A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Preparation of Silver-Palladium Alloyed Nanoparticles for Plasmonic Catalysis under Visible-Light Illumination
In This Article
Summary
Presented here is a protocol for the synthesis of silver-palladium (Ag-Pd) alloy nanoparticles (NPs) supported on ZrO2 (Ag-Pd/ZrO2). This system allows for harvesting energy from visible light irradiation to accelerate and control molecular transformations. This is illustrated by nitrobenzene reduction under light irradiation catalyzed by Ag-Pd/ZrO2 NPs.
Abstract
Localized surface plasmon resonance (LSPR) in plasmonic nanoparticles (NPs) can accelerate and control the selectivity of a variety of molecular transformations. This opens possibilities for the use of visible or near-IR light as a sustainable input to drive and control reactions when plasmonic nanoparticles supporting LSPR excitation in these ranges are employed as catalysts. Unfortunately, this is not the case for several catalytic metals such as palladium (Pd). One strategy to overcome this limitation is to employ bimetallic NPs containing plasmonic and catalytic metals. In this case, the LSPR excitation in the plasmonic metal can contribute to accelerate and control transformations driven by the catalytic component. The method reported herein focuses on the synthesis of bimetallic silver-palladium (Ag-Pd) NPs supported on ZrO2 (Ag-Pd/ZrO2) that acts as a plasmonic-catalytic system. The NPs were prepared by co-impregnation of corresponding metal precursors on the ZrO2 support followed by simultaneous reduction leading to the formation of bimetallic NPs directly on the ZrO2 support. The Ag-Pd/ZrO2 NPs were then used as plasmonic catalysts for the reduction of nitrobenzene under 425 nm illumination by LED lamps. Using gas chromatography (GC), the conversion and selectivity of the reduction reaction under the dark and light irradiation conditions can be monitored, demonstrating the enhanced catalytic performance and control over selectivity under LSPR excitation after alloying non-plasmonic Pd with plasmonic metal Ag. This technique can be adapted to a wide range of molecular transformations and NPs compositions, making it useful for the characterization of the plasmonic catalytic activity of different types of catalysis in terms of conversion and selectivity.
Introduction
Among the several applications of metal nanoparticles (NPs), catalysis deserves special attention. Catalysis plays a central role in a sustainable future, contributing to less energy consumption, better utilization of raw materials, and enabling cleaner reaction conditions1,2,3,4. Thus, progress in catalysis can provide tools for enhancing the atomic efficiency of chemical processes, making them cleaner, more economically viable, and more environmentally friendly. Metal NPs encompassing silver (Ag), gold (Au) or copper (Cu) can display interesting optical properties in the visible range that arise from the unique way these systems interact with light at the nanoscale via the localized surface plasmon resonance (LSPR) excitation5,6,7,8. In these NPs, referred to as plasmonic NPs, the LSPR comprises the resonant interaction between the incident photons (from an incoming electromagnetic wave) with the collective motion of electrons5,6,7,8. This phenomenon takes place at a characteristic frequency which is dependent on the size, shape, composition, and dielectric constant of the environment9,10,11. For example, for Ag, Au, and Cu, these frequencies can range from the visible to the near-IR, opening up possibilities for the utilization of solar energy to excite their LSPR5,6,7,8,12,13.
Recently, it has been demonstrated that the LSPR excitation in plasmonic NPs can contribute to accelerate the rates and control the selectivity of molecular transformations5,14,15,16,17,18,19. This gave birth to a field called plasmonic catalysis, which focus on using energy from light to accelerate, drive, and/or control chemical transformations5,14,15,16,17,18,19. In this context, it has been established that the LSPR excitation in plasmonic NPs can lead to the formation of energetic hot electrons and holes, referred to as LSPR-excited hot carriers. These carriers can interact with adsorbed species through electronic or vibrational activation15,16. In addition to increased reaction rates, this process can also provide alternative reaction pathways not accessible via traditional thermochemically-driven processes, opening up new avenues for the control over reaction selectivity20,21,22,23,24,25. Importantly, it is worth noting that the plasmon decay can also lead to thermal dissipation, leading to a temperature increase in the vicinity of the NPs which can also contribute to speed up reaction rates15,16.
Due to these interesting features, plasmonic catalysis has been successfully employed towards a variety of molecular transformations18. Nevertheless, an important challenge remains. While plasmonic NPs such as Ag and Au display excellent optical properties in the visible and near-IR ranges, their catalytic properties are limited in terms of the scope of transformations. In other words, they do not display good catalytic properties for several of transformations. Additionally, metals that are important in catalysis, such as palladium (Pd) and platinum (Pt), do not support LSPR excitation in the visible or near-IR ranges. To bridge this gap, bimetallic NPs containing a plasmonic and catalytic metal represents an effective strategy20,26,27,28,29. In these systems, the plasmonic metal can be employed as an antenna to harvest energy from the light excitation through the LSPR, which is then used to drive, accelerate, and control molecular transformations at the catalytic metal. Therefore, this strategy enables us to extend plasmonic catalysis beyond traditional plasmonic metal NPs20,26,27,28,29.
This protocol describes the facile synthesis of bimetallic silver-palladium (Ag-Pd) alloyed NPs supported on ZrO2 (Ag-Pd/ZrO2) that can act as a plasmonic-catalytic system for plasmonic catalysis. The Ag-Pd/ZrO2 NPs were prepared by co-impregnation of the corresponding metal precursors on the ZrO2 support followed by simultaneous reduction30. This approach led to the formation of bimetallic NPs around 10 nm in size (diameter) directly at the surface of the ZrO2 support. The NPs were composed of 1 mol% of Pd to minimize the utilization of the catalytic metal while maximizing the optical properties of the resulting Ag-Pd NPs. A protocol for the application of the Ag-Pd/ZrO2 NPs in plasmonic catalysis was demonstrated for the reduction of nitrobenzene. We employed 425 nm LED illumination for the LSPR excitation. Gas chromatography was performed to monitor the conversion and selectivity of the reduction reaction under the dark and light irradiation conditions. LSPR excitation led to enhanced catalytic performance and control over selectivity in Ag-Pd/ZrO2 NPs relative to purely thermally driven conditions. The method described in this protocol is based on a simple photocatalytic reaction setup coupled with gas chromatography and can be adapted to a wide range of molecular transformations and NPs compositions. Thus, this method makes possible the characterization of photocatalytic activity, in terms of conversion and reaction selectivity, of different NPs and for a myriad of liquid-phase transformations. We believe this article will provide important guidelines and insights to both newcomers and more experienced scientists in the field.
Access restricted. Please log in or start a trial to view this content.
Protocol
1. Synthesis of Ag-Pd/ZrO2 NPs
NOTE: In this procedure, the Pd mol% in Ag-Pd corresponded to 1%, and the Ag-Pd loading on ZrO2 corresponded to 3 wt.%.
- Place 1 g of ZrO2 powder in a 250 mL beaker.
- Add 50 mL of an AgNO3 (aq) (0.0059 mol/L) and 9.71 mL of a K2PdCl4 (aq) (0.00031 mol/L) solutions to the beaker under vigorous magnetic stirring (500 rpm) at room temperature.
- Add 10 mL of lysine (0.53 M) aqueous solution.
- Keep the mixture under vigorous stirring (500 rpm) for 20 min.
- After 20 min, use a pipette to add to the suspension 10 mL of a freshly prepared NaBH4 (aq) (0.035 M) solution dropwise, at a rate of 1 mL/min. Keep the suspension under stirring (500 rpm) throughout the process.
- Let the mixture stir for 30 min at room temperature.
2. Separation and purification of the catalyst
- Transfer the suspension to centrifuge tubes and separate the solids from the mixture by centrifugation at 3,260 x g for 10 min.
- Carefully remove the liquid phase with a pipette and add 15 mL deionized water to the tubes.
- Shake vigorously until dispersion of the solid is obtained. If good dispersion is not achieved, place the tubes in an ultrasonic bath for 5 min.
- Centrifuge the dispersion at 3,260 x g for 10 min.
- Repeat the washing steps (2.2. to 2.2.2.) two more times using deionized water, then once using ethanol instead of water.
- Remove the ethanol and dry the solid in an oven at 60 °C for 12 h.
- Characterize the prepared Ag-Pd/ZrO2 NPs by a variety of microscopy, elemental, and spectroscopic techniques.
3. Synthesis of Ag/ZrO2 NPs
NOTE: In this procedure, Ag loading on ZrO2 corresponded to 3 wt.%.
- Place 1 g of ZrO2 powder in a 250 mL beaker.
- Add 50 mL of an AgNO3 (aq) (0.0059 mol/L) solution to the beaker under vigorous magnetic stirring (500 rpm) at room temperature.
- Add 10 mL of lysine (0.53 M) aqueous solution.
- Keep the mixture under vigorous stirring (500 rpm) for 20 min.
- After 20 min, use a pipette to add to the suspension 10 mL of a freshly prepared NaBH4 (aq) (0.035 M) solution dropwise, at a rate of 1 mL/min. Keep the suspension under stirring (500 rpm) throughout the process.
- Let the mixture stir for 30 min under room temperature.
4. Separation and purification of the catalyst
- Transfer the suspension to centrifuge tubes and separate the solids from the mixture by centrifugation at 3,260 x g for 10 min.
- Carefully remove the liquid phase with a pipette and add 15 mL deionized water to the tubes.
- Shake vigorously until the dispersion of the solid is observed. If good dispersion is not achieved, place the tubes in an ultrasonic bath for 5 min.
- Centrifuge the dispersion at 3,260 x g for 10 min.
- Repeat the washing steps (4.2. to 4.2.2.) two more times using deionized water, then once using ethanol instead of water.
- Remove the ethanol and dry the solid in an oven at 60 °C for 12 h.
- The prepared Ag/ZrO2 NPs can then be characterized by a variety of microscopy, elemental, and spectroscopic techniques.
5. Investigation of plasmonic catalytic performance towards the nitrobenzene reduction under LSPR excitation (light illumination)
- Place 30 mg of catalyst in a 25 mL round-bottom flask along with a magnetic stirring bar.
- Add 5 mL of a solution of nitrobenzene (0.03 mol/L) in isopropyl alcohol (IPA) to the reactor.
- Then, add 11.22 mg of KOH powder (0.0002 mol).
- Purge the reactor by bubbling the suspension with an argon flow for 1 min. Immediately after purging, seal the flask.
- Place the reactor in an oil bath heated at 70 °C above a temperature-controlled magnetic stirrer (500 rpm).
- Irradiate the tube using 4 LED lamps with a wavelength of 425 nm as the light source, and a light intensity of 0.5 W/cm2. The distance from the lamps to the reaction flask should be 7 cm.
- Let the reaction proceed for 2.5 h at 70 °C under vigorous magnetic stirring (500 rpm).
- Then, turn the light off, open the reactor and use a syringe and a needle to collect a 1 mL sample. Filter it through a 0.45 μm filter, to remove the catalyst particulates, into a gas chromatography vial.
6. Reaction in the absence of LSPR excitation (dark conditions)
- Follow the same steps as described in 5, but without light irradiation. Wrap the reaction tube with aluminium foil to prevent any light exposure.
7. Gas chromatography (GC) analysis preparation
- Prepare an IPA solution containing approximately 30 mmol/L nitrobenzene (NB), 30 mmol/L of aniline (AN), and 30 mmol/L of azobenzene (AB).
- Run a GC analysis of the solution using a suitable method. Different methods can be tested by varying the column temperature and gas flow programs. The selected method should be able to separate the peaks corresponding to IPA, NB, AN, and AB in the minimum period of retention time.
- Once the method has been selected, prepare a set of solutions of 50 mM, 25 mM, 10 mM, 5 mM and 2.5 mM NB in IPA, and another set of solutions of AN and AB in IPA with the same concentrations.
- Run a GC analysis of the prepared solutions. Each chromatogram should present 2 peaks: the higher one corresponds to IPA and the lower one corresponds to NB, AN, or AB. For each chromatogram, note down the retention time and peak area of all the peaks.
- Trace the calibration curves of NB, AN, and AB by plotting the concentration versus peak area of each sample.
8. GC analysis
- Run a GC analysis on the samples collected in steps 5. and 6. with the same method used for steps 7.2. and 7.4.
- For each chromatogram, note down the retention time and peak area and use the calibration curves plotted previously to determine the concentration of NB, AN, and AB in the samples.
- Calculate the nitrobenzene conversion as well as the aniline and azobenzene selectivity using the equations:
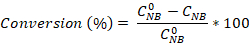
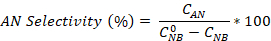
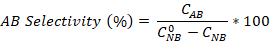
Where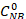 is the initial NB concentration (0.03 mol/L), and CNB, CAN, CAB correspond to the NB, AN, and AB concentrations, respectively, after 2.5 hours reaction by the GC analysis.
is the initial NB concentration (0.03 mol/L), and CNB, CAN, CAB correspond to the NB, AN, and AB concentrations, respectively, after 2.5 hours reaction by the GC analysis.
Access restricted. Please log in or start a trial to view this content.
Results
Figure 1A shows digital photographs of the solid samples containing the pure ZrO2 oxide (left) and the Ag-Pd/ZrO2 NPs (right). This change in color from white (in ZrO2) to brown (Ag-Pd/ZrO2) provides the initial qualitative evidence on the deposition of Ag-Pd NPs at the ZrO2 surface. Figure 1B shows the UV-visible absorption spectra from the Ag-Pd/ZrO2 NPs (blue trace) as well as ZrO2 (...
Access restricted. Please log in or start a trial to view this content.
Discussion
The findings described in this method demonstrate that the intrinsic catalytic activity of Pd (or other catalytic but not plasmonic metal) can be significantly enhanced by LSPR excitation via visible-light irradiation in bimetallic alloyed NPs35. In this case, Ag (or another plasmonic metal) is capable of harvesting energy from visible-light irradiation via LSPR excitation. The LSPR excitation leads to the formation of hot charge carriers (hot electrons and holes) and localized heating...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the University of Helsinki and the Jane and Aatos Erkko Foundation. S.H. thanks Erasmus+ EU funds for the fellowship.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| 2-Propanol (anhydrous, 99.5%) | Sigma-Aldrich | 278475 | CAS Number 67-63-0 |
| Aniline (for synthesis) | Sigma-Aldrich | 8.22256 | CAS Number 62-53-3 |
| Azobenzene (98%) | Sigma-Aldrich | 424633 | CAS Number 103-33-3 |
| Ethanol | Honeywell | 32221 | CAS Number 64-17-5 |
| Hydrochloric acid (37%) | VWR | PRLSMC310066 | CAS Number 7647-01-0 |
| L-Lysine (crystallized, ≥98.0% (NT)) | Sigma-Aldrich | 62840 | CAS Number 56-87-1 |
| Nitric acid (65%) | Merck | 100456 | CAS Number 7697-37-2 |
| Nitrobenzene | Sigma-Aldrich | 8.06770 | CAS Number 98-95-3 |
| Potassium hydroxide | Fisher | 10448990 | CAS Number 1310-58-3 |
| Potassium tetrachloropalladate (II) (98%) | Sigma-Aldrich | 205796 | CAS Number 10025-98-6 |
| Silver nitrate (ACS reagent, ≥99.0%) | Sigma-Aldrich | 209139 | CAS Number 7761-88-8 |
| Sodium borohydride (fine granular for synthesis) | Sigma-Aldrich | 8.06373 | CAS Number 16940-66-2 |
| Zirconium (IV) oxide (nanopowder, <100 nm particle size (TEM)) | Sigma-Aldrich | 544760 | CAS Number 1314-23-4 |
References
- Dunn, P. J., Hii, K. K., Krische, M. J., Williams, M. T. Sustainable Catalysis: Challenges and Pratices for the Pharmaceutical and Fine Chemical Industries. , Wiley-Blackwell. (2013).
- Tzouras, N. V., Stamatopoulos, I. K., Papastavrou, A. T., Liori, A. A., Vougioukalakis, G. C. Sustainable metal catalysis in C-H activation. Coordination Chemistry Reviews. 343, 25(2017).
- Polshettiwar, V., Varma, R. S. Green chemistry by nano-catalysis. Green Chemistry. 12 (5), 743(2010).
- Rodrigues, T. S., da Silva, A. G. M., Camargo, P. H. C. Nanocatalysis by noble metal nanoparticles: controlled synthesis for the optimization and understanding of activities. Journal of Materials Chemistry A. 7 (11), 5857-5874 (2019).
- Linic, S., Christopher, P., Ingram, D. B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nature Materials. 10 (12), 911-921 (2011).
- Nam, J. M., Liz-Marzán, L., Halas, N. Chemical Nanoplasmonics: Emerging Interdisciplinary Research Field at Crossroads between Nanoscale Chemistry and Plasmonics. Accounts of Chemical Research. 52 (11), 2995-2996 (2019).
- Brongersma, M. L., Halas, N. J., Nordlander, P. Plasmon-induced hot carrier science and technology. Nature Nanotechnology. 10 (1), 25-34 (2015).
- Smith, J. G., Faucheaux, J. A., Jain, P. K. Plasmon resonances for solar energy harvesting: A mechanistic outlook. Nano Today. 10 (1), 67-80 (2015).
- Hartland, G. V. Optical studies of dynamics in noble metal nanostructures. Chemical Reviews. 111 (6), 3858-3887 (2011).
- Kelly, K. L., Coronado, E., Zhao, L. L., Schatz, G. C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. Journal of Physical Chemistry B. 107 (3), 668-677 (2003).
- Hermoso, W., et al. Triangular metal nanoprisms of Ag, Au, and Cu: Modeling the influence of size, composition, and excitation wavelength on the optical properties. Chemical Physics. 423, 142-150 (2013).
- Kumar, A., et al. Rational Design and Development of Lanthanide-Doped NaYF4@CdS-Au-RGO as Quaternary Plasmonic Photocatalysts for Harnessing Visible-Near-Infrared Broadband Spectrum. ACS Applied Materials and Interfaces. 10 (18), 15565-15581 (2018).
- Reddy, K. L., Kumar, S., Kumar, A., Krishnan, V. Wide spectrum photocatalytic activity in lanthanide-doped upconversion nanophosphors coated with porous TiO2 and Ag-Cu bimetallic nanoparticles. Journal of Hazardous Materials. 367, 694-705 (2019).
- Ingram, D. B., Linic, S. Water splitting on composite plasmonic-metal/semiconductor photoelectrodes: Evidence for selective plasmon-induced formation of charge carriers near the semiconductor surface. Journal of the American Chemical Society. 133 (14), 5202-5205 (2011).
- Linic, S., Aslam, U., Boerigter, C., Morabito, M. Photochemical transformations on plasmonic metal nanoparticles. Nature Materials. 14 (6), 567-576 (2015).
- Aslam, U., Rao, V. G., Chavez, S., Linic, S. Catalytic conversion of solar to chemical energy on plasmonic metal nanostructures. Nature Catalyst. 1, 656-665 (2018).
- Araujo, T. P., Quiroz, J., Barbosa, E. C. M., Camargo, P. H. C. Understanding plasmonic catalysis with controlled nanomaterials based on catalytic and plasmonic metals. Current Opinion in Colloid and Interface Science. 39, 110-122 (2019).
- Gellé, A., et al. Applications of plasmon-enhanced nanocatalysis to organic transformations. Chemical Reviews. , 986-1041 (2020).
- Shaik, F., Peer, I., Jain, P. K., Amirav, L. Plasmon-Enhanced Multicarrier Photocatalysis. Nano Letters. 18 (7), 4370-4376 (2018).
- Quiroz, J., et al. Controlling Reaction Selectivity over Hybrid Plasmonic Nanocatalysts. Nano Letters. 18, 7289-7297 (2018).
- Peiris, E., et al. Plasmonic Switching of the Reaction Pathway: Visible-Light Irradiation Varies the Reactant Concentration at the Solid-Solution Interface of a Gold-Cobalt Catalyst. Angewandte Chemie - International Edition. 58 (35), 12032-12036 (2019).
- Yu, S., Wilson, A. J., Heo, J., Jain, P. K. Plasmonic Control of Multi-Electron Transfer and C-C Coupling in Visible-Light-Driven CO2 Reduction on Au Nanoparticles. Nano Letters. 18 (4), 2189-2194 (2018).
- Yu, S., Jain, P. K. Plasmonic photosynthesis of C 1 -C 3 hydrocarbons from carbon dioxide assisted by an ionic liquid. Nature Communications. 10, 2022(2019).
- Zhang, X., et al. Product selectivity in plasmonic photocatalysis for carbon dioxide hydrogenation. Nature Communications. 8, 1-9 (2017).
- Cortés, E. Efficiency and Bond Selectivity in Plasmon-Induced Photochemistry. Advanced Optical Materials. 5 (15), 1700191(2017).
- de Freitas, I. C., et al. Design-controlled synthesis of IrO 2 sub-monolayers on Au nanoflowers: marrying plasmonic and electrocatalytic properties. Nanoscale. , 23-27 (2020).
- Zhang, C., et al. Al-Pd Nanodisk Heterodimers as Antenna-Reactor Photocatalysts. Nano Letters. 16 (10), 6677-6682 (2016).
- Zhou, L., et al. Light-driven methane dry reforming with single atomic site antenna-reactor plasmonic photocatalysts. Nature Energy. 5, 61-70 (2020).
- Swearer, D. F., et al. Heterometallic antenna-reactor complexes for photocatalysis. Proceedings of the National Academy of Sciences. 113 (32), 8916-8920 (2016).
- Peiris, S., Sarina, S., Han, C., Xiao, Q., Zhu, H. -Y. Silver and palladium alloy nanoparticle catalysts: reductive coupling of nitrobenzene through light irradiation. Dalton Transactions. 46 (32), 10665-10672 (2017).
- Rahm, J. M., et al. A Library of Late Transition Metal Alloy Dielectric Functions for Nanophotonic Applications. Advanced Functional Materials. 2002122, 02122(2020).
- Zhang, C., Chen, B. Q., Li, Z. Y., Xia, Y., Chen, Y. G. Surface Plasmon Resonance in Bimetallic Core-Shell Nanoparticles. Journal of Physical Chemistry C. 119 (29), 16836-16845 (2015).
- Liu, Z., Huang, Y., Xiao, Q., Zhu, H. Selective reduction of nitroaromatics to azoxy compounds on supported Ag-Cu alloy nanoparticles through visible light irradiation. Green Chemistry. 18 (3), 817-825 (2016).
- Chaiseeda, K., Nishimura, S., Ebitani, K. Gold nanoparticles supported on alumina as a catalyst for surface plasmon-enhanced selective reductions of nitrobenzene. ACS Omega. 2 (10), 7066-7070 (2017).
- Peiris, S., et al. Metal nanoparticle photocatalysts: emerging processes for green organic synthesis. Catalysis Science and Technology. 6 (2), 320-338 (2016).
- García-García, I., et al. Silver-Based Plasmonic Catalysts for Carbon Dioxide Reduction. ACS Sustainable Chemistry and Engineering. 8 (4), 1879-1887 (2020).
- Agrawal, A., Johns, R. W., Milliron, D. J. Control of Localized Surface Plasmon Resonances in Metal Oxide Nanocrystals. Annual Review of Materials Research. 47 (1), 1-31 (2017).
- Lounis, S. D., Runnerstrom, E. L., Llordés, A., Milliron, D. J. Defect chemistry and Plasmon physics of colloidal metal oxide Nanocrystals. Journal of Physical Chemistry Letters. 5 (9), 1564-1574 (2014).
- Rej, S., et al. Determining Plasmonic Hot Electrons and Photothermal Effects during H2 Evolution with TiN-Pt Nanohybrids. ACS Catalysis. 10 (9), 5261-5271 (2020).
- Barragan, A. A., et al. Photochemistry of Plasmonic Titanium Nitride Nanocrystals. The Journal of Physical Chemistry C. 123 (35), 21796-21804 (2019).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved