Method Article
Implantation and Control of Wireless, Battery-free Systems for Peripheral Nerve Interfacing
In This Article
Summary
This is a protocol for the surgical implantation and operation of a wirelessly powered interface for peripheral nerves. We demonstrate the utility of this approach with examples from nerve stimulators placed on either the rat sciatic or phrenic nerve.
Abstract
Peripheral nerve interfaces are frequently used in experimental neuroscience and regenerative medicine for a wide variety of applications. Such interfaces can be sensors, actuators, or both. Traditional methods of peripheral nerve interfacing must either tether to an external system or rely on battery power that limits the time frame for operation. With recent developments of wireless, battery-free, and fully implantable peripheral nerve interfaces, a new class of devices can offer capabilities that match or exceed those of their wired or battery-powered precursors. This paper describes methods to (i) surgically implant and (ii) wirelessly power and control this system in adult rats. The sciatic and phrenic nerve models were selected as examples to highlight the versatility of this approach. The paper shows how the peripheral nerve interface can evoke compound muscle action potentials (CMAPs), deliver a therapeutic electrical stimulation protocol, and incorporate a conduit for the repair of peripheral nerve injury. Such devices offer expanded treatment options for single-dose or repeated dose therapeutic stimulation and can be adapted to a variety of nerve locations.
Introduction
Traumatic peripheral nerve injuries (PNIs) occur in the US with an annual incidence of approximately 200,000 per year1. Most patients who suffer PNIs are left with permanent functional impairments. At its worst, this may result in muscle paralysis and trigger treatment-refractory neuropathic pain so severe that patients are willing to undergo a limb amputation as treatment2. The biggest obstacle to improve PNI outcomes is that axon regeneration is too slow relative to the distances they must regrow. For example, an adult human axon grows at 1 mm/day but may have to regenerate over distances >1000 mm in the case of a lesion in a proximal limb.
In current clinical practice, ~50% of PNIs require surgical repair3. For successful nerve regeneration, axons must (i) grow across the lesion site (i.e., gap crossing) and then (ii) regenerate down the nerve pathway to reach an end-organ target (i.e., distal regrowth) (Figure 1). There are no FDA-approved drugs proven to accelerate nerve regeneration. The status quo of PNI clinical management has only changed incrementally over the past several decades and is limited to technical refinements to surgical methods such as distal motor nerve transfers to reduce the distance regenerating axons must travel4, or "off the shelf" synthetic nerve conduits for cases where the proximal nerve retracts and cannot be directly sutured back together5. However, there have been four randomized clinical trials on therapeutic electrical stimulation applied to nerves postoperatively, which were single-center studies led by Dr. K. Ming Chan at the University of Alberta that show significantly improved reinnervation of muscle6,7,8 or skin9. The foundational work for this electrical stimulation protocol was performed in rodents10,11, where it has been shown that electrical stimulation works specifically by enhancing gap crossing (Figure 1) but not distal regrowth12,13,14,15.
The surgical placement of transcutaneous wire electrodes used in all four electrical stimulation randomized clinical trials was necessary because its effects depend on the delivery of sufficient current to depolarize the neuron cell body at 20 Hz continuously for 1 h11. In clinical practice, this electrical stimulation protocol is not tolerable for most patients at the intensities required via surface-stimulating electrodes on the skin due to pain. There are non-trivial risks associated with running transcutaneous electrodes postoperatively, such as deep wound infection or accidental displacement of wires from the nerves during patient transport from the operating room (OR). Additionally, the high cost of OR time itself is a disincentive against attempting it in that setting rather than during acute postoperative recovery. A new class of wireless, battery-free, and fully implantable peripheral nerve interfaces is emerging to address this shortcoming of existing peripheral nerve interfaces.
This new class of wireless implantable electronic systems is poised to increase the ease and flexibility for electrical stimulation dosing and break down the barriers that preclude its broader clinical implementation. This paper describes methods to (i) surgically implant and (ii) wirelessly power and control this system in adult rat sciatic and phrenic nerve models. It shows how the peripheral nerve interface can evoke CMAPs, deliver a therapeutic electrical stimulation protocol, and even act as a conduit for the repair of peripheral nerves. The protocols here can be adapted for other variants of this technology that can deliver light pulses for optogenetic mediated neuromodulation16, controlled drug release17, or repeated bouts of electrical stimulation over time18,19.
Protocol
All procedures described in this protocol are carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of Northwestern University. This protocol follows the animal care guidelines of Northwestern University's Center for Comparative Medicine and IACUC. It is necessary to consult with IACUC when adapting the protocols.
1. Wireless electronic stimulator fabrication (Figure 2)
- Use copper/polyimide/copper (18 µm thick top and bottom copper, 75 µm thick polyimide) as a substrate for the radio frequency power harvester coil (i.e., wireless receiver antenna).
- Use direct laser ablation to pattern holes for the electrodes on the top and bottom copper layers and shape the device. Electrically connect the top and bottom layers using silver paste through the holes.
- Attach the electronic components with commercial packaging, such as diode and capacitor, via soldering.
- Use the bioresorbable dynamic covalent polyurethane (b-DCPU; 200 µm thick) encapsulated molybdenum (Mo; 15 µm thick; serpentine structure) as stretchable extension electrodes19.
- Form the cuff electrode for the interface between the device and the nerve using poly(lactic-co-glycolic acid) (PLGA) film (300 µm thick).
- After connecting the wireless receiver antenna and stretchable extension electrode, encapsulate the wireless receiver antenna and the connection with commercialized waterproof epoxy or polydimethysiloxane (PDMS). See Figure 2 (right) for the fully assembled device.
- Confirm the wireless operation of the device, using a waveform generator to generate monophasic electrical impulses via the primary coil (i.e., transmission coil).
NOTE: Examining the recruitment of peripheral axons and induction of axonal regeneration by monophasic and biphasic stimuli, prior studies reported a negligible effect due to the differences in waveform characteristic20, and this group has been able to achieve therapeutic electrical stimulation enhancement with the same monophasic current parameters in mice21 and rats18. Furthermore, prior studies examined biocompatibility in vivo and in vitro and did not find any evidence of tissue damage from heating effects or the materials themselves. Because of these findings and the limited duration of therapeutic electrical stimulation in the present study, monophasic, rather than biphasic, stimuli were used in this protocol. - Measure the resulting direct current output voltage with an oscilloscope connected to the cuff electrode.
2. Device preparation for implantation
- Place the implant devices into a sterile Petri dish and seal it with parafilm.
- Irradiate the devices with UV light for 30 min per side.
3. Surgical procedure of rat right sciatic nerve implantation of wireless, battery-free peripheral nerve interface for electrical stimulation (Figure 3)
NOTE: Maintain sterile conditions. Perform surgeries within the designated surgical area of an animal procedure room. The surgeon will don a facemask, coat, cap, and sterile gloves during surgery. If more than one surgery is performed, change sterile gloves between animals and use clean, sterile surgical instruments for each surgery. Sterilize tools between surgeries by heat sterilization (autoclave or glass bead sterilizer). Use adult Sprague-Dawley rats that weighing 200-250 g.
- Induce anesthesia using isoflurane gas anesthesia (3% induction, 1-3% maintenance) in oxygen (2 L/min), with subcutaneous administration of meloxicam (1-2 mg/kg). Cover the eyes of the rats with designated ophthalmic ointment to prevent drying.
- Place the rats in a prone position on disinfected surgical tables for subsequent procedures. For the remaining surgical duration, assess breathing rate (should be ~2/s), tissue color, and depth of anesthesia no less than every 15 min, and maintain isoflurane levels accordingly. Confirm the appropriate depth of anesthesia by checking pedal reflex (lack of response to a firm toe pinch). Monitor the mucous membranes, which should remain pink and moist.
- Shave the surgical area, including the right leg and lower half of the back. Scrub the shaved surgical area with a betadine pad, followed by a 70% medical ethanol swab, and repeat this scrub process three times for skin disinfection.
- Make a 1.5 – 2 cm incision in the skin parallel to the right femur bone using tissue scissors, followed by blunt separation of subcutaneous connective tissue on the back (directly medial to the incision) to clear a subcutaneous pocket for the receiver coil (Figure 4A). Make a subsequent incision (1.2-1.5 cm) on the right gluteal muscle parallel to the skin incision.
- Gently isolate the sciatic nerve with metal dissection probes with blunt ends (Figure 4B).
NOTE: The sciatic nerve is located deep to the biceps femoris and runs parallel to the femur. A dissecting scope is suggested. - Implant the wireless, battery-free device on the sciatic nerve (Figure 4C) by wrapping the cuff around the isolated right sciatic nerve, without putting the nerve under tension or distorting its path18,19,20. Mark out on the skin where the receiver coil is placed for further electrical stimulation.
- Suture the gluteal muscle incision using absorbable sutures (Figure 4D).
NOTE: The upper half of the receiver coil sits above the gluteal muscle and the cuff interface beneath it. - Close the skin incision with wound clips (or buried suture; Figure 4E). Match the skin edges.
- Deliver 1 h of continuous postsurgical 20 Hz electrical stimulation with 200 µs pulse width under anesthesia (Figure 4F). Return animals to their home cages after they have fully recovered from anesthesia.
NOTE: The detailed protocol is described below. The receiver coil is shown above the skin in Figure 4F. - Postsurgical treatment
- Place the rat in a recovery cage without bedding, lined with paper towels, with half the cage placed on an appropriate temperature-regulated heat source (approved heating pad).
- Monitor the rat carefully until it is ambulatory. Once ambulatory and assessed to be stable, return the rat to the home cage and watch for social reintegration.
- After acute recovery, monitor the rats for incision site infection and for symptoms of neurogenic pain, including but not limited to guarding, writhing, scratching, and self-mutilation. Monitor the rats daily for the 5-day postsurgical recovery period, and at least once every three days after that if the rats are not sacrificed at day 5.
- Administer meloxicam (1-2 mg/kg) subcutaneously once daily for two to three days after the surgery, depending on the animal's displayed level of pain/discomfort. If persistent pain is suspected, continue meloxicam beyond this period postoperatively, and if it proves refractory, euthanize the rat early in consultation with the veterinary team.
- Remove the skin sutures or wound clips 10-12 days post-surgery.
4. Surgical procedure of rat left phrenic nerve implantation of wireless stimulators (Figure 5A)
NOTE: Maintain sterile conditions, as in section 3. Use adult Sprague-Dawley rats weighing 200-250 g. Sterilize all surgical tools before use.
- Induce anesthesia using isoflurane gas anesthesia (3% induction, 1-3% maintenance) in oxygen (2 L/min), with subcutaneous administration of meloxicam (1-2 mg/kg). Cover the rat's eyes with designated ophthalmic ointment to prevent desiccation.
- Place the rats in a supine position on disinfected surgical tables for subsequent procedures. For the remaining surgical duration, assess breathing rate, tissue color, and depth of anesthesia no less than every 15 min, and maintain isoflurane levels accordingly. Confirm the appropriate depth of anesthesia by checking pedal reflex (lack of response to a firm toe pinch). Monitor the mucous membranes, which should remain pink and moist.
- Shave the surgical area on the ventral aspect of the neck. Scrub the shaved surgical area with a betadine pad, followed by a 70% medical ethanol swab, and repeat this scrub process three times for skin disinfection.
- Administer bupivacaine (2 mg/kg, diluted in saline not exceeding total volume 0.5mL) subcutaneously at midline on the neck, targeting the most superficial layer. Make a 3 cm midline incision through the skin and superficial cervical fascia to expose the sternohyoid and sternocleidomastoid muscles (Figure 5B).
NOTE: A dissecting scope is suggested. - Elevate the sternocleidomastoid using gentle blunt dissection with a probe and retract it laterally using a vessel loop (Figure 5C). Gently free and retract the omohyoid. Next, gently free and medially retract the vagus nerve and the carotid bundle beneath the omohyoid muscle.
NOTE: The major discrimination here is between the vagus nerve and the phrenic nerve. Cut the omohyoid if it is necessary to expose the beneath structures. - Isolate the phrenic nerve (Figure 5D).
NOTE: The phrenic nerve runs along the surface of the anterior scalene muscle, running as a notably small longitudinal nerve crossing perpendicularly to the brachial plexus. Unlike the sciatic nerve, the anatomy around the phrenic nerve at the neck is more complex. Perform electrophysiological confirmation (step 4.7) before implantation for best results. - Place the recording electrode subcutaneously, just caudally to the rib cage, ipsilateral to the isolated phrenic nerve (Figure 5E). Place the stimulators on the phrenic nerve and confirm via synchronous signaling (Figure 6).
NOTE: Evoking a maximal response with a stimulus intensity of ~3-6 mA and stimulus duration of 0.02 ms is typical. - Verify complete transection of the phrenic nerve by showing complete abolishment of the evoked response when an electrical stimulus is applied on the proximal nerve end relative to the transection site (Figure 6).
- Implant a wireless, battery-free device on the phrenic nerve (Figure 5F) by placing the receiver coil of the implantable device on the sternohyoid, deep relative to the bilateral sternocleidomastoid muscles, with the cuff around the phrenic nerve and the contact electrodes positioned perpendicular to the nerve.
- Close the superficial cervical fascia with simple running absorbable sutures (Figure 5G). Close the skin with interrupted inverted absorbable sutures in the deep dermis. Return the animals to their home cages only after they have fully recovered from anesthesia.
- For postsurgical treatment, follow step 3.10.
5. Wireless delivery of therapeutic electrical stimulation
- Apply electrical stimulation for 1 h to the rats under general anesthesia. For the wireless stimulation, place a waveform/function generator (voltage: 1-15 Vpp) and optional amplifier above the animal to provide electrical power to an external inductive coil (i.e., transmission coil) (two-dimensional spiral coil with 5 turns; diameter: 2 cm) to ensure good inductive coupling with the implanted receiver coil. Deliver monophasic, 200 µs pulses at 20 Hz for 1 h duration.
- To verify and quantify electrical stimulation delivery, record CMAPs from the tibial anterior muscle, adjusting the stimulation voltage to deliver supramaximal activation of the sciatic nerve. Use concentric needle electrodes for all recordings.
NOTE: If function-generated maximal voltage is insufficient to evoke a maximal response, use an amplifier.
6. Euthanasia
- Primary method
- Place the cage under a CO2 delivery chamber, set to a rate of 8-12 LPM (or appropriate flow rate based on the chamber size). Monitor the rats for unconsciousness and then for at least 1 min of cessation of breathing.
- Secondary method
- Perform cervical dislocation or bilateral thoracotomy.
Results
In the sciatic nerve injury model, the implant is placed around the right sciatic nerve prior to end-to-end repair of the tibial nerve branch (Figure 3, Figure 4A, and Figure 7A). A 30 G concentric needle electrode is placed in the right tibialis anterior muscle to define the stimulus parameters needed for maximal intensity electrical stimulation. These experiments include elevating the stimulation intensity until the response magnitude plateaus at the maximum. As the tibialis anterior is innervated by the fibular branch of the sciatic nerve, it is spared in the tibial nerve transection injury. Thus, recording from tibialis anterior enables continuous monitoring of the electrical stimulation treatment.
For a single-stimulus pulse delivered by a wire electrode to the right sciatic nerve (5 mA, 0.02 ms), a maximal CMAP response is elicited with a 5.4 mV negative peak amplitude recorded on ipsilateral tibialis anterior (Figure 7B; black trace). For a comparable stimulus pulse delivered by the wireless, battery-free implant, a comparable CMAP response is elicited with a 4.6 mV negative peak amplitude (Figure 7B; orange trace). This is consistent with a recent report that wireless nerve stimulation achieves on average 88% of the CMAP from wire-based nerve stimulation21, well above the threshold required for therapeutic effects in clinical studies6,7,8,9. In the example shown, the longer latency of the wireless stimulator vs. wired stimulator was due to its greater distance from the recorded muscle.
In the phrenic nerve model, the implant is placed around the right phrenic nerve prior to transection (Figure 5). To define the stimulus parameters needed for maximal intensity electrical stimulation, a 30 G concentric needle electrode is placed subcutaneously at the right (ipsilateral) anterior costal margin to record from the right hemidiaphragm. The experiments involve elevating the stimulation voltage till the response magnitude plateaus at its maximum. As the phrenic nerve can be challenging to isolate from surrounding neurovascular structures, its identity can be confirmed by evoking a twitch response (Figure 6; orange trace). The specificity of stimulation can be further verified by transection of the phrenic nerve distal to the nerve electrode cuff with subsequent abolishment of the twitch response (Figure 6; black trace).
Repetitive, low-frequency electrical stimulation therapy can be delivered to the sciatic nerve for 1 h using an established protocol that enhances axon regeneration (6,7,8,9,10,11; Figure 8). The cuff interface of the wireless implant was placed on the right sciatic nerve, and the 30 G concentric needle electrode was placed on the right tibialis anterior muscle to monitor the treatment. Figure 8A shows four sequential spikes in the recorded electromyography at the beginning (0 min) of the 1 h 20 Hz electrical stimulation. Figure 8B shows four other spikes recorded at the 40 min of the 1 h electrical stimulation with a slight decrease in peak amplitude, which is consistent with the fatigue pattern noted with wire-based electrical stimulation therapy15,21.
The degree of peripheral nerve regeneration can be assessed using retrograde tracers applied distally to the nerve lesion site. Because peripheral axons sprout multiple collateral sprouts, retrograde tracing and counts of the motor neuron soma in the spinal cord allow a more accurate assessment of the number of regenerating neurons than counting regenerating axons within the nerve itself31. To demonstrate this, the sciatic nerve trunk was transected by a crush injury. After 3 weeks of recovery, two different fluorescent retrograde dyes were administered on two branches of the sciatic nerve: fibular nerve (green) and tibial nerve (red), respectively (Figure 9A). Figure 9B-D show lit-up subgroups of lower motoneurons in the lumbar spinal cord anterior horn that form either the tibial nerve (Figure 9B) or the fibular nerve (Figure 9C). The overlay image shows two distinct columns of labeled neurons in the anterior horn of the spinal cord, which can be quantified in terms of spatial distribution and the count of motor neurons that have regenerated an axon distal to the lesion site (Figure 9D).
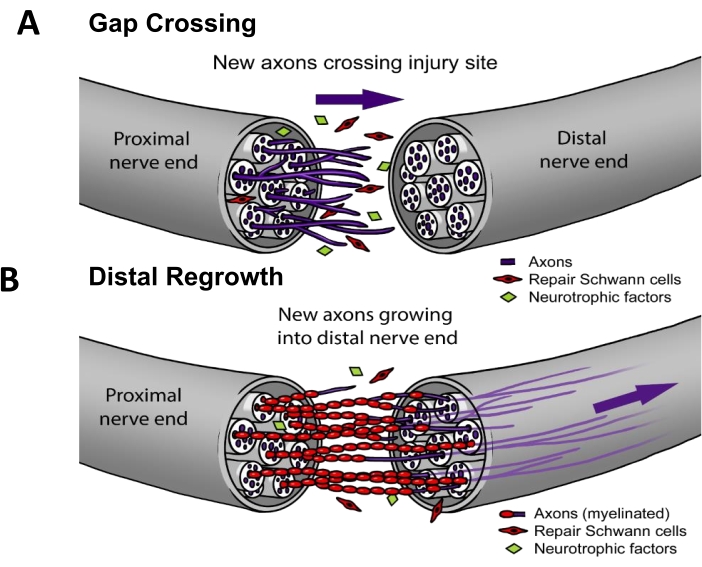
Figure 1: Nerve regeneration model. (A) Gap crossing occurs early after nerve repair when axons grow from proximal to distal nerve end after repair. (B) The duration of distal regrowth is related to the distance to the target end-organ (e.g., skin, muscle) and the rate of axon regrowth. Most therapies for improving nerve repair target one or both of these processes. Please click here to view a larger version of this figure.
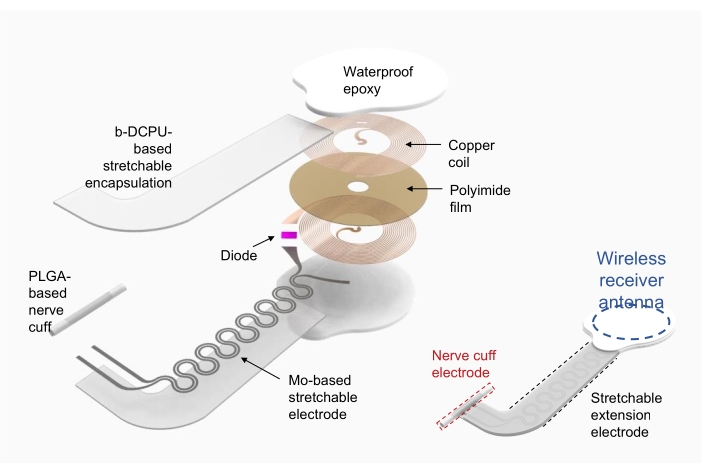
Figure 2: Illustration of a wireless electronic stimulator fabrication. Left, detailed layers of the structure of the device, including a circular radio frequency power harvester coil, a stretchable extension electrode, and a nerve cuff wrapping around a nerve of interest. Right, a simplified illustration showing three parts of the device. Abbreviations: PLGA = poly(lactic-co-glycolic acid); b-DCPU = bioresorbable dynamic covalent polyurethane. Please click here to view a larger version of this figure.
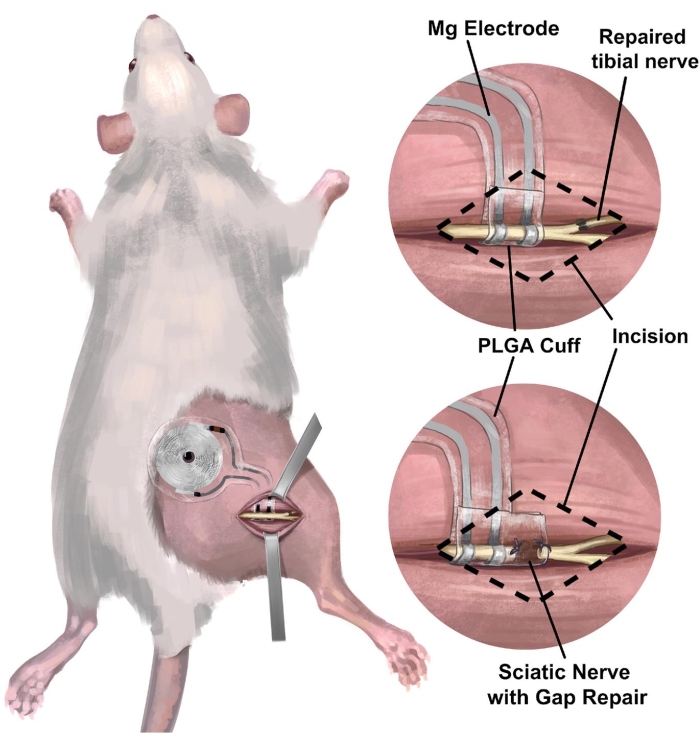
Figure 3: Implantation of wireless, battery-free nerve interface in the rat sciatic nerve model. (A) The illustration depicts a fully implantable system in the right sciatic nerve of a rat. (B) The top panel shows an electrode interface positioned on the sciatic nerve just proximal to the end-to-end repair of the right tibial nerve. The bottom panel shows an electrode interface with an extended nerve cuff bridging gap repair between the proximal end and the distal nerve stump. Abbreviation: PLGA = poly(lactic-co-glycolic acid). Please click here to view a larger version of this figure.
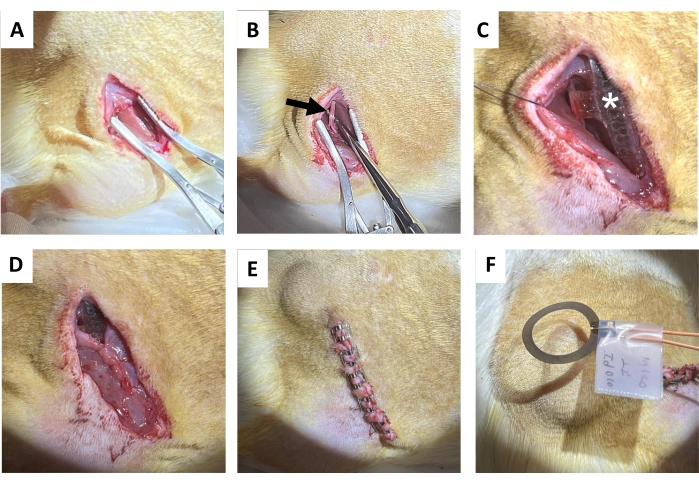
Figure 4: Sciatic nerve implantation procedure. (A) Incision on the skin, subcutaneous connective tissue, and the gluteal muscle to expose the hamstring. (B) Isolated sciatic nerve (black arrow). (C) Device post-implantation with nerve cuff, wires (white asterisk), and implant visible (star). (D) Closure of the connective tissue by suture. (E) Closure of the incision by wound clips. (F) Wireless electrical stimulation generated by a coil above the skin. Please click here to view a larger version of this figure.
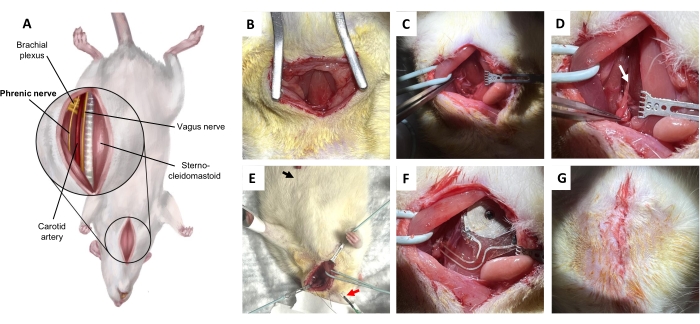
Figure 5: Phrenic nerve implantation procedure. (A) Ventral view of the neck in the supine position. (B) Incision on the skin and subcutaneous connective tissue to expose sternohyoid muscle. (C) Dissecting through the potential space between the omohyoid muscle and the sternocleidomastoid muscle. (D) Phrenic nerve (arrow), isolated from the brachial plexus. (E) Diaphragmatic electromyographic confirmation of the phrenic nerve. Black arrow, recording electrode. Red arrow, stimulators. (F) Implantation. (G) Closure of the skin with deep dermal stitches. Please click here to view a larger version of this figure.
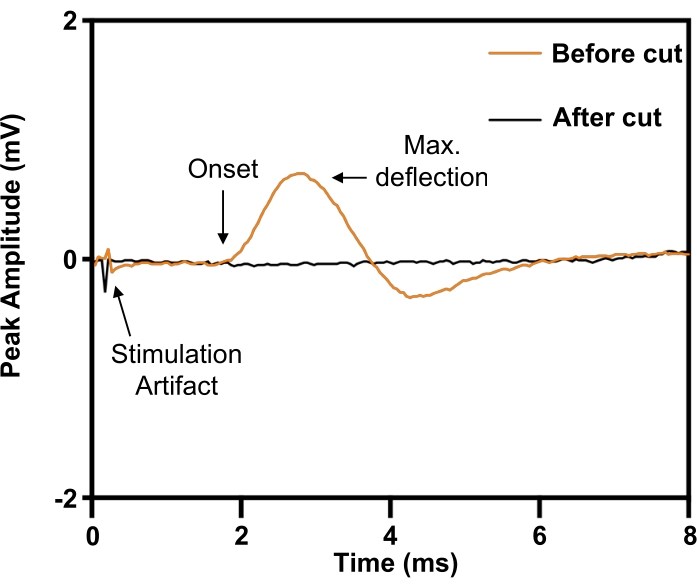
Figure 6: Confirmation of complete phrenic nerve transection injury by evoked compound muscle action potentials from the diaphragm. Before phrenic nerve transection (ORANGE), electrical stimulation of the phrenic nerve evoked compound muscle action potentials on the ipsilateral diaphragm, which was abolished by phrenic nerve transection (BLACK). Please click here to view a larger version of this figure.
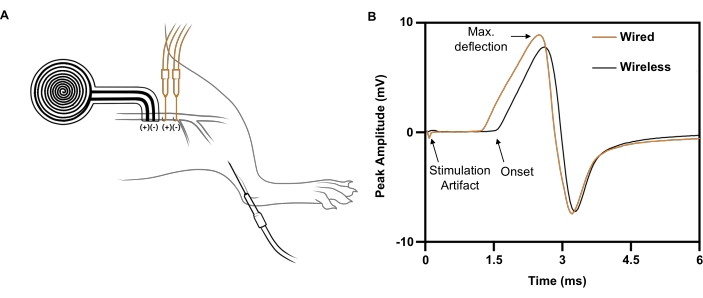
Figure 7: Representative nerve conduction studies comparing wireless to wire-based electrical stimulation. (A) Illustration of wireless (BLACK) and wired (ORANGE) devices placements on the sciatic nerve. The recording electrode was placed in the tibialis anterior. (B) Compound muscle action potentials evoked by wired implant (ORANGE) vs. wireless implant (BLACK). Please click here to view a larger version of this figure.
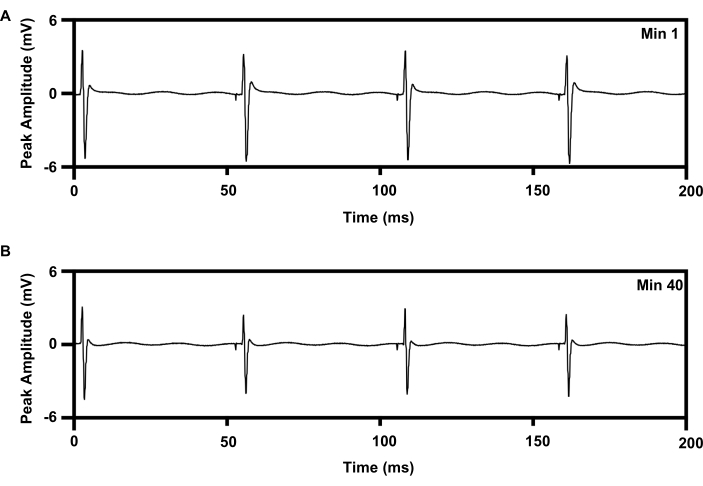
Figure 8: EMG recording from TA muscle with 20 Hz repetitive electrical stimulation for 1 h from implants. (A) Trace of EMG at min 1 of e-stim. (B) Trace of EMG at min 40 of e-stim. Abbreviations: EMG = electromyography; TA = tibialis anterior; e-stim = electrical stimulation; min = minute. Please click here to view a larger version of this figure.
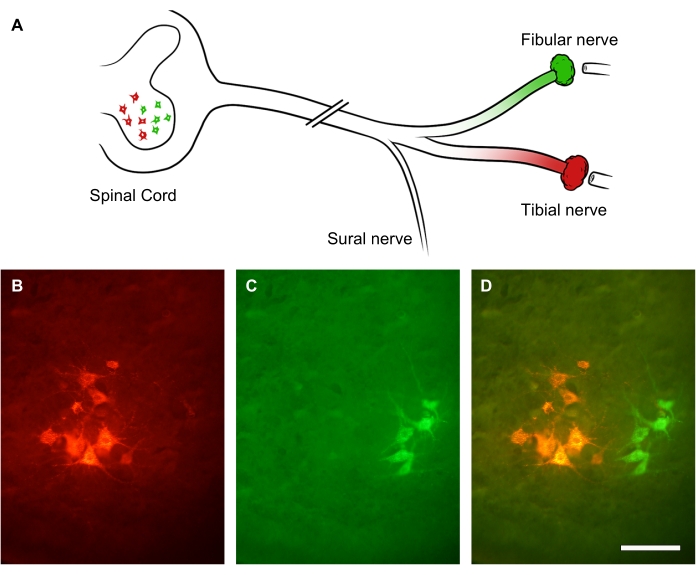
Figure 9: Representative images of sciatic nerve regeneration. (A) Illustration of sciatic nerve injury and fluorescent retrograde labeling. The sciatic nerve axons were transected by crush injury. After 3 weeks of recovery, its distal branches-the fibular nerve (in green) and tibial nerve (in red)-were retrogradely labeled. (B-D) Images of a lumbar spinal cord showing neuronal soma within the ipsilesional anterior horn. Scale bars = 30 µm. Please click here to view a larger version of this figure.
Discussion
This paper describes the steps in the surgical implantation and operation of a wireless, battery-free, and fully implantable peripheral nerve interfaces in the rat sciatic and phrenic nerve model. We demonstrate how this novel class of biomedical implants can be used to deliver a therapeutic electrical stimulation paradigm shown to enhance axon regeneration in preclinical and clinical studies (for review, see22). This protocol is uncomplicated and can be extrapolated to smaller animal models, such as mice21, as well as other wireless, battery-free, and fully implantable devices with functionality that includes optoelectronic and microfluidic peripheral nerve interfaces18,23,24,25,26,27,28,29,30. Also demonstrated is the approach using the rodent sciatic nerve, which is the most common experimental model31.
The versatility of this approach has been shown when it is adapted to interface with the phrenic nerve, which is rarely employed as a model of peripheral nerve injury32, perhaps because it is a vastly under-recognized clinical issue33,34,35. Phrenic nerve injury diagnosis and rehabilitation have become an important issue during the COVID-19 pandemic36,37,38. It is currently unknown whether regeneration of phrenic axons and recovery from diaphragm paralysis can be augmented by this brief, low-frequency electrical stimulation paradigm. However, phrenic nerve electrical stimulation for diaphragm muscle pacing is an established option for respiratory failure in patients with tetraplegia from high cervical spinal cord injury39,40,41,42,43. Other indications are being explored, including ventilator weaning after critical illness44.
Several critical steps should be emphasized to ensure good operation of the implanted system. First, it is important to avoid applying too much force on the thin electronic components of the devices when handling them to prevent lead de-insulation, kinking, or breakage. Next, it is important to accurately mark the location of the radio frequency power harvester coil on the overlying skin. Third, careful alignment of the transmission coil of the external radio frequency power supply over the power harvester coil of the implanted device with a gooseneck clamp allows for stable operation. Finally, to confirm electrical stimulation in addition to visual observation of muscle twitching, periodic neurophysiological monitoring is recommended. In the case of the more complex anatomy of the phrenic nerve in the neck, electrophysiological confirmation helps demonstrate that the correct nerve has been isolated (Figure 6).
Besides the wireless, battery-free electrical stimulators shown in this paper18,19,21, many other devices potentially share the same procedures. For instance, because electrodes designed to implant to the glossopharyngeal and vagus nerves to chronically record signals from sympathetic and parasympathetic nervous systems30,45,46 share a similar surgical area with the phrenic nerve, this protocol can be adapted for their implantation. Wireless long-term biocompatible stimulators for peripheral nerves, such as ReStore, are great tools to remain in place and stimulate nerves as needed25,47,48,49,50. Relevant multi-channel wireless recording implants have also been reported51. Overall, we believe these surgical and electrical stimulation protocols can be adapted as a standard for all wireless peripheral nerve interfacing related to electrical stimulation or recording.
Disclosures
The authors have no conflicts of interest.
Acknowledgements
This work used the NUFAB facility of Northwestern University's NUANCE Center, which has received support from the SHyNE Resource (NSF ECCS-1542205), the IIN, and Northwestern's MRSEC program (NSF DMR-1720139). This work made use of the MatCI Facility supported by the MRSEC program of the National Science Foundation (DMR-1720139) at the Materials Research Center of Northwestern University. C.K.F acknowledges support from Eunice Kennedy Shriver Institute of Child Health and Human Development of the NIH (grant no. R03HD101090) and the American Neuromuscular Foundation (Development Grant). Y.H. acknowledges support from NSF (grant no. CMMI1635443). This work was supported by the Querrey Simpson Institute for Bioelectronics at Northwestern University.
Materials
| Name | Company | Catalog Number | Comments |
| Amplifier | Electronics & Innovation | 201L | |
| Arbitrary Waveform Generator | RIGOL | DG1032Z | 30 MHz, 2 Channel, 200 MS/s, 14bit Resolution, 8 Mpts |
| Bupivacaine | Pfizer | 655317 | Marcaine, 0.5% |
| Copper/polyimide/copper | Pyralux | AP8535R | 18 µm thick top and bottom copper, 75 µm thick polyimide |
| EMG recording device | Natus | Nicolet VikingQuest | |
| EPOXY MARINE | Loctite | ||
| Isoflurane, USP | Butler Schein Animal Health | 1040603 | ISOTHESIA |
| Meloxicam | covetrus | 5mg/ml | |
| Needle electrodes | Technomed USA Inc. | TE/B50600- 001 | |
| PDMS (Silicone Elastomer Kit) | DOW | SYLGARD™ 184 | |
| ProtoLaser U4 | LPKF | U4 | |
| Puralube Vet Ointment Sterile Ocular Lubricant | Puralube | 83592 | |
| Waveform generator | Agilent Technologies | Agilent 33250A |
References
- Scholz, T., et al. Peripheral nerve injuries: an international survey of current treatments and future perspectives. Journal of Reconstructive Microsurgery. 25 (6), 339-344 (2009).
- Ayyaswamy, B., et al. Quality of life after amputation in patients with advanced complex regional pain syndrome: a systematic review. EFORT Open Reviews. 4 (9), 533-540 (2019).
- Kim, D. H., et al. Management and outcomes in 353 surgically treated sciatic nerve lesions. Journal of Neurosurgery. 101 (1), 8-17 (2004).
- Mackinnon, S. E. Donor distal, recipient proximal and other personal perspectives on nerve transfers. Hand Clinics. 32 (2), 141-151 (2016).
- Safa, B., Buncke, G. Autograft substitutes: conduits and processed nerve allografts. Hand Clinics. 32 (2), 127-140 (2016).
- Barber, B., et al. Intraoperative Brief Electrical Stimulation of the Spinal Accessory Nerve (BEST SPIN) for prevention of shoulder dysfunction after oncologic neck dissection: a double-blinded, randomized controlled trial. Journal of Otolaryngology - Head & Neck Surgery. 47 (1), 7(2018).
- Power, H. A., et al. Postsurgical electrical stimulation enhances recovery following surgery for severe cubital tunnel syndrome: a double-blind randomized controlled trial. Neurosurgery. 86 (6), 769-777 (2020).
- Gordon, T., et al. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Experimental Neurology. 223 (1), 192-202 (2010).
- Wong, J. N., et al. Electrical stimulation enhances sensory recovery: a randomized controlled trial. Annals of Neurology. 77 (6), 996-1006 (2015).
- Nix, W. A., Hopf, H. C. Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Research. 272 (1), 21-25 (1983).
- Al-Majed, A. A., et al. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. Journal of Neuroscience. 20 (7), 2602-2608 (2000).
- Witzel, C., et al. Electrical nerve stimulation enhances perilesional branching after nerve grafting but fails to increase regeneration speed in a murine model. Journal of Reconstructive Microsurgery. 32 (6), 491-497 (2016).
- Witzel, C., Rohde, C., Brushart, T. M. Pathway sampling by regenerating peripheral axons. Journal of Comparative Neurology. 485 (3), 183-190 (2005).
- Brushart, T. M., et al. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. Journal of Neuroscience. 22 (15), 6631-6638 (2002).
- Franz, C. K., Rutishauser, U., Rafuse, V. F. Intrinsic neuronal properties control selective targeting of regenerating motoneurons. Brain. 131, Pt 6 1492-1505 (2008).
- Park, S. I., et al. stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nature Biotechnology. 33 (12), 1280-1286 (2015).
- Koo, J., et al. Wirelessly controlled, bioresorbable drug delivery device with active valves that exploit electrochemically triggered crevice corrosion. Science Advances. 6 (35), (2020).
- Koo, J., et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nature Medicine. 24 (12), 1830-1836 (2018).
- Choi, Y. S., et al. Stretchable, dynamic covalent polymers for soft, long-lived bioresorbable electronic stimulators designed to facilitate neuromuscular regeneration. Nature Communications. 11 (1), 5990(2020).
- Hingne, P. M., Sluka, K. A. Differences in waveform characteristics have no effect on the antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) in rats with joint inflammation. Journal of Pain. 8, 251-255 (2007).
- Guo, H., et al. Advanced materials in wireless, implantable electrical stimulators that offer rapid rates of bioresorption for peripheral axon regeneration. Advanced Functional Materials. 31 (29), 2102724(2021).
- Zuo, K. J., et al. Electrical stimulation to enhance peripheral nerve regeneration: Update in molecular investigations and clinical translation. Experimental Neurology. 332, 113397(2020).
- Zhang, Y., et al. Battery-free, fully implantable optofluidic cuff system for wireless optogenetic and pharmacological neuromodulation of peripheral nerves. Science Advances. 5 (7), (2019).
- Montgomery, K. L., et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nature Methods. 12 (10), 969-974 (2015).
- Seo, D., et al. Wireless recording in the peripheral nervous system with ultrasonic neural dust. Neuron. 91 (3), 529-539 (2016).
- Neely, R. M., et al. Recent advances in neural dust: towards a neural interface platform. Current Opinion in Neurobiology. 50, 64-71 (2018).
- Mickle, A. D., et al. A wireless closed-loop system for optogenetic peripheral neuromodulation. Nature. 565 (7739), 361-365 (2019).
- Khalifa, A., et al. The microbead: a 0.009 mm(3) implantable wireless neural stimulator. IEEE Transactions on Biomedical Circuits and Systems. 13 (3), 971-985 (2019).
- Jeong, J. W., et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell. 162 (3), 662-674 (2015).
- Yao, G., et al. Effective weight control via an implanted self-powered vagus nerve stimulation device. Nature Communications. 9 (1), 5349(2018).
- Repair Brushart, M. Nerve Repair. , Oxford University Press. (2012).
- Laskowski, M. B., Sanes, J. R. Topographically selective reinnervation of adult mammalian skeletal muscles. Journal of Neuroscience. 8 (8), 3094-3099 (1988).
- Boon, A. J., et al. Sensitivity and specificity of diagnostic ultrasound in the diagnosis of phrenic neuropathy. Neurology. 83 (14), 1264-1270 (2014).
- Farr, E., D'Andrea, D., Franz, C. K. Phrenic nerve involvement in neuralgic amyotrophy (Parsonage-Turner syndrome). Sleep Medicine Clinics. 15 (4), 539-543 (2020).
- Mandoorah, S., Mead, T. Phrenic Nerve Injury. StatPearls. , StatPearls Publishing. Treasure Island, FL. (2021).
- Patel, Z., et al. Diaphragm and phrenic nerve ultrasound in COVID-19 patients and beyond: imaging technique, findings, and clinical applications. Journal of Ultrasound in Medicine. , (2021).
- Farr, E., et al. Short of breath for the long haul: diaphragm muscle dysfunction in survivors of severe COVID-19 as determined by neuromuscular ultrasound. medRxiv. , (2020).
- Fernandez, C. E., et al. Imaging review of peripheral nerve injuries in patients with COVID-19. Radiology. 298 (3), 117-130 (2021).
- Elefteriades, J. A., et al. Long-term follow-up of bilateral pacing of the diaphragm in quadriplegia. New England Journal of Medicine. 326 (21), 1433-1444 (1992).
- Elefteriades, J. A., et al. Long-term follow-up of pacing of the conditioned diaphragm in quadriplegia. Pacing and Clinical Electrophysiology: PACE 2002. 25 (6), 897-906 (2002).
- Glenn, W. W., et al. Ventilatory support by pacing of the conditioned diaphragm in quadriplegia. New England Journal of Medicine. 310 (18), 1150-1155 (1984).
- Garrido-Garcia, H., et al. Treatment of chronic ventilatory failure using a diaphragmatic pacemaker. Spinal Cord. 36 (5), 310-314 (1998).
- Romero, F. J., et al. Long-term evaluation of phrenic nerve pacing for respiratory failure due to high cervical spinal cord injury. Spinal Cord. 50 (12), 895-898 (2012).
- Vashisht, R., Chowdhury, Y. S. Diaphragmatic Pacing. StatPearls. , StatPearls Publishing. Treasure Island, FL. (2021).
- McCallum, G. A., et al. Chronic interfacing with the autonomic nervous system using carbon nanotube (CNT) yarn electrodes. Scientific Reports. 7 (1), 11723(2017).
- Zhang, Y., et al. Climbing-inspired twining electrodes using shape memory for peripheral nerve stimulation and recording. Science Advances. 5 (4), 1066(2019).
- Sivaji, V., et al. ReStore: A wireless peripheral nerve stimulation system. Journal of Neuroscience Methods. 320, 26-36 (2019).
- Tanabe, Y., et al. High-performance wireless powering for peripheral nerve neuromodulation systems. PLoS One. 12 (10), 0186698(2017).
- MacEwan, M. R., et al. Therapeutic electrical stimulation of injured peripheral nerve tissue using implantable thin-film wireless nerve stimulators. Journal of Neurosurgery. 130 (2), 486-495 (2019).
- Lee, B., et al. An implantable peripheral nerve recording and stimulation system for experiments on freely moving animal subjects. Scientific Reports. 8 (1), 6115(2018).
- Deshmukh, A., et al. Fully implantable neural recording and stimulation interfaces: Peripheral nerve interface applications. Journal of Neuroscience Methods. 333, 108562(2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved