A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Localized Bathless Metal-Composite Plating via Electrostamping
In This Article
Summary
Presented here is a protocol of bathless electroplating, where a stagnant metal salt paste containing composite particles are reduced to form metal composites at high loading. This method addresses the challenges faced by other common forms of electroplating (jet, brush, bath) of embedding composites particles into the metal matrix.
Abstract
Composite plating with particles embedded into the metal matrix can enhance the properties of the metal coating to make it more or less conductive, hard, durable, lubricated or fluorescent. However, it can be more challenging than metal plating, because the composite particles are either 1) not charged so they do not have a strong electrostatic attraction to the cathode, 2) are hygroscopic and are blocked by a hydration shell, or 3) too large to remain stagnate at the cathode while stirring. Here, we describe the details of a bathless plating method that involves anode and cathode nickel plates sandwiching an aqueous concentrated electrolyte paste containing large hygroscopic phosphorescent particles and a hydrophilic membrane. After applying a potential, the nickel metal is deposited around the stagnant phosphor particles, trapping them in the film. The composite coatings are characterized by optical microscopy for film roughness, thickness and composite surface loading. In addition, fluorescence spectroscopy can be used to quantify the illumination brightness of these films to assess the effects of various current densities, coating duration and phosphor loading.
Introduction
Traditional electroplating is widely used to deposit thin films of a variety of metals, alloys, and metal-composites onto conductive surfaces to functionalize them for the intended application1,2,3,4,5,6,7,8,9,10,11,12. This method adds a metal finish to parts used in the manufacturing of aerospace, automotive, military, medical, and electronic equipment. The object to be plated, the cathode, is submerged in an aqueous bath containing metal salt precursors, which are reduced to metal at the surface of the object by the application of a chemical or electrical potential. Non-charged composite particles can be incorporated into the metal film by adding these to the bath during coating to enhance the film properties for increased hardness in the case of metal oxides and carbides, smoothness with polymers or lubrication with liquid oils12,13. However, because these particles lack an inherent attraction to the cathode, the ratio of composite that is incorporated into the metal remains low for bath plating13,14,15. This is especially problematic for large particles that do not adsorb to the cathode long enough to be embedded by the growing metal film. Additionally, hygroscopic particles solvate in aqueous solutions and their hydration shell acts as a physical barrier impeding contact with the cathode16.
Some promising methods have been shown to mitigate this effect by using dry non-polar solvents to remove the hydration barrier completely17, or by decorating the composite particles with charged surfactant molecules16 that disrupt the hydration shell to allow contact between the particle and the cathode. However, because these methods involve organic materials, carbon contamination is possible in the film and breakdown of these organic materials could occur at the electrodes. For example, the organic solvents used (DMSO2 and acetamide) are heated to 130 °C in an inert atmosphere for air-free coating; however, we found them to be unstable during coating in air. Due to resistive heating at the electrodes, redox reactions with organic materials may result in impurities or sites for heterogeneous nucleation and growth of metal nanoparticles18. As a result, there is a need for an organic-free aqueous electroplating method that addresses the long-standing challenge of particle-cathode adsorption. So far, metal-composite bath coating has been shown to embed particles up to a few micrometers in diameter19 and as high as 15 % loading16,17.
In response to this, we describe an inorganic bathless electrostamping method that forces composite particles to become embedded into the film at high surface coverages despite their large size and hygroscopic nature20. By removing the bath, the process does not involve containers of hazardous coating liquids and the object to be plated does not need to be submerged. Therefore, large, cumbersome or otherwise corrosion- or water-sensitive objects, can be plated or “stamped” in select areas with the composite material. In addition, the removal of excess water requires less clean-up of liquid hazardous waste.
Here, we demonstrate this method to produce bright fluorescent metal films by co-depositing non-toxic and air-stable europium and dysprosium doped, strontium aluminate (87 ± 30 µm) with nickel at high loadings (up to 80%). This comes in contrast to previous examples that were plated in a bath and therefore were limited to small (nanometers to a few micrometers) phosphors12. In addition, previously reported electrodeposited films fluoresce only under short-wave UV-light, with the exception of a recent report that grew 1 – 5 µm luminescent strontium aluminate crystals in an alumina film with plasma electrolyte oxidation21. Fluorescent metal films could have far-reaching applications in many industries involving dim-light environments including road sign illumination21, aircraft maintenance equipment location and identification20, automobile and toy decorations, invisible messages, product authentication22, safety lighting, mechanochromic stress identification10 and tribological wear visual inspection12,16. Despite these potential uses for glowing metal surfaces, this method could also be expanded to include additional large and/or hygroscopic composite particles to produce a new variety of metal-composite functional coatings that were previously not possible via electroplating.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Preparing coating salts
CAUTION: Nickel salts and boric acid are toxic and should be handled with proper personal protective equipment including nitrile gloves, goggles and a lab coat. Strong acids and bases should be handled in the fume hood, and all waste chemicals should be disposed of as hazardous waste.
- Using a balance, weigh out the following powders in these ratios: 10.000 g of NiSO4·6H2O, 2.120 g of NiCl2·6H2O, 1.600 g of H3BO3 and combine in a vial together. See Table 1 for concentrations.
- Weigh out 1.800 g of SrAl2O4:Eu2+, Dy3+ phosphor or alternative phosphors including europium doped yttrium oxide, europium doped barium magnesium aluminate, or replace with alternative oxide, metal, or organic composite material depending on the desired effect.
NOTE: The amount added here may vary based on the properties of the composite material and the desired qualities of the metal-composite film. - Using a porcelain mortar and pestle, grind the composite powder for approximately 10 minutes until it becomes a fine powder.
NOTE: This does not change the particle size, but does separate aggregated particles. - Likewise, grind the salt mixture from step 1.1 in batches until it becomes a fine powder.
- Combine the ground phosphor with the ground salt mixture in a container for storage.
- Weigh out 0.188 grams of the mixture per cm2 of coating area, as prepared in step 1.5 and add to a container with an open top that is easy to access.
- To this, add 40 µL of water per cm2 of coating area, and stir to partially dissolve the salts forming a thick paste. Set this aside.
NOTE: The protocol can be paused here.
2. Preparing the electrodes
- Using scissors, cut the anode to the size and shape that matches the object to be plated. In this example, we prepare a 4 cm2 nickel foil to be coated, and a 4 cm2 nickel anode is cut to match this.
NOTE: Other objects can be coated including large objects. In this case, select the area on the object to be coated, and cut out the anode to match the coating area. - Using a cotton swab or a cloth, clean the surface of the anode foil and the cathode (coating object surface) with concentrated (10 M) potassium hydroxide or sodium hydroxide base to remove organic material. Next, rinse the surfaces with water to remove excess base.
- Using a cotton swab or a cloth, activate the object surface with concentrated acid. In the case of nickel, 37% vol/vol HCl is used, although for steel, 10% by volume aqueous HCl may be more appropriate. Please refer to the recommendations for activating metal surfaces provided elsewhere to determine the appropriate method for activating specific metals or alloys23,24.
NOTE: After this step, the metal surface is reactive and the surface will start to react with oxygen in the air to form an oxide layer. This will cause the surface to be inactive, so the following steps (2.4 – 3.5) should be performed in the next 5 minutes; otherwise, step 2.3 should be repeated before continuing.
CAUTION: This step should be performed in a fume hood to avoid exposure to HCl vapors. - Quickly, deposit the coating paste onto the cathode object. In this case, the cathode is a 4 cm2 nickel foil on the benchtop. Cover the object area to be plated evenly and try to avoid gaps in the paste.
NOTE: In this example, we are painting on this paste with two scoopulas, however, other options may include spraying, dipping or doctor blading to increase the speed and efficiency of this step. - Using a cotton swab or a cloth, activate the anode with concentrated acid by dipping the swab in the acid and gently rubbing the cathode surface. In the case of nickel, 70% vol/vol HNO3 can be used.
NOTE: However, other acids may be more appropriate for specific metals and alloys. Please refer to the recommendations provided elsewhere for the appropriate reagent to activate specific anode surfaces23,24.
CAUTION: This step should be performed in a fume hood to avoid exposure to NO2, a toxic brown gas that is formed during the reaction. Continue treating the surface until the surface becomes grey and textured. After this step, the metal surface is reactive and the surface will start to react with oxygen in the air to form an oxide layer, so the following steps should be performed quickly to avoid inactivation of the anode. - If calculating the current efficiency is desired, use an analytical balance to record the mass of the anode and the cathode.
3. Assembly and coating
- Pre-set a power supply to the desired current in constant current mode or voltage, if constant voltage mode is desired. In this example, constant current mode is used with a current of 0.1 Amperes (0.1 A per 4 cm2 = 0.025 A/cm2).
NOTE: For larger or irregularly shaped objects, the coating area can be predetermined with a grid or using a photo with scale bar and an imaging software like ImageJ. The applied current can be scaled to deliver the same current density required for the coating area. - Cut a piece of nylon sheet (or alternative hydrophilic membrane) to a size larger than the anode so that the anode does not make direct contact with the cathode object.
- Place the nylon sheet on top of the coating paste, and then add a small amount of paste to this.
- Next, add 1-2 drops of water from a pipette to allow the salt to partially dissolve. Steps 3.2.1 – 3.3 make the nylon sheet conductive and allow for the mass transport of ions through the electrolyte, which is necessary to balance charge in the coating reaction.
- Finally, add the activated anode on top and attach the negative lead to the cathode object and the positive lead to the anode.
NOTE: It might be helpful to tape down these leads so that the setup remains stationary, especially if the experiment involves small pieces of metal foil. This is less important for large objects. - Cover the system with plastic or seal to help retain water, and apply moderate pressure (~100 g per cm2 area), turn on the power supply and continue coating for the desired duration.
- Turn off the power supply and expose the system.
- Disconnect the leads, separate the electrodes and rinse the cathode object with water into a waste container.
- Soak the other items in water to remove salts and dispose of this aqueous solution in the properly labeled hazardous waste container
- Wearing gloves, gently rub the cathode object by hand to remove any uncoated composite particles. The coating is complete and ready for characterization.
- Using an analytical balance, record the mass of the anode and the cathode and find the difference between these values and their original mass.
- Use Faraday’s Laws of electrolysis to calculate the current efficiency. The theoretical moles of metal coating can be determined using Equation 1.
 Equation 1
Equation 1
where n is the amount of metal deposited (units: mol), I is the applied current, t is the coating time, F is Faraday’s constant (96485 coulombs per mole) and z is the charge of the metal ion. Calculate this value based on the experimental parameters. - Divide the experimentally determined deposit mass obtained from the masses of the cathode or anode (Steps 2.6 and 3.7.3) by the theoretical mass lost (anode) or gained (cathode) to calculate the current efficiency using Equation 2.
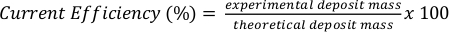 Equation 2
Equation 2
[NOTE: At a current efficiency of 100%, under constant voltage, a theoretical deposit mass is expected of approximately 1.095 g of nickel or 12.3 µm of nickel per hour, given 0.04 A and 4 cm2 area. Likewise, under constant current, approximately 614.6 µm nickel would theoretically deposit per unit of 1 A∙cm-2 after 30 min.]
4. Characterization with electrochemistry
- Use chronopotentiometry to monitor changes in voltage under constant current, and chronoamperometry to monitor changes in current under constant voltage.
- Turn on the potentiostat and designate the duration and the applied current or voltage.
- Repeat steps 3.2 – 3.5 to prepare the coating.
- Use a calibrated 3-electrode system to normalize the voltage to a reference standard.
- Place a platinum wire pseudo reference electrode between on top of the nylon sheet and below the anode. In order to ensure that the reference electrode does not make direct contact with the anode, use a separate nylon sheet (or alternative membrane) placed on top of the reference followed by a few drops of water, a small amount of coating paste (repeat steps 3.2 – 3.3) and then the anode.
- Connect the leads to the electrodes, seal, press, begin coating and monitor changes in voltage or current.
5. Characterization with quantum yield fluorescence spectroscopy
- If the coating contains fluorescent composite particles, use a fluorometer equipped with an integrating sphere to obtain absolute quantum yield measurements.
- Place the coating into the fluorimeter stage with the fluorescent coating facing 45° from the excitation source and 315° from the detector.
- Record the fluorescence spectra starting at a wavelength below the excitation wavelength to record the areas of the excitation peak and the fluorescence peak.
- Remove the sample from the fluorometer, and repeat step 5.1.2 to record the blank excitation peak. Calculate the quantum yield (QY) from the ratios of the areas of the excitation and emission peaks (Equation 3 and Figure 3B).
 Equation 3
Equation 3
where Aem, Aex and Ao are the peak areas at the emission wavelength of the sample, the excitation wavelength of the sample and the excitation wavelength of the blank, respectively.
6. Characterization with optical microscopy
- Place the sample on the stage of a calibrated optical microscope with the coating side facing the lenses and bring the surface into focus.
- Record surface images at the desired magnifications and surface sample sites.
- Using image analysis software (ex. ImageJ (IJ 1.46r)), calculate and plot the surface coverage and average composite particle size.
- To determine coating thickness and cross-section features, cut the cathode foil with scissors. Set the coating on the side and re-adjust the focus. Repeat steps 6.2 – 6.3.
Access restricted. Please log in or start a trial to view this content.
Results
After following this protocol, a thin coating of metal should become plated onto the cathode surface and contain the composite particles that were added to the coating paste. Fluorescent or colored particle incorporation can be observed by visual inspection as a result of a change in appearance compared to the uncoated surface (Figure 1A1-A3). To investigate the percent surface coverage of the composite particles and to observe the surface morphology of the coating, optical ...
Access restricted. Please log in or start a trial to view this content.
Discussion
Critical steps of electrostamping. Bathless electrostamping shares many of the same critical steps with traditional bath electroplating. These include proper cleaning of the electrodes, mixing metal ions into the electrolyte and applying and external or chemical (electroless plating) potential to cause reduction of metal onto the cathode. In addition, the oxidation of the anode and cathode should be avoided after acid activation by quickly rinsing with water and adding these electrodes to the setup.
...Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Aircraft Equipment Reliability and Maintainability Improvement Program and the Patuxent Partnership. Townsend was supported by an ONR Faculty Research Fellowship. The authors also acknowledge the general support of the SMCM Chemistry and Biochemistry Department faculty and students, including support from the SMCM football team.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 37% M Hydrochloric Acid (aq) | SigmaAldrich | 320331-500ML | corrosive - handle in fume hood |
| 70% Nitric Acid (aq) | SigmaAldrich | 438073-500ML | corrosive - handle in fume hood |
| Barium magnesium aluminate, europium doped (s) | SigmaAldrich | 756512-25G | fine powder |
| Boric Acid (s) | SigmaAldrich | B6768-500G | toxic |
| Cotton Swab | Q-tips | Q-tips Cotton Swabs | |
| ImageJ | National Institutes of Health | IJ 1.46r | free software |
| Nickel (II) chloride hexahydrate (s) | SigmaAldrich | 223387-500G | toxic |
| Nickel (II) sulfate hexahydrate (s) | SigmaAldrich | 227676-500G | toxic |
| Nickel foil (s) | AliExpress | Ni99.999 | |
| Nitrile gloves | Fisher Scientific | 19-149-863B | |
| nylon membrane (s) | Tisch Scientific | RS10133 | |
| Optical Microscope equipped with FTIC filter (470 ± 20 nm) | Nikon | Eclipse 80i | |
| Plastic Wrap | Fisher Scientific | 22-305654 | |
| Porcelain Mortar | Fisher Scientific | FB961A | |
| Porcelain Pestle | Fisher Scientific | FB961K | |
| Potassium Hydroxide (s) | SigmaAldrich | 221473-25G | corrosive |
| Potentiostat with platinum wire | Gamry Instruments | 1000E | |
| Scoopula | Fisher Scientific | 14-357Q | |
| Spectrofluorometer | Photon Technology International | QM-40 | |
| Strontium aluminate, europium and dysprosium doped (s) | GloNation | 756539-25G | powder |
| Variable linear DC power supply | Tekpower | TP3005T | |
| Yttrium oxide, europium doped (s) | SigmaAldrich | 756490-25G | fine powder |
References
- Hunt, W. H., et al. Comprehensive Composite Materials. , Elsevier Ltd. (2000).
- Hovestad, A., Janssen, L. J. J. Electrochemical codeposition of inert particles in a metallic matrix. Journal of Applied Electrochemistry. 25 (6), 519-527 (1995).
- Zimmerman, A. F., Clark, D. G., Aust, K. T., Erb, U. Pulse electrodeposition of Ni-SiC nanocomposite. Materials Letters. 52 (1), 85-90 (2002).
- Devaneyan, S. P., Senthilvelan, T. Electro Co-deposition and Characterization of SiC in Nickel Metal Matrix Composite Coatings on Aluminium 7075. Procedia Engineering. 97, 1496-1505 (2014).
- Lekka, M., Kouloumbi, N., Gajo, M., Bonora, P. L. Corrosion and wear resistant electrodeposited composite coatings. Electrochimica Acta. 50 (23), 4551-4556 (2005).
- Balaraju, J. N., Sankara Narayanan, T. S. N., Seshadri, S. K. Electroless Ni-P composite coatings. Journal of Applied Electrochemistry. 33 (9), 807-816 (2003).
- Jugović, B., Stevanović, J., Maksimović, M. Electrochemically deposited Ni + WC composite coatings obtained under constant and pulsating current regimes. Journal of Applied Electrochemistry. 34 (2), 175-179 (2004).
- Hilla, F., et al. Fabrication of self-lubricating cobalt coatings on metal surfaces. Nanotechnology. 18 (11), 115703(2007).
- Abi-Akar, H., Riley, C., Maybee, G. Electrocodeposition of Nickel-Diamond and Cobalt-Chromium Carbide in Low Gravity. Chemistry of Materials. 8 (11), 2601-2610 (1996).
- Zhang, X., Chi, Z., Zhang, Y., Liu, S., Xu, J. Recent Advances in Mechanochromic Luminescent Metal Complexes. Journal of Materials Chemistry C. 1, 3376-3390 (2013).
- Lancsek, T., Feldstein, M. Composite electroless plating. US Patent. , 20060251910A1 United States (2006).
- Walsh, F. C., Ponce de Leon, C. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: an established and diversifying technology. Transactions of the Institute of Materials Finishing. 92 (2), 83-98 (2014).
- Roos, J. R., Celis, J. P., Fransaer, J., Buelens, C. The development of composite plating for advanced materials. Journal of The Minerals, Metals and Materials Society. 42 (11), 60-63 (1990).
- Guglielmi, N. Kinetics of the Deposition of Inert Particles from Electrolytic Baths. Journal of The Electrochemical Society. 119 (8), 1009-1012 (1971).
- Celis, J. P., R, J. R., Buelens, C. A Mathematical Model for the Electrolytic Codeposition of Particles with a Metallic Matrix. Journal of The Electrochemical Society. 134 (6), 1402-1408 (1987).
- He, Y., et al. The monitoring of coating health by in situ luminescent layers. RSC Advances. 5 (53), 42965-42970 (2015).
- Ganapathi, M., et al. Electrodeposition of luminescent composite metal coatings containing rare-earth phosphor particles. Journal of Materials Chemistry. 22 (12), 5514-5522 (2012).
- Monnens, W., Deferm, C., Sniekers, J., Fransaer, J., Binnemans, K. Electrodeposition of indium from non-aqueous electrolytes. Chemical Communications. 55 (33), 4789-4792 (2019).
- Low, C. T. J., Wills, R. G. A., Walsh, F. C. Electrodeposition of composite coatings containing nanoparticles in a metal deposit. Surface and Coatings Technology. 201 (1), 371-383 (2006).
- Gerwitz, C. N., David, H. M., Yan, Y., Shaw, J. P., Townsend, T. K. Bathless Inorganic Composite Nickel Plating: Dry-Cell Stamping of Large Hygroscopic Phosphor Crystals. Advanced Materials Interfaces. 7 (4), (2020).
- Bite, I., et al. Novel method of phosphorescent strontium aluminate coating preparation on aluminum. Materials and Design. 160 (15), 794-802 (2018).
- Feldstein, M. D. Coatings with identification and authentication properties. US Patent. , 20120021120A1 (2012).
- Rose, I., Whittingham, C. Nickel Plating Handbook. , Nickel Institute. (2014).
- Anderson, D. M., et al. Electroplating Engineering Handbook. , Springer US. New York, NY. (1996).
- Helle, K., Walsh, F. Electrodeposition of Composite Layers Consisting of Inert Inclusions in a Metal Matrix. Transactions of the Institute of Metal Finishing. 75 (2), 53-58 (1997).
- Kerr, C., Barker, D., Walsh, F., Archer, J. The Electrodeposition of Composite Coatings based on Metal Matrix-Included Particle Deposits. Transactions of the Institute of Metal Finishing. 78 (5), 171-178 (2000).
- Walsh, F. C., Wang, S., Zhou, N. The electrodeposition of composite coatings: Diversity, applications and challenges. Current Opinion in Electrochemistry. 20, 8-19 (2020).
- Feldstein, N. Functional coatings comprising light emitting particles. US Patent. , US/1996/5514479A (1996).
- Feldstein, N. Composite plated articles having light-emitting properties. US Patent. , US/1998/5834065A (1998).
- Zimmerman, E. M. Method of Jet Plating. US Patent. , US/1957/2873232A (1957).
- Schwartz, B. J. Method of Electroplating. United States Patent. , US/1961/3313715A (1961).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved