A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Standard Membrane Feeding Assay for the Detection of Plasmodium falciparum Infection in Anopheles Mosquito Vectors
In This Article
Summary
The standard membrane feeding assay (SMFA) is regarded as the gold standard for the assessment and identification of potential antimalarial compounds. This artificial feeding system is used to infect mosquitoes to further evaluate the effects of such compounds on the intensity and prevalence of the Plasmodium falciparum parasite.
Abstract
Malaria remains one of the most devastating diseases worldwide and, to date, the African region is still responsible for 94% of all cases worldwide. This parasitic disease requires a protozoan parasite, an Anopheles mosquito vector, and a vertebrate host. The Anopheles genus comprises more than 500 species, of which 60 are known as vectors of the parasite. The Plasmodium parasite genus consists of 250 species, and 48 of these are involved in disease transmission. Furthermore, the Plasmodium falciparum parasite has contributed toward an estimated 99.7% of malaria cases in sub-Saharan Africa in recent years.
Gametocytes form part of the sexual stage of the parasite and are ingested by the female mosquito upon feeding on an infected human host. Further development of the parasite within the mosquito is enhanced by favorable environmental conditions in the midgut of the mosquito. Here, the fusion of the female and male gametes takes place, and the motile ookinetes originate. The ookinetes enter the midgut epithelium of the mosquito, and mature ookinetes form oocysts, which, in turn, produce motile sporozoites. These sporozoites migrate to the mosquito's salivary glands and are injected as a mosquito takes a blood meal.
For drug discovery purposes, mosquitoes were artificially infected with gametocyte-infected blood in the standard membrane feeding assay (SMFA). To detect infection within the mosquito and/or to assess the efficacy of antimalarial compounds, the midguts of the female mosquitoes were removed post infection and were stained with mercurochrome. This method was used to enhance the visual detection of oocysts under the microscope for the accurate determination of oocyst prevalence and intensity.
Introduction
Malaria, known as one of the most destructive diseases worldwide, still poses a great threat to several countries-especially those within the African region-and contributes toward approximately 95% of cases worldwide1. This disease is caused by a protozoan parasite and, together with its Anopheles mosquito vector, these culprits can cause great harm to the human host2. More specifically, the falciparum species of the Plasmodium parasite genus is responsible for an estimated 99% of malaria cases in sub-Saharan Africa1. In addition to this, several major Anopheles mosquito vectors (including An. gambiae Giles, An. arabiensis Patton, An. coluzzii Coetzee & Wilkerson sp.n., and An. funestus Giles) could be blamed for more than 95% of parasite transmission globally3,4,5,6,7,8. For the ideal parasite-vector companionship to be established, the mosquito vector should be susceptible to the parasite and be able to transmit it9. Furthermore, both the vector and parasite should overcome physical barriers to form the perfect infective combination-the mosquito vector should be able to sustain parasite development, and the parasite should have the ability to overcome the host's defense mechanisms10,11.
Gametocytes, the sexual stage of the P. falciparum parasite, play a crucial role in connecting the vector and parasite partners12. Sexual development takes place in vivo, and gametocytogenesis describes the process of the differentiation of mature gametocytes into motile male microgametes and female macrogametes13. Another process that takes place within the mosquito is exflagellation-the process during which the male gametocyte transforms into gametes and emerges from the red blood cells taken up during a blood meal11. The exflagellation process is further suggested to be enhanced by a favorable change in the environment of the mosquito midgut14. After exflagellation, a zygote is formed by the fusion of the male and female gametes13. From the zygote, a motile ookinete arises and moves from the blood meal to the epithelium of the mosquito midgut13. Here, the ookinete matures, and an oocyst is formed, which, in turn, produces motile sporozoites13,15. The sporozoites then migrate to the mosquito salivary glands and, as the mosquito takes a blood meal from its host, these sporozoites are injected into the host's bloodstream15.
Malaria control interventions, combining vector control strategies and the use of effective antimalarial drugs, have become crucial in combatting this disease15. With a rise in parasite and mosquito resistance, the urgency for the identification of novel antimalarial compounds is increasing16. Therefore, the in vivo evaluation of transmission-blocking compounds is important16. After the development of such effective transmission-blocking drugs, the SMFA has been used to assess whether these compounds inhibit the sexual development of P. falciparum in the Anopheles mosquito17,18,19. This assay has gained recognition since the 1970-1980s as the gold standard for evaluating transmission blocking20,21. This assay provides a cheaper alternative than other assays such as RT-qPCR, which requires specialized equipment. Furthermore, no patients are needed to execute the experiments. This assay also involves the provision of gametocyte-induced blood to female mosquitoes, which are then dissected to evaluate whether oocyst development is present21. This allows for gametocyte quantification and the detection of deformed oocysts because of the compounds22. For a compound to be classified as effective, the prevalence (the proportion of mosquitoes that harbor at least one oocyst in the midgut) and the number of oocysts (intensity) in the mosquito midgut must be evaluated to assess infection inhibition17,21,22.
Access restricted. Please log in or start a trial to view this content.
Protocol
Refer to Figure 1 for an illustration of the protocol. Ethical clearance was obtained from the University of Pretoria Health Sciences Ethics Committee (506/2018) for the withdrawal and use of human blood.
1. Gametocyte culture
NOTE: Prior to setting up the SMFA, a gametocyte culture was prepared at the University of Pretoria (see Reader et al.22 for the complete protocol).
- Prepare a gametocyte culture that consists of stage V gametocytes from the NF54 parasite strain.
- Ensure that the gametocytemia of the culture is between 1.5% and 2.5%, with a 50% hematocrit in A+ male serum, to which fresh red blood cells are added.
- Separate the culture into different flasks and add 2 µM of each compound for each respective treatment 48 h prior to conducting the SMFA. Leave the control group untreated.
- Assess the gametocyte culture shortly before conducting the SMFA to ensure the exflagellation of male gametes, with the presence of a 3:1 female:male ratio.
2. Artificial infection of mosquitoes through the SMFA
NOTE: Biosafety: infected mosquitoes should be housed in a biosafety level 2 (BSL2) facility with restricted access.
- Using a mouth aspirator, place 25 unfed female An. gambiae mosquitoes into a 350 mL feeding cup. Do the same for each treatment cup and label the cups clearly according to whether they are to be used as control or treatment groups. Choose the number of cups per treatment according to the number of technical replicates included.
NOTE: Colony mosquitoes between 5 and 7 days old are used in a typical transmission-blocking compound evaluation. Starving mosquitoes for 3-4 h or longer prior to blood feeding will facilitate the uptake of blood during SMFA. - Connect the glass feeder system to the water bath and maintain the temperature at 37 °C.
NOTE: The glass feeder consists of two arms, which are connected to the silicone tubing to which the water bath is connected (Figure 2). The hollow structure of the feeder allows water to circulate through and maintenance of the temperature of the blood. - Prepare cow intestine (or synthetic membrane) by rinsing it in tap water and cut it into pieces that are fitted for each feeder. Cover each feeder and fasten the membrane with an elastic band.
NOTE: No ethical clearance was needed for the intestine, as it was bought from a local butchery, where it is sold to the public for food preparation. - Place the infection cups underneath the feeders, with the membrane laying on top of the net of the cup.
- Add 1 mL of gametocyte-infected blood to the feeders of the control cups and gametocyte-infected blood with added compound to each corresponding compound feeder and cup.
- Leave the mosquitoes to feed for approximately 40 min with the feeders uncovered.
NOTE: Feedings take place under insectary conditions (25 °C, 80% relative humidity) in the dark. The diameter of a feeder is approximately 13 mm. - After feeding, remove the feeders from the cups, rinse the feeders, and treat the excess blood with hypochlorite.
- Remove the unfed mosquitoes from the cup by knocking all the mosquitoes down on ice (for 1-2 min) and separating the unfed mosquitoes from those that have taken a blood meal. Look for swollen and red abdomens (indicating blood) to distinguish the fed, fully engorged mosquitoes from the unfed ones (Figure 3).
- Place the infection cups in the biosafety chamber (Supplemental Figure S1) and provide each cup with a 10% sugar water pad, replacing the sugar water on alternate days for 8-10 days.
3. Preparation of infected mosquitoes
NOTE: This part of the protocol takes place within the BSL2 infection room. Only authorized, trained staff are permitted to enter the infection room where infected mosquitoes are housed. Mosquitoes are kept in modified cups that contain only one entry point, which automatically seals when the mouth aspirator is removed. These cups are placed inside a transparent, thermoplastic container to prevent escape. The container is located in the infection room behind a double-door system. All necessary protocols must be in place for accidental exposure to infected mosquitoes (Supplemental File S1). The protocols are country-specific and depend on the requirements of the institution.
- On days 8-10 post infection-feeding, knock the infected mosquitoes down by placing them on ice and transferring them to labeled tubes with 70% ethanol (keeping the mosquitoes of each control and treatment group separate).
- Ensure that all mosquitoes are dead before leaving the infection room.
4. Dissections of infected mosquitoes
NOTE: This part of the protocol is conducted in the laboratory.
- Transfer the mosquitoes to labeled Petri dishes lined with filter paper, keeping the control and test groups separate.
- Place a droplet of phosphate-buffered saline (PBS) on a microscope slide (marked according to the control/test group) and transfer an individual mosquito from the filter paper to the PBS.
- Remove the midgut from the immobilized, infected specimen by pinning the thorax of the mosquito with the dissecting needle whilst pulling the 7th abdominal segment with the forceps.
- With the gut being exposed and visible, look for the Malpighian tubules (Figure 4A,B) to distinguish the gut from the ovaries. Remove it from the PBS, transfer it to a droplet of 0.1% mercurochrome on a new microscope slide, and leave the gut to stain for 8-10 min.
- After staining, place a coverslip on the stained gut and view the gut under brightfield illumination at 20x-40x magnification (Figure 4C,D).
- Record the presence of and the number of oocysts per midgut for each control and treatment group (Supplemental File S2).
- Calculate the transmission-blocking activity using Equation (1):
%TBA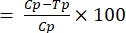 (1)
(1)
where TBA = transmission-blocking activity (reduction in oocyst prevalence); p = oocyst prevalence; C = control; and T = treatment. - Calculate the transmission-reducing activity using Equation (2):
%TRA =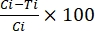 (2)
(2)
where TRA = transmission-reducing activity (reduction in oocyst intensity); I = oocyst intensity; C = control; and T = treatment.
NOTE: The TBA might not be significantly reduced, but a significant difference might be observed in the TRA and vice versa. This is dependent on the chemical material being evaluated. - Perform statistical analysis using the non-parametric t-test (Mann-Whitney).
Access restricted. Please log in or start a trial to view this content.
Results
The total number of control specimens dissected was 47, with an average to 89% prevalence and an intensity of 9.5 oocysts per midgut (Table 1, as published previously22). For the compound MMV1581558, the sample size reached a total of 42 specimens, with a 36% oocyst prevalence and an average intensity of 1.5 oocysts. This shows a reduction in oocyst prevalence of 58% and a TRA of 82% across all three biological replicates (Table 1).
Access restricted. Please log in or start a trial to view this content.
Discussion
For this protocol to be executed successfully, attention should be given to each step, even though it might be a tedious and laborious process. One of the most important steps is to ensure that the gametocyte culture is of good quality and that it consists of mature gametocytes, with the correct male:female ratio, prior to starting the SMFA23,24. During the SMFA, it is also crucial to maintain the gametocyte culture at the correct temperature to prevent male game...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors would like to acknowledgeProf. Lyn-Mari Birkholtz and Dr. Janette Reader from the Department of Biochemistry, Genetics and Microbiology, Institute for Sustainable Malaria Control, at the University of Pretoria, for culturing and supplying the gametocyte culture. The parasite strain was obtained from the latter department (not part of this publication). The Department of Science and Innovation (DSI) and the National Research Foundation (NRF); South African Research Chairs Initiative (UID 64763 to LK and UID 84627 to LMB); the NRF Communities of Practice (UID 110666 to LMB and LK); and the South African Medical Research Council Strategic Health Innovation Partnerships (SHIP) are also acknowledged for funds from the DSI.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Bovine intestine/ | Butchery | ||
| Compound MMV1581558 | MMV | Pandemic response box | |
| Dissecting needles | WRIM | Custom made | |
| falcon tube | Lasec | ||
| Glass feeders | Glastechniek Peter Coelen B.V. | ||
| Graphpad Prism (8.3.0) | Graphpad | ||
| Mercurochrome | Merck (Sigma-Aldrich) | 129-16-8 | |
| Microscope slides | Merch (Sigma-Aldrich) | S8902 | |
| Parafilm | Cleansafe | ||
| PBS tablets | ThermoFisher Scientific | BP2944 | |
| Perspex biosafety cabinet | Wits University | Made by the contractors at Wits | |
| Plastic cups (350 mL) | Plastic Land |
References
- World Malaria Report. World Health Organization. , Available from: https://www.who.int/publications/i/item/9789240040496 (2021).
- Takken, W., Verhulst, N. O. Host preferences of blood feeding mosquitoes. Annual Review of Entomology. 58, 433-453 (2013).
- Gillies, M. T., Coetzee, M. Supplement to the Anophelinae of Africa south of the Sahara Afrotropical region. Publications of the South African Institute for Medical Research. 55, 1(1987).
- Gillies, M. T., De Meillon, B. The Anophelinae of Africa south of the Sahara. Publications of the South African Institute for Medical Research. 54, (1968).
- Antonio-Nkondjio, C., et al. Complexity of the malaria vectorial system in Cameroon: contribution of secondary vectors to malaria transmission. Journal of Medical Entomology. 43, 1215-1221 (2006).
- Sinka, M. E., et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasites and Vectors. 3, 117(2010).
- Coetzee, M., Hunt, R. H., Wilkerson, R., Della Torre, A., Coulibaly, M. B., Besansky, N. J. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 3619, 246-274 (2013).
- Kyalo, D., Amratia, P., Mundia, C. W., Mbogo, C. M., Coetzee, M., Snow, R. W. A geo-coded inventory of anophelines in the Afrotropical Region south of the Sahara: 1898-2016. Wellcome Open Research. 2, 57(2017).
- Cohuet, A., Harris, C., Robert, V., Fontenille, D. Evolutionary forces on Anopheles: What makes a malaria vector. Trends in Parasitology. 309, (2009).
- Weathersby, A. B. The role of the stomach wall in the exogenous development of Plasmodium gallinaceum as studies by means of haemocoel injections of susceptible and refractory mosquitoes. The Journal of Infectious Diseases. 91, 198-205 (1952).
- Ally, A. S. I., Vaughan, A. M., Kappe, S. H. I. Malaria parasite development in the mosquito and infection of the mammalian host. The Annual Review of Microbiology. 63, 195-221 (2009).
- Delves, M. J., et al. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. American Society for Microbiology Journal. , (2013).
- Sinden, R. E. Sexual development of malarial parasites in their mosquito vector. Transactions of the Royal Society of Tropical Medicine and Hygiene. 75, (1981).
- Garcia, G. E., Wirtz, R. A., Barr, J. R., Woolfitt, A. Xanthurenic acid induces gametogenesis in Plasmodium, the malaria parasite. Journal of Biological Chemistry. 15, 12003-12005 (1998).
- Oaks, S. C. Jr, Mitchell, V. S., Pearson, G. W., et al. Malaria: Obstacles and Opportunities. , National Academic Press. ISBN 0-309-54389-4 (1991).
- Le Manach, C., et al. Identification and profiling of a novel Diazaspirol[3.4]octane chemical series active against multiple stages of the human malaria parasite Plasmodium falciparum and optimization efforts. Journal of Medicinal Chemistry. 64, 2291-2309 (2021).
- Cibulskis, R. E., et al. Malaria: global progress 2000-2015 and future challenges. Infect Diseases of Poverty. 5, 61(2016).
- Smith, T. A., Chitnis, N., Briet, O. J., Tanner, M. Uses of mosquito-stage transmission-blocking vaccines against Plasmodium falciparum. Trends in Parasitology. 27, 190-196 (2011).
- Boyd, M. F. Epidemiology: factors related to the definitive host. Malariology. Boyd, M. F. , W.B. Saunders. Philadelphia. 608-697 (1949).
- Ponnudurai, T., van Gemert, G. J., Bensink, T., Lensen, A. H., Meuwissen, J. H. Transmission blockade of Plasmodium falciparum: its variability with gametocyte numbers and concentration of antibody. Transactions of The Royal Society of Tropical Medicine. 81, 491-493 (1987).
- Rutledge, L. C., Ward, R. A., Gould, D. J. Studies on the feeding response of mosquitoes to nutritive solutions in a new membrane feeder. Mosquito News. 24 (4), (1964).
- Reader, J., et al. Multistage and transmission-blocking targeted antimalarials discovered from the open-source MMV Pandemic Response Box. Nature Communications. 12, 269(2021).
- Bousema, T., et al. Mosquito feeding assays for natural infections. PLoS One. 7 (8), (2012).
- Churcher, T., et al. Measuring the blockade of malaria transmission - An analysis of the standard membrane feeding assay. International Journal for Parasitology. 42, 1037-1044 (2012).
- Medley, G. F., et al. Heterogeneity in patterns of malarial oocyst infections in the mosquito vector. Parasitology. 106, 441-449 (1993).
- Miura, K., et al. Transmission-blocking activity is determined by transmission reducing activity and number of control oocysts in Plasmodium falciparum standard membrane-feeding assay. Vaccine. 34, 4145-4151 (2016).
- Sattabongkot, J., Maneechai, N., Rosenberg, R. Plasmodium vivax: gametocyte infectivity of naturally infected Thai adults. Parasitology. 102 (01), 27-31 (1991).
- Vallejo, A. F., Garcia, J., Amado-Garavito, A. B., Arevalo-Herrera, M., Herrera, S. Plasmodium vivax gametocyte infectivity in sub-microscopic infections. Malaria Journal. 15 (1), 48(2016).
- Ponnudurai, T., Lensen, A. H. W., van Gemert, G. J. A., Bolmer, M. G., Meuwissen, J. H. E. Feeding behavior and sporozoite ejection by infected Anopheles stephensi. Transactions of the Royal Society of Tropical Medicine and Hygiene. 85, 175-180 (1991).
- Miura, K., et al. Qualification of standard membrane-feeding assay with Plasmodium falciparum malaria and potential improvements for future assays. PLoS One. 8, 57909(2013).
- Griffin, P., et al. Safety and reproducibility of a clinical trial system using induced blood stage Plasmodium vivax infection and its potential as a model to evaluate malaria transmission. PLoS Neglected Tropical Diseases. 10, 0005139(2016).
- Delves, M. J., Sinden, R. E. A semi-automated method for counting fluorescent malaria oocysts increases the throughput of transmission blocking studies. Malaria Journal. 9, 35(2010).
- vander Kolk, M., et al. Evaluation of the standard membrane feeding assay (SMFA) for the determination of malaria transmission-reducing activity using empirical data. Parasitology. 130, 13-22 (2005).
- van der Kolk, M., de Vlas, S. J., Sauerwein, R. W. Reduction and enhancement of Plasmodium falciparum transmission by endemic human sera. International Journal for Parasitology. 36, 1091-1095 (2006).
- Singh, M., et al. Plasmodium's journey through the Anopheles mosquito: A comprehensive review. Biochimie. 181, 176-190 (2021).
- Vos, M. W., et al. A semi-automated luminescence based standard membrane feeding assay identifies novel small molecules that inhibit transmission of malaria parasites by mosquitoes. Scientific Reports. 5, 18704(2015).
- Azevedo, R., et al. Bioluminescence method for in vitro screening of Plasmodium transmission-blocking compounds. Antimicrobial Agents and Chemotherapy. 61, (2017).
- Okell, L. C., Bousema, T., Griffin, J. T., Ouedraogo, A. L., Ghani, A. C., Drakeley, C. J. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nature Communications. 3, 1237(2012).
- Pasay, C. J., et al. Piperaquine monotherapy of drug-susceptible Plasmodium falciparum infection results in rapid clearance of parasitemia but is followed by the appearance of gametocytemia. The Journal of Infectious Diseases. 214, 105-113 (2016).
- Stone, W. J., et al. A scalable assessment of Plasmodium falciparum transmission in the standard membrane-feeding assay, using transgenic parasites expressing green fluorescent protein-luciferase. The Journal of Infectious Diseases. 210, 1456-1463 (2014).
- Hasan, A. U., et al. Implementation of a novel PCR based method for detecting malaria parasites from naturally infected mosquitoes in Papua New Guinea. Malaria Journal. 8, 182(2009).
- Stone, W. J., et al. The relevance and applicability of oocyst prevalence as a read-out for mosquito feeding assays. Scientific Reports. 3, 3418(2013).
- Marquart, L., Baker, M., O'Rourke, P., McCarthy, J. S. Evaluating the pharmacodynamic effect of antimalarial drugs in clinical trials by quantitative PCR. Antimicrobial Agents and Chemotherapy. 59, 4249-4259 (2015).
- McCarthy, J. S., et al. tolerability, pharmacokinetics, and activity of the novel long-acting antimalarial DSM265: a two-part first-in-human phase 1a/1b randomised study. TheLancet Infectious Diseases. 17, 626-635 (2017).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved