A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Use of a Percutaneous Ventricular Assist Device/Left Atrium to Femoral Artery Bypass System for Cardiogenic Shock
In This Article
Summary
The following article describes the stepwise procedure for placement of a device (e.g., Tandemheart) in cardiogenic shock (CS) that is a percutaneous left ventricular assist device (pLVAD) and a left atrial to femoral artery bypass (LAFAB) system that bypasses and supports the left ventricle (LV) in CS.
Abstract
The left atrial to femoral artery bypass (LAFAB) system is a mechanical circulatory support (MCS) device used in cardiogenic shock (CS) that bypasses the left ventricle by draining blood from the left atrium (LA) and returning it to the systemic arterial circulation via the femoral artery. It can provide flows ranging from 2.5-5 L/min depending on the size of the cannula. Here, we discuss the mechanism of action of LAFAB, available clinical data, indications for its use in cardiogenic shock, steps of implantation, post-procedural care, and complications associated with the use of this device and their management.
We also provide a brief video of the procedural component of device therapy, including the pre-placement preparation, percutaneous placement of the device via transseptal puncture under echocardiographic guidance and the post-operative management of device parameters.
Introduction
Cardiogenic shock (CS) is a state of tissue hypoperfusion with or without concomitant hypotension, in which the heart is unable to deliver sufficient blood and oxygen to meet the body's demands, resulting in organ failure. It is classified into stages A to E by the Society of Cardiovascular Angiography and Interventions (SCAI): stage A - patients at risk for CS; stage B - patients at beginning stage of CS with hypotension or tachycardia without hypoperfusion; stage C - classic CS with cold and wet phenotype requiring inotropes/vasopressors or mechanical support to maintain perfusion; stage D - deteriorating on current medical or mechanical support requiring escalation to more advanced devices; and stage E - includes patients with circulatory collapse and refractory arrhythmias who are actively experiencing cardiac arrest with ongoing cardiopulmonary resuscitation1. The most common causes of CS are acute MI (AMI) representing 81% of cases in a recently reported analysis2, and acute decompensated heart failure (ADHF). CS is classically characterized by congestion and impaired perfusion, manifested by elevated filling pressures (pulmonary capillary wedge pressure [PCWP], left ventricular end-diastolic pressure [LVEDP], central venous pressure [CVP], and right ventricular end-diastolic pressure [RVEDP]), decreased cardiac output (CO), cardiac index (CI), cardiac power output (CPO), and end-organ malfunction3. In the past, the only available treatments for AMI complicated by CS were early revascularization and medical management with inotropes and/or vasopressors4. More recently, with the advent of mechanical circulatory support (MCS) devices and the recognition that escalation of vasopressors is associated with increased mortality, there has been a paradigm shift in the treatment of both AMI and ADHF related CS5,6.
In the current era of percutaneous ventricular assist devices (pVAD), there are a number of MCS device platforms/configurations available, which provide univentricular or biventricular circulatory and ventricular support with and without oxygenation capability7. Despite steady increases in the use of pVADs to treat both AMI and ADHF CS, mortality rates have remained largely unchanged5. With emerging evidence for possible clinical benefits to early unloading of the left ventricle (LV) in AMI8 and early use of MCS in AMI CS9, the use of MCS continues to increase.
The Left Atrial to Femoral Artery Bypass (LAFAB) MCS device bypasses the LV by draining blood from the left atrium (LA) and returning it to the systemic arterial circulation via the femoral artery (Figure 1). It is supported by an external centrifugal pump that offers 2.5-5.0 liters per minute (L/m) flow (new generation pump, designated as LifeSPARC, capable of up to 8 L/m flow) depending on the size of the cannulas. Once the blood is extracted from the LA via the transseptal venous cannula, it passes through the external centrifugal pump which recirculates the blood back into the patient's body via the arterial cannula placed in the femoral artery.

Figure 1: LAFAB setup. Image courtesy of TandemLife, a wholly owned subsidiary of LivaNova US Inc. Please click here to view a larger version of this figure.
Protocol
This procedure and protocol have been approved by the institutional review board and the United States Food and Drug Administration (FDA).
1. Patient criteria
- Include patients with CS stage B and above as defined by the SCAI consensus statement1.
- Include as bridge to transplant or durable left ventricular assist system in stage D heart failure.
- Include as bridge to recovery in AMI complicated by CS.
- Exclude contra-indication to systemic anticoagulation.
- Exclude life-expectancy <6 months (active malignancy).
- Exclude if there is the presence of LA thrombus.
- Exclude if patients have peripheral vascular disease (PVD) with small arteries that cannot accommodate the large cannulas.
- Exclude if patients have an irreversible neurological injury/coma.
- Exclude if patients have severe aortic insufficiency (AI).
- Exclude if patients have a ventricular septal defect (VSD).
NOTE: The placement of the LAFAB device includes three separate processes: 1) setting up the controller and the pump; 2) placement of arterial and venous cannulas and transeptal access under transesophageal echocardiogram (TEE) or intracardiac echocardiogram (ICE); and 3) connecting the system to the circuit.
2. Placement of the left atrium to femoral artery bypass device
- Setting up the controller
NOTE: This step can be performed as the patient is being transported to the lab and the table is being set up for the procedure. Usually, the device representative and perfusionist team is present to assist with the process.- Open the box and initiate the controller setup with the following steps.
- Prior to powering on the controller, place 2 batteries in the controller by opening the battery door. Insert each battery by placing the logo facing away from the controller screen. The groove on the battery should align with the key battery housing. Make sure that the batteries are well seated.
- Install the other 2 batteries in the dock following the same process.
- Attach the controller to the dock, verify it is fully seated on the dock and connect the power cord to the power outlet and plug it in to the wall socket for AC power. The controller can operate in the dock connected to AC power or when separated from the dock, it can operate using battery power.
- Mount the dock and controller on an intravenous pole using the clamp.
- Turn on the controller using the buttons on the side.
- Setting up the pump – Priming the system
NOTE: Pump priming requires two people — the primary operator is scrubbed (sterile operator) and stays in the sterile field. The secondary operator (non-sterile operator) handles the controller in the non-sterile field. Pump de-airing is a crucial step and needs to be performed very carefully.- Have the secondary operator open the package and present the priming tray to the primary operator. Have the primary operator then open the sterile drape and lay out the components of the priming tray on the sterile table. The pump, oxygenator and green oxygen tubing are included in the tray.
- Have the primary operator hand the pump driveline to the secondary operator who then plugs it to the controller.
- Have the sterile operator remove the protective caps on the tubing and inserts the ends to the basin. The blue tubing is the inflow tubing which goes into the blue port. The red tubing is the outflow tubing which goes into the red port of the basin.
- Place the basin in the fill ready position tilted back and away from the blue port.
- Have the secondary operator fill the basin with 4 liters of saline.
- Have the primary operator lift the basin and tilt it back to prime ready position to gravity prime the pump.
- Make sure that all the air has been removed from the tubing and the pump. Gently tap the tubing and the pump to remove any small air bubbles. This is a crucial step.
- Then turn on the pump from the non-sterile end.
- Remove any tiny air bubbles in the oxygenator by gently tapping the oxygenator and positioning the outflow tubing at a higher level (12 o’clock position) for the air bubbles to rise above and escape out.
- Stop the pump once all the air bubbles are removed.
- Clamp the inflow and outflow tubing. Remove the inflow and outflow tubing from the basin and then attach the green oxygen supply tubing to the gas in port on the oxygenator. The circuit is now ready.
- Transseptal access10,32
- Prepare and drape the patient in a sterile manner.
- Perform the procedure under general anesthesia with the anesthesia team.
- Once the patient is intubated and sedated adequately, pass the TEE probe into the esophagus and obtain the basic images. If using ICE, then obtain the images after venous access.
- Identify the ideal spot for septostomy on the inter-atrial septum (IAS) using TEE or ICE. Use the bicaval view on TEE showing the membranous part of the IAS at the region of the fossa ovalis to expose the IAS better.
- Confirm the absence of any thrombus in the LA where the inflow cannula will be positioned using TEE or ICE.
- Obtain femoral venous access via ultrasound guidance with modified Seldinger technique and insert an 0.035” guidewire
- Advance the guidewire to the inferior vena cava (IVC) – right atrial junction and then direct it towards the IAS under fluoroscopic and TEE or ICE guidance. Using multiple angiographic projections (Right or Left Anterior Oblique), identify the optimal site for transeptal puncture. Ideally, it should be done in the region of the foramen ovale to minimize complications. In patients with thick or aneurysmal septa or an IAS which has been previously patched or instrumented surgically or closed percutaneously, one may consider use of an energized or radiofrequency transseptal needle to insure precise puncture without needle deflection.
- Anticoagulate the patient (ACT more than 250 seconds). Perform transseptal puncture using a transseptal needle, insert guidewire to the LA.
- Dilate the venous access and the IAS with a 2-stage dilator. Insert the transseptal cannula and advance it into LA, remove introducer and guidewire, wait for back-bleed, and clamp. Secure cannula to the patient.
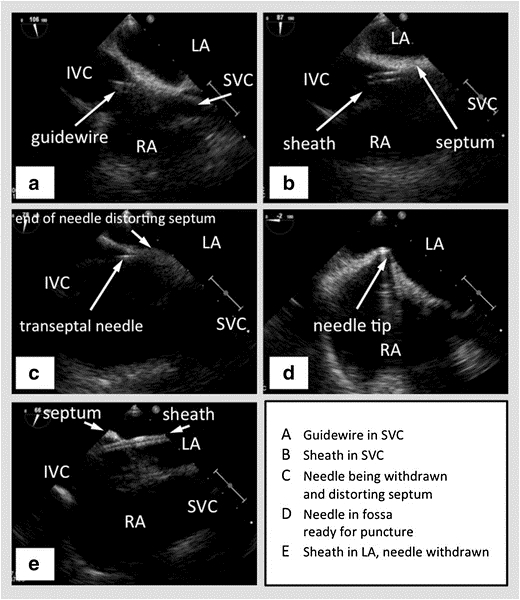
Figure 2: TEE with biplane in the bicaval view showing the SVC to the right, the interatrial septum horizontal in the middle with the left atrium above and the right atrium below, and the IVC towards the left. (A) - Guidewire passing into the SVC. (B) - Sheath passing over the wire into the SVC. (C) - Transseptal needle passing through the sheath. (D) - Transseptal needle tenting the interatrial septum. (E) - Sheath passing through the interatrial septum into the left atrium, after the needle has been withdrawn. Picture courtesy47
SVC – Superior Vena Cava, IVC – Inferior Vena Cava, RA – Right Atrium, LA – Left Atrium
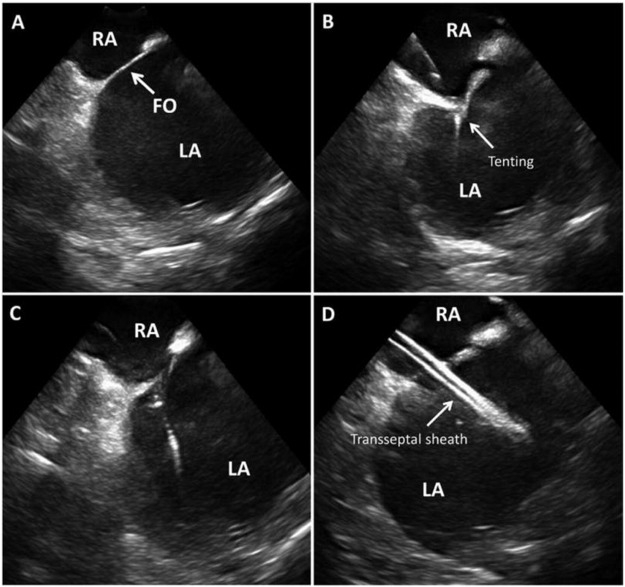
Figure 3: ICE for transeptal access ICE guided trans septal access showing inter-atrial septum and fossa ovalis (FO) in (A), septal tenting as the needle engages in (B), loss of tenting as the needle crosses in (C), transseptal sheath in the left atrium in D. Picture courtesy48 .
RA – Right Atrium, LA – Left Atrium, FO – Foramen Ovale
- Arterial access
- Obtain femoral arterial access via the modified Seldinger technique using ultrasound and angiographic guidance at the level of the femoral head. Insert an 0.035” guidewire.
- Consider use of “pre-closure” technique using the variously commercially available vascular access closure devices prior to upsizing arterial access.
- Serially dilate the arterial access site appropriate to the size of the arterial cannula selected. Insert the arterial cannula, remove the introducer and guidewire, wait for back-bleed, and then clamp. Secure the cannula to the patient using the holders.
- Connecting the components
- Make wet-to-wet connections to the cannulas to avoid introduction of any air bubbles in the circuit. This is a crucial step.
- Use saline immersion or constant infusion of saline (“waterfall”) over the two ends of the cannulas as they are being connected.
- Connect the transseptal cannula (venous) to the pump inlet which is marked in blue and the arterial cannula to the pump outlet which is marked in red.
- First, remove the venous clamps and start the pump (from the controller box). Then release the other clamps sequentially, constantly checking for any air, releasing the arterial clamp last.
- Adjust pump speed (by adjusting the RPMs) to optimize flow. Confirm the position of the cannula under fluoroscopy and TEE or ICE and secure the circuit to patient.
- Maintain therapeutic anticoagulation (activated clotting time ACT at 180–220s or activated partial thromboplastin time aPTT at 65–80s) for as long as the pump is in place to prevent pump thrombosis and stroke. This is a crucial step.
3. Right Atrium to Pulmonary Artery Bypass (RAPAB) system placement
- Initiate the controller, prime the lower chamber of the pump and check for any air bubbles - same steps described as above.
- Prime the upper chamber of the pump, check for any air and clamp it - same steps described as above.
- Patient procedure
- Obtain venous access at the right internal jugular (RIJ) vein via modified Seldinger technique under ultrasound guidance.
- Insert a Pulmonary Artery (PA) Catheter with a 0.035" lumen and advance it to the main PA just before the bifurcation. Insert a stiff-bodied 0.035" guidewire and remove the PA Catheter.
- Anticoagulate the patient (ACT > 250 s).
- Dilate the venous access site sequentially using the stepwise dilators provided in the package until the desired size is reached (29 French or 31 French).
- Insert the venous cannula (e.g., ProtekDuo) over the guidewire.
- Remove the guidewire, wait for back-bleed, and then clamp the distal port which is marked as "Distal."
- Remove the hemostasis cap, wait for back-bleed, and clamp the proximal port which is marked as "Proximal." Secure the cannula to patient via sutures.
- Verify the cannulas and make wet-to-wet connections from the pump to the cannula.
- Connect the proximal cannula to the pump inlet which is marked in blue and the distal cannula to the pump outlet which is marked in red. Turn on the pump (from the controller).
- Release clamps sequentially, constantly checking for any air bubbles. Adjust pump speed (by controlling the RPMs) to optimize flow.
- Confirm cannula position under fluoroscopy (may use TEE guidance to confirm position in the main PA) and secure the circuit to patient. Maintain therapeutic anticoagulation (ACT at 180-220 s; aPTT at 65-80 s).
4. Device removal
NOTE: Once the patient's end organ function has improved and hemodynamics have stayed stable with either LV recovery or advanced therapies such as durable LVAD placement/transplant, the device can be removed.
- Before removing the device, slowly turn down the speed by 0.5 L/min in a stepwise manner, carefully observing the hemodynamics to make sure there is adequate CO and normal filling pressures with less support (also known as the ramp down or turn down study).
- Turn off the pump once the turndown study is successful.
- Make sure the ACT is < 150 seconds before removing the arterial cannula. Tighten the previously placed intravascular suture to occlude the arteriotomy site or manual pressure can be held for at least 40 minutes to ensure hemostasis.
- Withdraw the transseptal cannula into the IVC and remove it slowly from the femoral vein. Apply a figure of 8 suture to the venous site and additionally, hold manual pressure over the site to achieve hemostasis.
NOTE: The atrial septal defect (ASD) is usually small and is not closed routinely. - Post procedure, perform continuous monitoring of patient's hemodynamics and end organ function to ensure stability.
- Removal of RAPAB.
- Similar to the LAFAB removal, when the patient's end-organ function has stabilized with recovery or advanced therapies, slowly turn down the pump by 0.5 L/min, carefully observing the hemodynamics.
- Turn off the pump when turn-down study is successful.
- Once ACT is < 150 seconds, remove the venous cannula from the neck and place a figure of 8 suture to secure the puncture site. Hold manual pressure in addition to the suture to achieve complete hemostasis.
- Post removal, closely monitor patient's hemodynamics and end-organ function to ensure stability.
| Complication | Risk factors | Timing of occurrence | Precaution | Management |
| Cardiac perforation and tamponade | Inadvertent advancement of needle or dilator or sheath along the posterior free wall of left atrium. | During transseptal puncture, placement of inflow cannula | Accurate assessment of inter-atrial septum on TEE or ICE and optimizing the site and angle of transseptal puncture via angiography and echo. | Immediate pericardiocentesis to relieve tamponade. May need surgical intervention. |
| Acute limb ischemia distal to arterial cannulation | Small caliber vessels housing large cannulas, pre-existing peripheral arterial disease | Immediately post procedure | Peripheral angiogram prior to cannulation. | Placement of distal perfusion catheter, vascular surgery assistance in severe cases. |
| Hemolysis, retroperitoneal bleeding, vascular complications such as pseudoaneurysm formation. | Higher pump speeds, pump thrombosis, DIC, anticoagulation | Anytime on the pump | Optimize pump speed for every patient individually. Avoid supratherapeutic anticoagulation. | Reducing pump speed, maintaining therapeutic range of anticoagulation. |
| Optimal site of arterial access at the femoral head in the common femoral artery. | ||||
| Residual atrial septal defect | Multiple attempts for transseptal access | After decannulation | Hemodynamically significant defects can be closed percutaneously. |
Table 1: Complications of LAFAB device33.
Results
Clinical applications of LAFAB device
The technique and feasibility of a percutaneous trans-atrial left ventricular bypass system were first described in the 1960s by Dennis et al.11,12. However transseptal puncture was not initially widely adopted due to complications with the septostomy technique. Over the last decade, with advancements in the field of percutaneous interventions, operators have accumulated experience with atrial septostom...
Discussion
Hemodynamics of LAFAB device:
The hemodynamic profile of the LAFAB device is distinct from other pVADs. By draining blood directly from the LA and returning it to the femoral artery, the device bypasses the LV completely. In doing so, it reduces LV end diastolic volume and pressure, contributing to improved LV geometry, and thereby effecting a decrease in LV stroke work. However, by returning the blood back into the iliac artery/descending aorta, afterload increases. This...
Disclosures
Sandeep Nathan - Disclosures: Consultant, Abiomed, Getinge, CSI, Inc.
Alexander Truesdell - Disclosures: Consultant, Abiomed Inc.
Poonam Velagapudi - Disclosures: Advisory board for Womens’ Health Initiative, Abiomed
Acknowledgements
To the TandemHeart team at LifeSparc.
Materials
| Name | Company | Catalog Number | Comments |
| For LAFAB (TandemHeart) | |||
| Factory Supplied Equipment for circuit connections. | TandemLife | ||
| ProtekSolo 15 Fr or 17 Fr Arterial Cannula | TandemLife | ||
| ProtekSolo 62 cm or 72 cm Transseptal Cannula | TandemLife | ||
| TandemHeart Controller | TandemLife | For adjusting flows/RPM | |
| TandemHeart Pump | LifeSPARC | Centrifugal pump | |
| For RAPAB (ProtekDuo) | |||
| Factory Supplied Equipment to complete the circuit. | TandemLife | ||
| ProtekDuo 29 Fr or 31 Fr Dual Lumen Cannula | TandemLife | ||
| TandemHeart Controller | TandemLife | For adjusting flows/RPM | |
| TandemHeart Pump | LifeSPARC | Centrifugal pump |
References
- Baran, D. A., et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheterization and Cardiovascular Interventions. 94 (1), 29-37 (2019).
- Harjola, V. -. P., et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. European Journal of Heart Failure. 17 (5), 501-509 (2015).
- Furer, A., Wessler, J., Burkhoff, D. Hemodynamics of Cardiogenic Shock. Interventional Cardiology Clinics. 6 (3), 359-371 (2017).
- Hochman, J. S., et al. Cardiogenic shock complicating acute myocardial infarction--etiologies, management and outcome: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK. Journal of the American College of Cardiology. 36 (3), 1063-1070 (2000).
- Shah, M., et al. Trends in mechanical circulatory support use and hospital mortality among patients with acute myocardial infarction and non-infarction related cardiogenic shock in the United States. Clinical Research in Cardiology. 107 (4), 287-303 (2018).
- van Diepen, S., et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 136 (16), 232-268 (2017).
- Alkhouli, M., et al. Mechanical Circulatory Support in Patients with Cardiogenic Shock. Current Treatment Options in Cardiovascular Medicine. 22 (2), 4 (2020).
- Basir, M. B., et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheterization and Cardiovascular Interventions. 91 (3), 454-461 (2018).
- Basir, M. B., et al. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheterization and Cardiovascular Interventions. 93 (7), 1173-1183 (2019).
- Alkhouli, M., Rihal, C. S., Holmes, D. R. Transseptal Techniques for Emerging Structural Heart Interventions. JACC: Cardiovascular Interventions. 9 (24), 2465-2480 (2016).
- Dennis, C., et al. Clinical use of a cannula for left heart bypass without thoracotomy: experimental protection against fibrillation by left heart bypass. Annals of Surgery. 156 (4), 623-637 (1962).
- Dennis, C., et al. Left atrial cannulation without thoracotomy for total left heart bypass. Acta Chirurgica Scandinavica. 123, 267-279 (1962).
- Fonger, J. D., et al. Enhanced preservation of acutely ischemic myocardium with transseptal left ventricular assist. Annals of Thoracic Surgery. 57 (3), 570-575 (1994).
- Thiele, H., et al. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral arterial bypass assistance. Circulation. 104 (24), 2917-2922 (2001).
- Burkhoff, D., et al. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. American Heart Journal. 152 (3), 469 (2006).
- Thiele, H., et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. European Heart Journal. 26 (13), 1276-1283 (2005).
- Gregoric, I. D., et al. TandemHeart as a rescue therapy for patients with critical aortic valve stenosis. Annals of Thoracic Surgery. 88 (6), 1822-1826 (2009).
- Kar, B., et al. The percutaneous ventricular assist device in severe refractory cardiogenic shock. Journal of the American College of Cardiology. 57 (6), 688-696 (2011).
- Patel, C. B., Alexander, K. M., Rogers, J. G. Mechanical Circulatory Support for Advanced Heart Failure. Current Treatment Options in Cardiovascular Medicine. 12 (6), 549-565 (2010).
- Tempelhof, M. W., et al. Clinical experience and patient outcomes associated with the TandemHeart percutaneous transseptal assist device among a heterogeneous patient population. Asaio Journal. 57 (4), 254-261 (2011).
- Gregoric, I. D., et al. The TandemHeart as a bridge to a long-term axial-flow left ventricular assist device (bridge to bridge). Texas Heart Institute Journal. 35 (2), 125-129 (2008).
- Bruckner, B. A., et al. Clinical experience with the TandemHeart percutaneous ventricular assist device as a bridge to cardiac transplantation. Texas Heart Institute Journal. 35 (4), 447-450 (2008).
- Agarwal, R., et al. Successful treatment of acute left ventricular assist device thrombosis and cardiogenic shock with intraventricular thrombolysis and a tandem heart. Asaio Journal. 61 (1), 98-101 (2015).
- Vetrovec, G. W. Hemodynamic Support Devices for Shock and High-Risk PCI: When and Which One. Current Cardiology Reports. 19 (10), 100 (2017).
- Al-Husami, W., et al. Single-center experience with the TandemHeart percutaneous ventricular assist device to support patients undergoing high-risk percutaneous coronary intervention. Journal of Invasive Cardiology. 20 (6), 319-322 (2008).
- Vranckx, P., et al. Clinical introduction of the Tandemheart, a percutaneous left ventricular assist device, for circulatory support during high-risk percutaneous coronary intervention. International Journal of Cardiovascular Interventions. 5 (1), 35-39 (2003).
- Vranckx, P., et al. The TandemHeart, percutaneous transseptal left ventricular assist device: a safeguard in high-risk percutaneous coronary interventions. The six-year Rotterdam experience. Euro Intervention. 4 (3), 331-337 (2008).
- Vranckx, P., et al. Assisted circulation using the TandemHeart during very high-risk PCI of the unprotected left main coronary artery in patients declined for CABG. Catheterization and Cardiovascular Interventions. 74 (2), 302-310 (2009).
- Thomas, J. L., et al. Use of a percutaneous left ventricular assist device for high-risk cardiac interventions and cardiogenic shock. Journal of Invasive Cardiology. 22 (8), 360 (2010).
- Vranckx, P., et al. Assisted circulation using the Tandemhear , percutaneous transseptal left ventricular assist device, during percutaneous aortic valve implantation: the Rotterdam experience. Euro Intervention. 5 (4), 465-469 (2009).
- Pitsis, A. A., et al. Temporary assist device for postcardiotomy cardiac failure. The Annals of Thoracic Surgery. 77 (4), 1431-1433 (2004).
- Singh, G. D., Smith, T. W., Rogers, J. H. Targeted Transseptal Access for MitraClip Percutaneous Mitral Valve Repair. Interventional Cardiology Clinics. 5 (1), 55-69 (2016).
- Subramaniam, A. V., et al. Complications of Temporary Percutaneous Mechanical Circulatory Support for Cardiogenic Shock: An Appraisal of Contemporary Literature. Cardiology and Therapy. 8 (2), 211-228 (2019).
- Morley, D., et al. Hemodynamic effects of partial ventricular support in chronic heart failure: Results of simulation validated with in vivo data. The Journal of Thoracic and Cardiovascular Surgery. 133 (1), 21-28 (2007).
- Naidu, S. S. Novel Percutaneous Cardiac Assist Devices. Circulation. 123 (5), 533-543 (2011).
- Kapur, N. K., et al. Hemodynamic Effects of Left Atrial or Left Ventricular Cannulation for Acute Circulatory Support in a Bovine Model of Left Heart Injury. ASAIO Journal. 61 (3), 301-306 (2015).
- Smith, L., et al. Outcomes of patients with cardiogenic shock treated with TandemHeart percutaneous ventricular assist device: Importance of support indication and definitive therapies as determinants of prognosis. Catheterization and Cardiovascular Interventions. 92 (6), 1173-1181 (2018).
- Ergle, K., Parto, P., Krim, S. R. Percutaneous Ventricular Assist Devices: A Novel Approach in the Management of Patients With Acute Cardiogenic Shock. The Ochsner Journal. 16 (3), 243-249 (2016).
- Sultan, I., Kilic, A., Kilic, A.Short-Term Circulatory and Right Ventricle Support in Cardiogenic Shock: Extracorporeal Membrane Oxygenation, Tandem Heart, CentriMag, and Impella. Heart Failure Clinics. 14 (4), 579-583 (2018).
- Bermudez, C., et al. . Percutaneous right ventricular support: Initial experience from the tandemheart experiences and methods (THEME) registry. , (2018).
- Aggarwal, V., Einhorn, B. N., Cohen, H. A. Current status of percutaneous right ventricular assist devices: First-in-man use of a novel dual lumen cannula. Catheterization and Cardiovascular Interventions. 88 (3), 390-396 (2016).
- Kapur, N. K., et al. Mechanical circulatory support devices for acute right ventricular failure. Circulation. 136 (3), 314-326 (2017).
- Kapur, N. K., et al. Mechanical Circulatory Support for Right Ventricular Failure. JACC: Heart Failure. 1 (2), 127-134 (2013).
- Geller, B. J., Morrow, D. A., Sobieszczyk, P. Percutaneous Right Ventricular Assist Device for Massive Pulmonary Embolism. Circulation: Cardiovascular Interventions. 5 (6), 74-75 (2013).
- Bhama, J., et al. Initial Experience with a Percutaneous Dual Lumen Single Cannula Strategy for Temporary Right Ventricular Assist Device Support Following Durable LVAD Therapy. The Journal of Heart and Lung Transplantation. 35 (4), 323 (2013).
- O'Neill, B., et al. Right ventricular hemodynamic support with the PROTEKDuo Cannula. Initial experience from the tandemheart experiences and methods (THEME) registry category. Miscellaneous. , (2018).
- O’Brien, B., et al. Fluoroscopy-free AF ablation using transesophageal echocardiography and electroanatomical mapping technology. Journal of Interventional Cardiac Electrophysiology. 50 (3), 235-244 (2017).
- O’Brien, B., et al. Transseptal puncture — Review of anatomy, techniques, complications and challenges. International Journal of Cardiology. 233, 12-22 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved