integral membrane pyrophosphatases: a novel drug target for human pathogens. AIMS Biophysics. 3 (1), 171-194 (2016).
- Baykov, A. A., Malinen, A. M., Luoto, H. H., Lahti, R. Pyrophosphate-Fueled Na+ and H+ Transport in Prokaryotes. Microbiology and Molecular Biology Reviews. 77 (2), 267-276 (2013).
- Serrano, A., Perez-Castineira, J. R., Baltscheffsky, M., Baltscheffsky, H. H+-PPases: yesterday, today and tomorrow. IUBMB Life. 59 (2), 76-83 (2007).
- Liu, J., et al. A vacuolar-H+-pyrophosphatase (TgVP1) is required for microneme secretion, host cell invasion, and extracellular survival of Toxoplasma gondii. Molecular Microbiology. 93 (4), 698-712 (2014).
- Lemercier, G., et al. A pyrophosphatase regulating polyphosphate metabolism in acidocalcisomes is essential for Trypanosoma brucei virulence in mice. Journal of Biological Chemistry. 279 (5), 3420-3425 (2004).
- Belogurov, G. A., et al. Membrane-bound pyrophosphatase of Thermotoga maritima requires sodium for activity. Biochemistry. 44 (6), 2088-2096 (2005).
- Vidilaseris, K., et al. Asymmetry in catalysis by Thermotoga maritima membrane-bound pyrophosphatase demonstrated by a nonphosphorus allosteric inhibitor. Science Advances. 5 (5), (2019).
- Lin, S. M., et al. Crystal structure of a membrane-embedded H+-translocating pyrophosphatase. Nature. 484 (7394), 399-403 (2012).
- Fiske, C. H., Subbarow, Y. The colorimetric determination of phosphorus. Journal of Biological Chemistry. 66 (2), 375-400 (1925).
- Nagul, E. A., McKelvie, I. D., Worsfold, P., Kolev, S. D. The molybdenum blue reaction for the determination of orthophosphate revisited: Opening the black box. Analytica Chimica Acta. 890, 60-82 (2015).
- Kellosalo, J., Kajander, T., Palmgren, M. G., Lopez-Marques, R. L., Goldman, A. Heterologous expression and purification of membrane-bound pyrophosphatases. Protein Expression and Purification. 79 (1), 25-34 (2011).
- Vidilaseris, K., Kellosalo, J., Goldman, A. A high-throughput method for orthophosphate determination of thermostable membrane-bound pyrophosphatase activity. Analytical Methods. 10 (6), 646-651 (2018).
- He, Z. Q., Honeycutt, C. W. A modified molybdenum blue method for orthophosphate determination suitable for investigating enzymatic hydrolysis of organic phosphates. Communications in Soil Science and Plant Analysis. 36 (9-10), 1373-1383 (2005).
- Martin, B., Pallen, C. J., Wang, J. H., Graves, D. J. Use of fluorinated tyrosine phosphates to probe the substrate specificity of the low molecular weight phosphatase activity of calcineurin. Journal of Biological Chemistry. 260 (28), 14932-14937 (1985).
- Strauss, J., Wilkinson, C., Vidilaseris, K., Harborne, S. P. D., Goldman, A. A simple strategy to determine the dependence of membrane-bound pyrophosphatases on K+ as a cofactor. Methods in Enzymology. 607, 131-156 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved