A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Fast Grid Preparation for Time-Resolved Cryo-Electron Microscopy
In This Article
Summary
Here, we provide a detailed protocol for the use of a rapid grid making device for both fast grid-making and for rapid mixing and freezing to conduct time-resolved experiments.
Abstract
The field of cryo-electron microscopy (cryo-EM) is rapidly developing with new hardware and processing algorithms, producing higher resolution structures and information on more challenging systems. Sample preparation for cryo-EM is undergoing a similar revolution with new approaches being developed to supersede the traditional blotting systems. These include the use of piezo-electric dispensers, pin printing and direct spraying. As a result of these developments, the speed of grid preparation is going from seconds to milliseconds, providing new opportunities, especially in the field of time-resolved cryo-EM where proteins and substrates can be rapidly mixed before plunge freezing, trapping short lived intermediate states. Here we describe, in detail, a standard protocol for making grids on our in-house time-resolved EM device both for standard fast grid preparation and also for time-resolved experiments. The protocol requires a minimum of about 50 µL sample at concentrations of ≥ 2 mg/mL for the preparation of 4 grids. The delay between sample application and freezing can be as low as 10 ms. One limitation is increased ice thickness at faster speeds and compared to the blotting method. We hope this protocol will aid others in designing their own grid making devices and those interested in designing time-resolved experiments.
Introduction
Background
Recent developments in cryo-electron microscopy (cryo-EM) have enabled structural studies of increasingly complex systems at high resolution. With few exceptions, such studies have been limited to biological macromolecules at equilibrium1 or relatively slow reactions2. Many processes in vivo occur on a faster timescale (milliseconds) and there is increasing interest in time-resolved cryo-EM (TrEM) on these timescales3. However, conventional cryo-EM sample preparation by the blotting method is too slow for millisecond TrEM.
The blotting method has other limitations besides poor time resolution. Proteins and protein complexes can suffer from denaturation or preferred orientation on grids4. Reducing the exposure time to the air-water interface during sample preparation has been shown to mitigate preferred orientation and protein denaturation5,6. Thus, fast grid preparation not only enables millisecond TrEM but can also improve grid quality.
Currently, there are three different approaches to automated grid preparation. The first approach uses a pin or capillary that holds a small amount of sample. After establishing contact between the liquid and the grid surface, the sample is 'written' onto the grid7,8. The sample application process is relatively slow and takes a few seconds. An alternative approach uses controlled droplet generation by a piezo dispenser and self-wicking grids9. This allows faster dispense to freeze times, but is still limited by droplet and wicking speed (currently reaching 54 ms). The fastest approach so far is the direct spray approach, in which the sample is atomized in a spray nozzle and the small (~ 10 - 20 µm) and fast (> 5 m/s) droplets spread upon contact with the cryo-EM grid. The sample spray can be generated through different ways such as airblast atomizers, surface acoustic waves or ultrasonic humidifiers10,11,12,13. In our experience, the ice thickness with the direct spraying approach is greater but direct spraying enables dispense to freeze times < 10 ms.
This protocol describes step-by-step how a time-resolved EM device (TED) equipped with a microfluidic spray nozzle can be used to prepare grids on a fast timescale14,15. The device has been used to prepare grids with a minimum delay time of 6 ms between sample application and freezing and to rapidly mix and freeze two samples. The design of the TED is based on a previous version16 and is similar to other spray-based time-resolved cryo-EM devices17.
First, the four main parts of the TED setup are described. The core of the TED is the liquid handling unit, which is responsible for sample aspiration and dispensing. A pneumatic plunger moves the grid through the spray into the liquid ethane. Generation of the spray is achieved with microfluidic spray nozzles and freezing is done in a liquid ethane container, which are described briefly. Lastly, the additional features to control the grid environment, especially humidity, are highlighted. This is followed by detailed protocols for the operation of the device and for conducting TrEM experiments. Representative results are given for fast grid preparation and a simple TrEM experiment.
Experimental Setup
The liquid handling unit
The liquid handling system of the TED is formed by three syringe drive pumps ('pumps 1 - 3'), each equipped with a rotary valve (Figure 1). A power supply provides pumps 1 - 3 with 24 V DC. Communication with the control software (written in Visual Basic and C++) is via a RS232 interface to pump 1. Commands are distributed through the serial I/O expansion ports from pump 1 to pumps 2-3. Pumps 1-3 are equipped with glass syringes ('syringes 1-3', we use 250 µL/zero dead volume syringes here). Each valve has two positions, 'load' and 'dispense'. The 'load' position is used to aspirate sample into the syringe. A short piece (~ 3 - 4 cm) of 1/16" O.D., 0.01′′ I.D. FEP tubing is connected via ETFE/ETFE flangeless fittings to the 'load' position of valves 1-3. This short piece of tubing reaches into the sample reservoir (typically a 1.5 mL or 0.5 mL plastic tube). The 'dispense' position leads to the spray nozzle. Connection between the 'dispense' outlet and the spray nozzle is made by PE tubing (~ 20-30 cm length, 0.043" O.D., 0.015" I.D.), with a short piece of sleeve tubing (~ 0.5 cm) and ETFE/ETFE flangeless fittings.
The pneumatic plunger
The TED uses a pneumatic plunger to accelerate the grid and move it through the sample spray into the liquid ethane container. Negative pressure tweezers hold the grid, screwed into a home-built holder which is mounted to a dual rod pneumatic cylinder (Figure 2A).
Pressure is supplied from a large nitrogen gas cylinder (size W), equipped with a multistage regulator (0 - 10 bar, 'main pressure'). Flexible reinforced PVC tubing (12 mm O.D.) connects the regulator to a 12-port manifold where pressurized nitrogen is delivered to the nozzle and the pneumatic plunger. Gas flow through the nozzle is constant, regulated directly at the nitrogen cylinder (main pressure). The connection to the nozzle is made with PU tubing (4 mm O.D., 2.5 mm I.D.), a short piece of PE tubing (~ 8 cm length, 0.043" O.D., 0.015" I.D.) and appropriate connectors. Pressure on the pneumatic plunger is controlled through a solenoid valve. PU tubing (4 mm O.D., 2.5 mm I.D.) connects the solenoid valve with a regulator and the pneumatic plunger, to allow a reduced plunge pressure (≤ main pressure). The solenoid valve is computer controlled. A schematic overview of the setup is given in Figure 2B.
Note that with this setup the plunge pressure is always equal or smaller than the spray gas pressure (main pressure). However, the setup can easily be changed by incorporating a second regulator upstream of the spray nozzle to allow higher plunge speeds at low spray gas pressure. High pressures (>> 2 bar) can damage the PDMS spray nozzle.
CAUTION: This is a pressurized system and the 'main pressure' should always be < 7 bar.
Pressures between 0.5 and 2 bar are typically used for the pneumatic plunger and show an approximately linear relation between pressure and speed (at the vertical position of the spray). Plunge speeds are measured with an oscilloscope, connected in line with a slide potentiometer (10 kΩ) and in parallel with a 2 kΩ resistor (Figure 2C). A power supply provides the potentiometer with 9 V DC. While the approximate plunge speed is set prior to the experiment by setting the plunge pressure, the potentiometer gives a precise readout of the speed after the experiment.
Spray nozzles and liquid ethane container
The fabrication and operation of gas-dynamic virtual nozzles for spray-based sample delivery has been described elsewhere in detail15. As described above, the 'dispense' outlets of valves 1-3 are connected to the liquid inlets of the nozzle (Figure 3A). The pressurized spray gas is connected to the gas inlet of the nozzle. The inlets in the PDMS spray nozzles are such that 0.043" O.D. PE tubing can be used directly without the need for fittings. Our nozzle design contains a 'jet-in-jet' geometry for mixing of two samples, similar to the device described in ref.18. A schematic of the design is shown in Figure 3B, a microscopic image of a nozzle is shown in Figure 3C. The layout of the microfluidic device requires the use of three syringes to mix two samples. The spray nozzle is typically positioned at 1-1.5 cm distance from the grid (during sample application).
We use liquid ethane as a cryogen, in a liquid ethane/nitrogen container as used for the standard blotting method. Vertical positioning of the liquid ethane cup is achieved with a laboratory lifting platform.
Control of the spray and grid environment
The plunger and spray nozzle are contained within a custom built PMMA (acrylic glass) box with a double door (Figure 4A). High relative humidity inside the box is achieved by an air-humidification system at the back of the TED (Figure 4B). Air is supplied by a pump and fed into a first 10" canister (typically used for under sink water purification). The canister is filled with a low (~ 5-10 cm) level of water and also houses a humidifier unit. Mains power to the humidifier is controlled by a digital humidity/temperature controller and a humidity/temperature sensor located inside the acrylic glass box. The controller is set to turn off the pump when the relative humidity reaches ≥ 90 %. Humidified air from the first canister is pumped through a diffuser, immersed in water in a second 10" canister and then enters the acrylic glass box.
CAUTION: Because the sample is aerosolized in the spray nozzle, hazardous biological or chemical specimen are not suitable as samples.
The run sequence
The Run Script button in the control software initiates the run sequence. This sequence of commands can be pre-defined in a script file and altered through the software. The most important variables are explained here:
Spray speed: The spray speed determines the liquid flowrate used by the syringe pump. The flowrate can be calculated as follows: The syringe pump motors used here have a fixed step size. The full range of the pump is divided into 48,000 steps. The second important factor is the syringe volume. We typically use 250 µL syringes. The spray speed in the control software is set as number of steps/second. A spray speed of 1000 steps/second corresponds to:
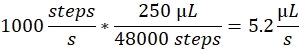
Spray volume: The spray volume determines the total volume to be sprayed. Thus, it also determines the duration of the spray. The spray volume in the control software is set as a number of steps. A spray volume of 2000 steps, at a spray speed of 1000 steps/second, leads to a spray duration of 2 s and a total volume of 10.4 µL.
Pre-spray time: This variable defines the time between initiation of the spray and plunge. It is important to choose the delay time such that the spray has sufficient time to stabilize before plunging the grid. Usually, the spray is given 1.5 - 4 s to stabilize before the grid is plunged. The spray is maintained until the grid has moved through. Usually, the liquid flow (and therefore the spray) is stopped 0.5 to 1 s after the grid has been plunged. Using a spray speed of 1000 steps/s and a spray volume of 2000 steps, a typical pre-spray time is 1.5 s, for example.
An exemplary sequence of commands is shown in Figure 5A, the grid position over time is illustrated in Figure 5B.
Protocol
1. Preparing the system
NOTE: The following protocol describes how to prepare grids of a single sample. Usually, a minimum of 2 replicate grids are prepared for each sample or condition. For faster plunge speeds (less than ~ 20 ms time delay), 3 or 4 replicate grids are typically prepared to account for a reduced number of thin ice areas.
- Dilute the protein sample to the target concentration in the desired buffer. Typically, final concentrations ≥ 2 mg/mL work well for grid preparation with the TED. Note that the sample can be kept on ice until step 10, from step 10 onwards the sample will spend considerable time at room temperature as it takes approximately 20 min to prepare 3-4 grids.
- Turn on the TED. Then turn on the control PC and start the control software.
- Initialize all syringe pumps by pressing the Initialize button (lower black button) on each syringe pump.
- Turn on the potentiometer power supply, set it to 9 V and start the oscilloscope control software.
- Ensure that the regulator valve of the N2-cylinder is closed. Open the cylinder valve. Then, slowly open the regulator valve setting the outlet pressure to the desired value for the spray gas pressure (typically 1-2 bar). For the PDMS nozzles used here, do not use pressures higher than ~ 2.5 bar to avoid damage to the nozzle. The continuously flowing gas prevents liquid from dripping and accumulating at the nozzle tip which can lead to irreproducible spraying.
- Ensure all syringe pump valves are in the 'load' position. This can be done by switching all valves to the 'dispense' position in the control software and then back to 'load'. Leave all valves switched to 'load'. Set all syringes to zero.
- Remove any air-bubbles present in the system at this stage. To do this, the syringes may have to be unscrewed, bubbles removed manually and the syringes mounted again when containing no more bubbles.
- The liquid handling components are usually stored in H2O. Before loading the sample solution, equilibrate the liquid system with buffer by washing the tubing with an excess of buffer. Use an appropriate maximum liquid flowrate to avoid overpressure on the spray nozzle. Liquid flowrates > 10 µL/s may cause damage to the PDMS nozzles described here.
- Place a 1.5 mL tube containing ≥ 200 µL buffer onto syringe 1 (the top must be pierced to attach to tubing).
- Make sure valve 1 is in 'load' position. Switch in the control software, if necessary.
- Aspirate the desired amount (typically 50-100 µL) of buffer with syringe 1, through the control software.
- Switch valve 1 to the 'dispense' position, through the control software.
- Dispense all of the liquid in syringe 1, through the control software.
- Return valve to 'load', reset the syringe position to '0' in the program and press initialize on syringe 1, to prepare the system for the next cycle.
NOTE: Steps 1.9.1 - 1.9.5 are usually repeated three times to ensure thorough washing of the tubing.
- Load tweezers with an EM grid (no requirement for any specific type) by loosening the plunging arm clamp, placing the tweezers in the clamp and tightening the clamp.
- Manually move the plunging arm so that grid and nozzle are at the same height (the 'sample application' position). If nozzle and grid are aligned, liquid will accumulate on the grid after spraying for an extended period. If necessary, adjust the nozzle position.
- Adjust the position of the liquid ethane cup by manually moving the plunging arm with mounted tweezers to its end position (reaching into the ethane cup). When set up correctly, a grid held by the tweezers will reach approximately the center of the liquid ethane cup.
- Check that the 'run script' will use the desired flowrates, volumes and timings. See section 'The run sequence' above for details.
- Make sure nothing is obstructing the path of the plunger. Aspirate the volume of buffer required for a single run into syringe 1. This is done in the control software, see section 'The run sequence' for typical setting and volumes. Then make sure valve 1 is switched to the 'dispense' position. Perform a test run by pressing Run Script in the control software.
CAUTION: Stay clear of the TED until the run sequence is finished. Moving parts could cause injury! - When the 'run sequence' is finished, set the pressure on the plunging arm to the desired value (typically 1-2 bar). Only then press OK in the control software to release pressure from the plunging arm. If the 'run sequence' or pressure on the plunging arm need to be adjusted, these settings may be changed at this stage and steps 1.13 - 1.15 repeated.
2. Fast Grid Preparation
- Fill the liquid ethane/nitrogen container first with liquid nitrogen. When sufficiently cold and free of liquid nitrogen, fill the cup with liquid ethane. Avoid solidification of the liquid ethane. This step is the same as for conventional grid preparation.
NOTE: To minimize ethane contamination, ensure that steps 2.2 - 2.13 are performed as quickly as possible
CAUTION: Liquid ethane is a cryogen and flammable. Care should be taken when handling. - Prepare cryo-EM grids for glow-discharge. We typically use holey carbon grids and glow-discharge for 90 s, at 0.1 mbar air pressure and 10 mA. Usually, no more than 4 grids are glow discharged at a time. The grids are used within 30 min of glow-discharging.
- Equilibrate the tubing with sample (following steps 1.9.1 - 1.9.5, using sample instead of buffer). If the available sample volume is low, the tubing may be equilibrated with only 1 dead volume.
- Aspirate the amount of sample that is needed for a single run into syringe 1 in the control software (see section 'The run sequence' for details). Then switch valve 1 to the 'dispense' position.
- Check that the relative humidity has reached the desired value (we typically prepare grids at 60 % relative humidity or higher). Once ≥ 60 % humidity is reached, open the humidity chamber only for a minimal amount of time, to maintain high humidity.
- Place the tweezers, holding a glow-discharged grid, in the pneumatic plunger and fix them. Move the plunger to its start position (at the top).
- Make sure the slider of the potentiometer is in the start position, ready for the measurement, by moving it manually to contact the plunger. Set the trigger on the oscilloscope in the oscilloscope software.
- Place the liquid ethane/nitrogen container.
- Press Run Script in the control software. When prompted, click OK in the control software to start the run.
CAUTION: Stay clear of the TED until the run sequence is finished. Moving parts could cause injury! - Once the 'run sequence' is completed, release the pressure on the pneumatic plunger by clicking OK in the control software.
- Open humidity chamber, loosen the connection between plunging arm and tweezers with one hand while securing the tweezers with the other. When the tweezers are free, move the plunging arm up while keeping the grid in the liquid ethane. Then transfer the grid to its storage space in the surrounding liquid N2. After freezing, the grid needs to be kept at liquid N2 temperature at all times.
- Save the oscilloscope measurement. Manually reset the position of the potentiometer slider and plunger afterwards.
- Repeat steps 2.4-2.12 to prepare replicate grids
- Transfer the grids into long-term storage until grid clipping and data collection.
- Wash the system with buffer, according to steps 1.9.1 - 1.9.5. Then wash the system with H2O, according to steps 1.9.1 - 1.9.5.
- Turn off the main nitrogen gas regulator and turn off the power.
- Place the ethane/nitrogen container in a fume hood to let it warm up and let the liquid ethane and N2 evaporate.
3. Time-resolved cryo-EM
NOTE: When time-resolved experiments are conducted with the TED, there are additional aspects to be considered, although the basic setup and variables remain the same. It is assumed here that two solutions are mixed in a 1:1 (v/v) ratio to produce the final mixture which is deposited on the grid. Follow the protocol described in '1. Fast grid preparation with the TED', with the following changes:
- Use higher stock concentrations for mixing experiments than for simple spray experiments. Mixing in a 1:1 (v/v) ratio will result in a 2x dilution of each component.
- For a rapid mixing experiment, use all three syringes rather than just a single one.
- Attach tubing to syringe pumps 2-3.
- Attach tubing from syringe pumps 2-3 to the spray nozzle.
- Equilibrate all three syringes in buffer and sample separately. Typically, syringe 1 is filled with sample A and syringes 2-3 are filled with sample B (Figure 3).
- Change the run sequence. An example for a run sequence using all 3 syringes for a rapid mixing experiment is given in Figure 6. See also section 'The run sequence' for details.
- Different time delays can be achieved in two ways:
- Change the plunger speed. By adjusting the plunger speed, the time delay can be changed in a relatively narrow range. For example, with a spray/ethane distance of 2 cm, the plunger can be moved at 1 m/s or 2 m/s to give a time delay of 20 ms or 10 ms, respectively. This is done as described in step 1.15.
- Change the spray/ethane position by adjusting the (vertical) position of the spray nozzle. If the nozzle is positioned at a 5 cm distance from the ethane surface, for example, a plunge speed of 1 m/s gives a time delay of 50 ms. Achieving significantly longer time delays requires further modifications to the setup.
NOTE: Due to laminar flow in the flow focussing region of the nozzle, we do not expect significant mixing in this part of the nozzle. Instead, we expect that mixing occurs during spray generation, in droplets en route to the grid and during droplet spreading on the grid. The time of flight for spray droplets to reach the grid is estimated ≤ 1 ms (for a droplet speed of ≥ 10 m/s and a nozzle-grid distance of 1 cm). Thus, only the time between droplet landing and vitrification is considered the 'time delay'.
Results
Fast grid preparation with the TED
As a test specimen for fast grid preparation, we have used apoferritin from equine spleen at 20 µM in 30 mM HEPES, 150 mM NaCl, pH 7.5. A reconstruction at 3.5 Å resolution was obtained from 690 micrographs as described in ref.15 (Figure 7A). The defocus range was chosen so that particles can easily be identified in the raw images (Figure 7B). A typical grid prepared with...
Discussion
The protocols in this work can be used for fast grid preparation by direct spraying and TrEM experiments. Fast grid preparation can be used to reduce particle interactions with the air water interface5. The main limitations are the available sample concentration and ice thickness on the grid. Within these limits and provided that the sample quality is good, the protocol produces grids suitable for high resolution cryo-EM.
Troubleshooting
Liquid fl...
Disclosures
None.
Acknowledgements
We would like to thank Molly S.C. Gravett for helpful discussions and the ABSL facility staff for help with cryo-EM data collection. David P. Klebl is a PhD student on the Wellcome Trust 4-year PhD program in The Astbury Centre funded by The University of Leeds. The FEI Titan Krios microscopes were funded by the University of Leeds (UoL ABSL award) and Wellcome Trust (108466/Z/15/Z). This work was funded by a BBSRC grant to Stephen P. Muench (BB/P026397/1) and supported by research grants to Howard D. White from the American Heart Association (AMR21-236078) and Howard D. White and Vitold Galkin from the U.S. National Institutes of Health (171261).
Materials
| Name | Company | Catalog Number | Comments |
| Time resolved device | |||
| acrylic glass box | USA scientific | ||
| digital humidity/temperature controller | THE20 digital humidity/temperature controller | ||
| dual rod pneumatic cylinder | dual rod pneumatic cylinder TN 10x70 | ||
| FEP tubing | Upchurch Scientific 1/16” O.D., 0.01'' I.D. FEP tubing | ||
| flangeless fittings | Upchurch Scientific ETFE/ETFE flangeless fittings | ||
| flexible reinforced PVC tubing | 12 mm OD. flexible reinforced PVC tubing | ||
| glass syringes | Kloehn 250 µL zero-dead volume | ||
| humidifier pump | Interpret Aqua Air AP3 | ||
| liquid ethane container | from Thermo/FEI VitrobotTM Mark IV | ||
| multistage regulator | GASARC class 3 multistage regulator | ||
| negative pressure tweezers | Dumont N5 Inox B negative pressure tweezers | ||
| oscilloscope | Hantek 6022BE oscilloscope | ||
| PE tubing | Scientific Commodities Inc. 0.043” O.D., 0.015” I.D. PE tubing | ||
| power supply | Mean Well GSM160A24-R7B | ||
| power supply | Wanptek KPS305D power supply | ||
| PU tubing | SMC TU0425 4 mm O.D., 2.5 mm I.D. PU tubing | ||
| regulator | Norgren R72G-2GK-RMN | ||
| slide potentiometer | PS100 slide potentiometer | ||
| solenoid valve | SMC NVJ314M solenoid valve | ||
| syringe drive pumps | Kloehn V6 48K model | ||
| Reagents & Materials | |||
| apoferritin from equine spleen | Sigma-Aldrich, A3660 | ||
| ATP | Sigma-Aldrich, A2383 | ||
| cryo-EM grids | Quantifoil 300 mesh Cu, R 1.2/1.3 | ||
| EGTA | Sigma Aldrich E3889 | ||
| F-actin | Provided by H.D. White (for preparation procedure, see ref. 1) | ||
| glow-discharger | Cressington 208 carbon coater with a glow-discharge unit | ||
| HEPES | Sigma-Aldrich, H7006 | ||
| KAc | Sigma-Aldrich, P1190 | ||
| MgCl2 | Sigma-Aldrich, M8266 | ||
| MOPS | Sigma-Aldrich, M1254 | ||
| NaCl | Sigma-Aldrich, S9888 | ||
| Skeletal muscle myosin S1 | Provided by H.D. White (for preparation procedure, see ref. 2) | ||
| Ref 1 | Spudich, J. A. & Watt, S. The regulation of rabbit skeletal muscle contraction I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. Journal of biological chemistry 246, 4866-4871 (1971). | ||
| Ref 2 | White, H. & Taylor, E. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry 15, 5818-5826 (1976). |
References
- Murphy, B. J., et al. Rotary substates of mitochondrial ATP synthase reveal the basis of flexible F1-Fo coupling. Science. 364, (2019).
- Benton, D. J., Gamblin, S. J., Rosenthal, P. B., Skehel, J. J. Structural transitions in influenza haemagglutinin at membrane fusion pH. Nature. , 1-4 (2020).
- Dance, A. Molecular motion on ice. Nature Methods. , 1-5 (2020).
- D'Imprima, E., et al. Protein denaturation at the air-water interface and how to prevent it. Elife. 8, 42747 (2019).
- Noble, A. J., et al. Reducing effects of particle adsorption to the air-water interface in cryo-EM. Nature Methods. 15, 793-795 (2018).
- Klebl, D. P., et al. Need for speed: Examining protein behaviour during cryoEM grid preparation at different timescales. BioRxiv. , (2020).
- Ravelli, R. B., et al. Cryo-EM structures from sub-nl volumes using pin-printing and jet vitrification. Nature Communications. 11, 1-9 (2020).
- Arnold, S. A., et al. Blotting-free and lossless cryo-electron microscopy grid preparation from nanoliter-sized protein samples and single-cell extracts. Journal of Structural Biology. 197, 220-226 (2017).
- Razinkov, I., et al. A new method for vitrifying samples for cryoEM. Journal of Structural Biology. 195, 190-198 (2016).
- Feng, X., et al. A fast and effective microfluidic spraying-plunging method for high-resolution single-particle cryo-EM. Structure. 25, 663-670 (2017).
- Ashtiani, D., et al. Delivery of femtolitre droplets using surface acoustic wave based atomisation for cryo-EM grid preparation. Journal of Structural Biology. 203, 94-101 (2018).
- Rubinstein, J. L., et al. Shake-it-off: a simple ultrasonic cryo-EM specimen-preparation device. Acta Crystallographica Section D: Structural Biology. 75, (2019).
- Mäeots, M. -. E., et al. Modular microfluidics enables kinetic insight from time-resolved cryo-EM. Nature Communications. 11, 1-14 (2020).
- Kontziampasis, D., et al. A cryo-EM grid preparation device for time-resolved structural studies. IUCrJ. 6, (2019).
- Klebl, D. P., et al. Sample deposition onto cryo-EM grids: from sprays to jets and back. Acta Crystallographica Section D: Structural Biology. 76, (2020).
- White, H., Thirumurugan, K., Walker, M., Trinick, J. A second generation apparatus for time-resolved electron cryo-microscopy using stepper motors and electrospray. Journal of Structural Biology. 144, 246-252 (2003).
- Kaledhonkar, S., Fu, Z., White, H., Frank, J. . Protein Complex Assembly. , 59-71 (2018).
- Trebbin, M., et al. Microfluidic liquid jet system with compatibility for atmospheric and high-vacuum conditions. Lab on a Chip. 14, 1733-1745 (2014).
- Klebl, D. P., Sobott, F., White, H. D., Muench, S. P. On-grid and in-flow mixing for time-resolved Cryo-EM. Acta Crystallographica Section D: Structural Biology. , (2021).
- He, S., Scheres, S. H. Helical reconstruction in RELION. Journal of Structural Biology. 198, 163-176 (2017).
- Millar, N. C., Geeves, M. A. The limiting rate of the ATP-mediated dissociation of actin from rabbit skeletal muscle myosin subfragment 1. FEBS Letters. 160, 141-148 (1983).
- Kasas, S., Dumas, G., Dietler, G., Catsicas, S., Adrian, M. Vitrification of cryoelectron microscopy specimens revealed by high-speed photographic imaging. Journal of Microscopy. 211, 48-53 (2003).
- Glaeser, R. M., et al. Defocus-dependent Thon-ring fading. bioRxiv. , (2020).
- Bagshaw, C. A beginner's guide to flow kinetics. The Biochemist. 42, (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved