A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Standardized Data Acquisition for Neuromelanin-Sensitive Magnetic Resonance Imaging of the Substantia Nigra
* These authors contributed equally
In This Article
Summary
This protocol shows how to acquire neuromelanin-sensitive magnetic resonance imaging data of the substantia nigra.
Abstract
The dopaminergic system plays a crucial role in healthy cognition (e.g., reward learning and uncertainty) and neuropsychiatric disorders (e.g., Parkinson's disease and schizophrenia). Neuromelanin is a byproduct of dopamine synthesis that accumulates in dopaminergic neurons of the substantia nigra. Neuromelanin-sensitive magnetic resonance imaging (NM-MRI) is a noninvasive method for measuring neuromelanin in those dopaminergic neurons, providing a direct measure of dopaminergic cell loss in the substantia nigra and a proxy measure of dopamine function. Although NM-MRI has been shown to be useful for studying various neuropsychiatric disorders, it is challenged by a limited field-of-view in the inferior-superior direction resulting in the potential loss of data from the accidental exclusion of part of the substantia nigra. In addition, the field is lacking a standardized protocol for the acquisition of NM-MRI data, a critical step in facilitating large-scale multisite studies and translation into the clinic. This protocol describes a step-by-step NM-MRI volume placement procedure and online quality control checks to ensure the acquisition of good-quality data covering the entire substantia nigra.
Introduction
Neuromelanin (NM) is a dark pigment found in dopaminergic neurons of the substantia nigra (SN) and noradrenergic neurons of the locus coeruleus (LC)1,2. NM is synthesized by the iron-dependent oxidation of cytosolic dopamine and norepinephrine and is stored in autophagic vacuoles in the soma3. It first appears in humans around 2-3 years of age and accumulates with age1,4,5.
Within the NM-containing vacuoles of SN and LC neurons, NM forms complexes with iron. These NM-iron complexes are paramagnetic, allowing for noninvasive visualization of NM using magnetic resonance imaging (MRI)6,7. MRI scans that can visualize NM are known as NM-sensitive MRI (NM-MRI) and use either direct or indirect magnetization transfer effects to provide contrast between regions with high NM concentration (e.g., the SN) and the surrounding white matter8,9.
Magnetization transfer contrast is the result of the interaction between macromolecular-bound water protons (which are saturated by the magnetization transfer pulses) and the surrounding free water protons. In NM-MRI, it is believed that the paramagnetic nature of NM-iron complexes shortens the T1 of the surrounding free water protons, resulting in reduced magnetization-transfer effects so that regions with higher NM concentration appear hyperintense on NM-MRI scans10. Conversely, the white matter surrounding the SN has a high macromolecular content, resulting in large magnetization-transfer effects so that these regions appear hypointense on NM-MRI scans, thus providing high contrast between the SN and surrounding white matter.
In the SN, NM-MRI can provide a marker of dopaminergic cell loss11 and dopamine system function12. These two processes are relevant for several neuropsychiatric disorders and are supported by a vast body of clinical and preclinical work. For example, abnormalities in dopamine function have been widely observed in schizophrenia; in vivo studies using positron emission tomography (PET) have shown increased striatal dopamine release13,14,15,16 and increased dopamine synthesis capacity17,18,19,20,21,22. Furthermore, post-mortem studies have shown that patients with schizophrenia have increased levels of tyrosine hydroxylase—the rate-limiting enzyme involved in dopamine synthesis—in the basal ganglia23 and SN24,25.
Several studies have investigated patterns of dopaminergic cell loss, particularly in Parkinson's disease. Post-mortem studies have revealed that the pigmented dopaminergic neurons of the SN are the primary site of neurodegeneration in Parkinson's disease26,27, and that, while SN cell loss in Parkinson's disease is not correlated with cell loss in normal aging28, it is correlated with the duration of the disease29. Unlike most methods for investigating the dopaminergic system, the non-invasiveness, cost-effectiveness, and lack of ionizing radiation make NM-MRI a versatile biomarker30.
The NM-MRI protocol described in this paper was developed to increase both within-subject and across-subject reproducibility of NM-MRI. This protocol ensures full coverage of the SN despite the limited coverage of NM-MRI scans in the inferior-superior direction. The protocol makes use of sagittal, coronal, and axial three-dimensional (3D) T1-weighted (T1w) images, and the steps should be followed to achieve proper slice stack placement. The protocol outlined in this paper has been utilized in multiple studies31,32 and was extensively tested. Wengler et al. completed a study of the reliability of this protocol in which NM-MRI images were acquired twice in each participant across multiple days32. Intra-class correlation coefficients demonstrated excellent test-retest reliability of this method for region of interest (ROI)-based and voxelwise analyses, as well as high contrast in the images.
Protocol
NOTE: The research conducted to develop this protocol was performed in compliance with New York State Psychiatric Institute Institutional Review Board guidelines (IRB #7655). One subject was scanned for recording the protocol video, and written informed consent was obtained. Refer to the Table of Materials for details about the MRI scanner used in this protocol.
1. MRI acquisition parameters
- Prepare to acquire high-resolution T1w images using a 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence with the following parameters: spatial resolution = 0.8 x 0.8 x 0.8 mm3; field-of-view (FOV) = 176 x 240 x 240 mm3; echo time (TE) = 3.43 ms; repetition time (TR) = 2462 ms; inversion time (TI) = 1060 ms; flip angle = 8°; in-plane parallel imaging factor (ARC) = 2; through-plane parallel imaging factor (ARC) = 233; bandwidth = 208 Hz/pixel; total acquisition time = 6 min 39 s.
- Prepare to acquire NM-MRI images using a two-dimensional (2D) gradient recalled echo sequence with magnetization transfer contrast (2D GRE-MTC) with the following parameters: resolution = 0.43 x 0.43 mm2; FOV = 220 x 220 mm2; slice-thickness = 1.5 mm; 20 slices; slice gap = 0 mm; TE = 4.8 ms; TR = 500 ms; flip angle = 40°; bandwidth = 122 Hz/pixel; MT frequency offset = 1.2 kHz; MT pulse duration = 8 ms; MT flip angle = 670°; number of averages = 5; total acquisition time = 10 min 4 s.
NOTE: Although the displayed results used these MRI acquisition parameters, this protocol is valid for various T1w and NM-MRI imaging protocols. The NM-MRI protocol should cover ~25 mm in the inferior-superior direction to guarantee complete coverage of the SN.
2. Placement of NM-MRI volume
- Acquire a high-resolution T1w image (≤1 mm isotropic voxel size). Use online reformatting directly after image acquisition to create high-resolution T1w images aligned to the anterior commissure-posterior commissure (AC-PC) line and the midline.
- Carry out online reformatting using the vendor-provided software (e.g., if acquiring data on a GE scanner: MultiPlanar Reconstruction (MPR) in Planning; if acquiring data on a Siemens scanner: MPR in the 3D Task Card; if acquiring data on a Philips scanner: MPR in the Render Mode of the VolumeView Package).
- Create multiplanar reconstructions of the 3D T1w image in the axial plane perpendicular to the AC-PC line to cover the whole brain with minimal slice-gap.
- Create multiplanar reconstructions of the 3D T1w image in the coronal plane perpendicular to the AC-PC line to cover the whole brain with minimal slice-gap.
- Create multiplanar reconstructions of the 3D T1w image in the sagittal plane parallel to the AC-PC line to cover the whole brain with minimal slice-gap.
- Carry out online reformatting using the vendor-provided software (e.g., if acquiring data on a GE scanner: MultiPlanar Reconstruction (MPR) in Planning; if acquiring data on a Siemens scanner: MPR in the 3D Task Card; if acquiring data on a Philips scanner: MPR in the Render Mode of the VolumeView Package).
- Load the sagittal, coronal, and axial views of the reformatted high-resolution T1w image and ensure that reference lines depicting the location of each displayed slice are present.
- Identify the sagittal image that shows the largest separation between the midbrain and thalamus (Figure 1A). To do this, visually inspect the sagittal slices of the reformatted T1w image until the slice showing this greatest separation is identified.
- Using the sagittal image from the end of step 2.3, visually identify the coronal plane that delineates the most anterior aspect of the midbrain (Figure 1B).
- Using the coronal image from the end of step 2.4, visually identify the axial plane that delineates the inferior aspect of the third ventricle (Figure 1C).
- On the sagittal image from the end of step 2.3, align the superior boundary of the NM-MRI volume to the axial plane identified in step 2.5 (Figure 1D).
- Move the superior boundary of the NM-MRI volume 3 mm in the superior direction (Figure 1E).
- Align the NM-MRI volume to the midline in the axial and coronal images (Figure 1F).
- Acquire the NM-MRI images.
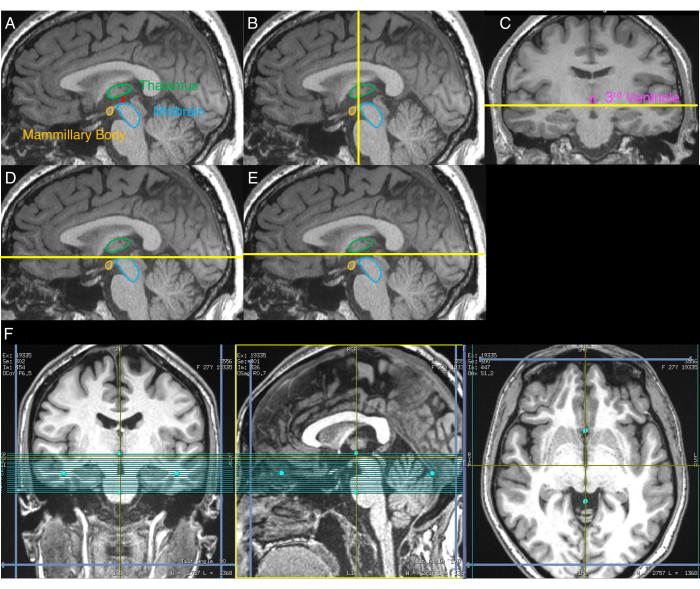
Figure 1: Images displaying the step-by-step NM-MRI volume placement procedure. Yellow lines indicate the location of the slices used for volume placement as described in the protocol. (A) First, the sagittal image with the greatest separation between the midbrain and thalamus is identified (step 2.3 of the protocol). (B) Second, using the image from A, the coronal plane delineating the most anterior aspect of the midbrain is identified (step 2.4). (C) Third, on the coronal image from the plane identified in B, the axial plane delineating the inferior aspect of the third ventricle is identified (step 2.5). (D) Fourth, the axial plane identified in C is displayed on the sagittal image from A (step 2.6). (E) Fifth, the axial plane from D is shifted 3 mm in the superior direction, and this plane indicates the superior boundary of the NM-MRI volume (step 2.7). (F) The final NM-MRI volume placement where the coronal image corresponds to C, the sagittal image corresponds to A, and the axial image corresponds to the axial plane in E. The NM-MRI volume is aligned to the brain midline in the coronal and axial images and the AC-PC line in the sagittal image (step 2.8). Part of this figure has been reprinted with permission from Elsevier from 30. Abbreviations: NM-MRI = neuromelanin-sensitive magnetic resonance imaging; AC-PC = anterior commissure-posterior commissure. Please click here to view a larger version of this figure.
3. Quality control checks
- Ensure that the acquired NM-MRI images cover the entire SN and that the SN is visible in the central images but not in the most superior or most inferior images of the NM-MRI volume. Otherwise (Figure 2), repeat steps 2.3-2.9 to ensure correct NM-MRI volume placement. If the participant has moved significantly since the acquisition of the high-resolution T1w scan, repeat steps 2.1-2.9.
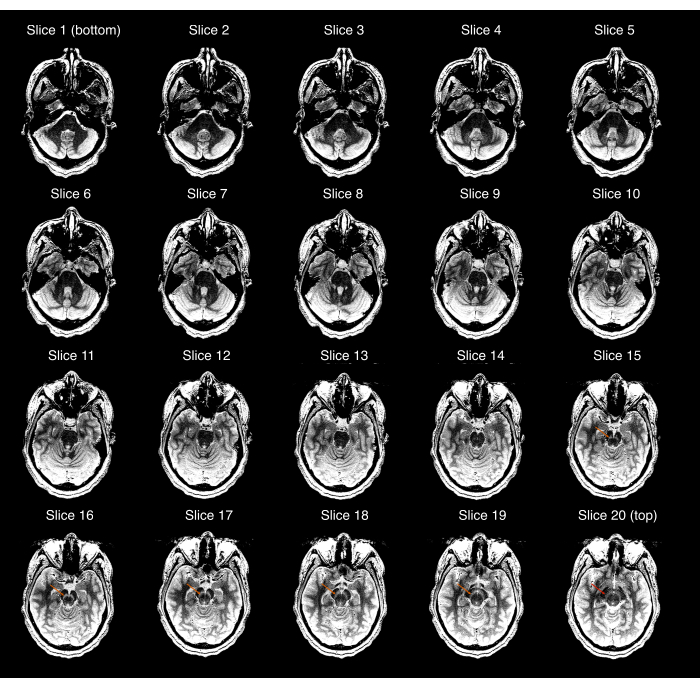
Figure 2: Example of an NM-MRI acquisition that failed the first quality control check (step 3.1 of the protocol). Each of the 20 NM-MRI slices displayed from most inferior (top left image) to most superior (bottom right image); the image window/level was set to exaggerate the contrast between the substantia nigra and crus cerebri. The orange arrows in slices 15-19 show the location of the substantia nigra in those slices. The red arrow in the most superior slice (slice 20) shows that the substantia nigra is still visible in this slice, and thus, the acquisition fails the quality check. Abbreviation: NM-MRI = neuromelanin-sensitive magnetic resonance imaging. Please click here to view a larger version of this figure.
- Check for artifacts, particularly ones that go through the SN and the surrounding white matter, by visually inspecting each slice of the acquired NM-MRI scan.
- Look for abrupt changes in signal intensity with a linear pattern that does not respect normal anatomical boundaries. For example, this may appear as a low-intensity region that is flanked by two high-intensity regions.
- If the artifact is the result of blood vessels (Figure 3A), retain the NM-MRI images because these artifacts will most likely always be present.
- If the artifacts are the result of participant head motion (Figure 3B), remind the participant to stay as still as possible and reacquire the NM-MRI images according to step 3.2.5.
- If the artifacts are ambiguous (Figure 3C), reacquire the NM-MRI images according to step 3.2.5. Upon reacquisition, if the artifacts remain present, proceed with these images as they are likely biological rather than a result of acquisition issues.
- If the NM-MRI images pass the quality control check in step 3.1, copy the previous NM-MRI volume placement. If the NM-MRI images fail the quality control check in step 3.1, repeat steps 2.3-2.9 to ensure correct NM-MRI volume placement (or steps 2.1-2.9 if the participant moved significantly).

Figure 3: Examples of NM-MRI acquisitions that failed the second quality control check (step 3.2 of the protocol). Only one representative slice is shown for each case. (A) An NM-MRI acquisition that fails the quality control check due to a blood vessel artifact (red arrows) that is the result of the blood vessel identified by the blue arrows. (B) An NM-MRI acquisition that fails the quality control check due to motion artifacts (red arrows). (C) An NM-MRI acquisition that fails the quality control check due to an ambiguous artifact (red arrows). Abbreviation: NM-MRI = neuromelanin-sensitive magnetic resonance imaging. Please click here to view a larger version of this figure.
Results
Figure 4 shows the representative results from a 28-year-old female participant with no psychiatric or neurological disorders. The NM-MRI protocol ensures complete coverage of the SN, achieved by following step 2 of the protocol outlined in Figure 1, and satisfactory NM-MRI images by following step 3 of the protocol. Excellent contrast between the SN and neighboring white matter regions with negligible NM concentration (i.e., crus cerebri) can be seen. Thes...
Discussion
The dopaminergic system plays a crucial role in healthy cognition and neuropsychiatric disorders. The development of noninvasive methods that can be used to repeatedly investigate the dopaminergic system in vivo is critical for the development of clinically meaningful biomarkers. The protocol described here supplies step-by-step instructions for acquiring good-quality NM-MRI images of the SN, including placement of the NM-MRI volume and quality control checks to ensure usable data.
Ev...
Disclosures
Drs Horga and Wengler each reported having patents for analysis and use of neuromelanin imaging in central nervous system disorders (WO2021034770A1, WO2020077098A1), licensed to Terran Biosciences, but have received no royalties.
Acknowledgements
Dr. Horga received support from the NIMH (R01-MH114965, R01-MH117323). Dr. Wengler received support from NIMH (F32-MH125540).
Materials
| Name | Company | Catalog Number | Comments |
| 3T Magnetic Resonance Imaging | General Electric | GE SIGNA Premier with 48-channel head coil |
References
- Zecca, L., et al. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proceedings of the National Academy of Sciences of the United States of America. 105 (45), 17567-17572 (2008).
- Zucca, F. A., et al. The neuromelanin of human substantia nigra: physiological and pathogenic aspects. Pigment Cell Research. 17 (6), 610-617 (2004).
- Sulzer, D., et al. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proceedings of the National Academy of Sciences of the United States of America. 97 (22), 11869-11874 (2000).
- Cowen, D. The melanoneurons of the human cerebellum (nucleus pigmentosus cerebellaris) and homologues in the monkey. Journal of Neuropathology & Experimental Neurology. 45 (3), 205-221 (1986).
- Zecca, L., et al. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson's disease. FEBS Letters. 510 (3), 216-220 (2002).
- Sulzer, D., et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson's disease. NPJ Parkinson's Disease. 4 (1), 11 (2018).
- Zucca, F. A., et al. Neuromelanin organelles are specialized autolysosomes that accumulate undegraded proteins and lipids in aging human brain and are likely involved in Parkinson's disease. NPJ Parkinson's Disease. 4 (1), 17 (2018).
- Chen, X., et al. Simultaneous imaging of locus coeruleus and substantia nigra with a quantitative neuromelanin MRI approach. Magnetic Resonance Imaging. 32 (10), 1301-1306 (2014).
- Sasaki, M., et al. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson's disease. Neuroreport. 17 (11), 1215-1218 (2006).
- Trujillo, P., et al. Contrast mechanisms associated with neuromelanin-MRI. Magnetic Resonance in Medicine. 78 (5), 1790-1800 (2017).
- Kitao, S., et al. Correlation between pathology and neuromelanin MR imaging in Parkinson's disease and dementia with Lewy bodies. Neuroradiology. 55 (8), 947-953 (2013).
- Cassidy, C. M., et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 116 (11), 5108-5117 (2019).
- Abi-Dargham, A., et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. American Journal of Psychiatry. 155 (6), 761-767 (1998).
- Laruelle, M., et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Sciences of the United States of America. 93 (17), 9235-9240 (1996).
- Breier, A., et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proceedings of the National Academy of Sciences of the United States of America. 94 (6), 2569-2574 (1997).
- Abi-Dargham, A., et al. Increased baseline occupancy of D-2 receptors by dopamine in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 97 (14), 8104-8109 (2000).
- Hietala, J., et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 346 (8983), 1130-1131 (1995).
- Lindström, L. H., et al. Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(β-11C) DOPA and PET. Biological Psychiatry. 46 (5), 681-688 (1999).
- Meyer-Lindenberg, A., et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nature Neuroscience. 5 (3), 267-271 (2002).
- McGowan, S., Lawrence, A. D., Sales, T., Quested, D., Grasby, P. Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F] fluorodopa study. Archives of General Psychiatry. 61 (2), 134-142 (2004).
- Bose, S. K., et al. Classification of schizophrenic patients and healthy controls using [18F] fluorodopa PET imaging. Schizophrenia Research. 106 (2-3), 148-155 (2008).
- Kegeles, L. S., et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Archives of General Psychiatry. 67 (3), 231-239 (2010).
- Toru, M., et al. Neurotransmitters, receptors and neuropeptides in post-mortem brains of chronic schizophrenic patients. Acta Psychiatrica Scandinavica. 78 (2), 121-137 (1988).
- Perez-Costas, E., Melendez-Ferro, M., Rice, M. W., Conley, R. R., Roberts, R. C. Dopamine pathology in schizophrenia: analysis of total and phosphorylated tyrosine hydroxylase in the substantia nigra. Frontiers in Psychiatry. 3, 31 (2012).
- Howes, O. D., et al. Midbrain dopamine function in schizophrenia and depression: a post-mortem and positron emission tomographic imaging study. Brain. 136 (11), 3242-3251 (2013).
- Bernheimer, H., Birkmayer, W., Hornykiewicz, O., Jellinger, K., Seitelberger, F. Brain dopamine and the syndromes of Parkinson and Huntington Clinical, morphological and neurochemical correlations. Journal of the Neurological Sciences. 20 (4), 415-455 (1973).
- Hirsch, E., Graybiel, A. M., Agid, Y. A. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 334 (6180), 345 (1988).
- Fearnley, J. M., Lees, A. J. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 114 (5), 2283-2301 (1991).
- Damier, P., Hirsch, E., Agid, Y., Graybiel, A. The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 122 (8), 1437-1448 (1999).
- Horga, G., Wengler, K., Cassidy, C. M. Neuromelanin-sensitive magnetic resonance imaging as a proxy marker for catecholamine function in psychiatry. JAMA Psychiatry. 78 (7), 788-789 (2021).
- Wengler, K., et al. Cross-scanner harmonization of neuromelanin-sensitive MRI for multisite studies. Journal of Magnetic Resonance Imaging. , (2021).
- Wengler, K., He, X., Abi-Dargham, A., Horga, G. Reproducibility assessment of neuromelanin-sensitive magnetic resonance imaging protocols for region-of-interest and voxelwise analyses. NeuroImage. 208, 116457 (2020).
- Griswold, M. A., et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic Resonance in Medicine. 47 (6), 1202-1210 (2002).
- vander Pluijm, M., et al. Reliability and reproducibility of neuromelanin-sensitive imaging of the substantia nigra: a comparison of three different sequences. Journal of Magnetic Resonance Imaging. 53 (5), 712-721 (2020).
- Cassidy, C. M., et al. Evidence for dopamine abnormalities in the substantia nigra in cocaine addiction revealed by neuromelanin-sensitive MRI. American Journal of Psychiatry. 177 (11), 1038-1047 (2020).
- Wengler, K., et al. Association between neuromelanin-sensitive MRI signal and psychomotor slowing in late-life depression. Neuropsychopharmacology. 46, 1233-1239 (2020).
- Biondetti, E., et al. Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson's disease. Brain. 143 (9), 2757-2770 (2020).
- Shibata, E., et al. Use of neuromelanin-sensitive MRI to distinguish schizophrenic and depressive patients and healthy individuals based on signal alterations in the substantia nigra and locus ceruleus. Biological Psychiatry. 64 (5), 401-406 (2008).
- Fabbri, M., et al. Substantia nigra neuromelanin as an imaging biomarker of disease progression in Parkinson's disease. Journal of Parkinson's Disease. 7 (3), 491-501 (2017).
- Matsuura, K., et al. Neuromelanin magnetic resonance imaging in Parkinson's disease and multiple system atrophy. European Neurology. 70 (1-2), 70-77 (2013).
- Watanabe, Y., et al. Neuromelanin magnetic resonance imaging reveals increased dopaminergic neuron activity in the substantia nigra of patients with schizophrenia. PLoS One. 9 (8), 104619 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved