A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Characterizing Salmonella Typhimurium-induced Septic Peritonitis in Mice
In This Article
Summary
This protocol describes the induction of Gram-negative monobacterial sepsis in a mouse model system. The model is useful in investigating the inflammatory and lethal host responses during sepsis.
Abstract
Sepsis is a dysregulated host immune response to microbial invasion or tissue damage, leading to organ injury at a site distant from that of the infection or damage. Currently, the widely used mice models of sepsis include lipopolysaccharide (LPS)-induced endotoxemia, cecal ligation and puncture (CLP), and monobacterial infection model systems. This protocol describes a method to study the host responses during Salmonella Typhimurium infection-induced septic peritonitis in mice. S. Typhimurium, a Gram-negative intracellular pathogen, causes typhoid-like disease in mice.
This protocol elaborates the culture preparation, induction of septic peritonitis in mice through intraperitoneal injection, and methods to study systemic host responses. Furthermore, the assessment of bacterial burden in different organs and the flow cytometric analysis of increased neutrophil numbers in the peritoneal lavage is presented. Salmonella Typhimurium-induced sepsis in mice leads to an increase in proinflammatory cytokines and rapid infiltration of neutrophils in the peritoneal cavity, leading to lower survival.
Every step in this protocol has been optimized, resulting in high reproducibility of the pathogenesis of septic peritonitis. This model is useful for studying immunological responses during bacterial sepsis, the roles of different genes in disease progression, and the effects of drugs to attenuate sepsis.
Introduction
Sepsis is defined as a dysregulated systemic inflammatory and immune response to microbial invasion or tissue damage, leading to organ injury distant from the site of infection or damage. Septic shock is a subset of sepsis characterized by hypotension persisting during volume resuscitation, with a substantially increased risk of mortality1. The general public has become more aware of this disorder during the COVID-19 pandemic. Despite its high associated mortality, comprehensive epidemiological data on the global burden of sepsis is lacking because of the complexity of its diagnosis. In 2017, there were 48.9 million sepsis incidences and 11 million deaths worldwide, accounting for 19.7% of all global deaths2. Further, a study on the extended prevalence of infection and related sepsis in intensive care unit patients found that 62% of the positive isolates from patients were Gram-negative organisms3.
Initially, the investigations on sepsis focused on delineating microbial pathogenesis. However, understanding the "danger hypothesis", which dictates how the host distinguishes self and non-self, led to the tilting of the balance of sepsis research toward understanding the host response to an invading pathogen. The widely used mice models of sepsis include the lipopolysaccharide (LPS)-induced endotoxemia model, polymicrobial sepsis models, cecal ligation and puncture (CLP) and colon ascendens stent peritonitis (CASP), and monobacterial infection models4.
We have standardized a mouse model system by inducing peritoneal sepsis using Salmonella Typhimurium. This model is advantageous over others because Salmonella Typhimurium is an intracellular pathogen that mimics the clinically relevant condition of Gram-negative sepsis. The outcome of peritonitis sepsis in this model is systemic, with 100% mortality within 96 h post infection. Therefore, this model is instrumental in studying the inflammatory and lethal host responses. In this model, sepsis is induced by intraperitoneally injecting 0.5 million colony-forming units (CFU) of Salmonella Typhimurium into an 8-10-week-old C57BL/6 mouse. Systemic infection can be confirmed by assessing organ bacterial burden ~16 h post infection. This article demonstrates Salmonella Typhimurium-induced peritonitis sepsis in mice, characterizes the resulting alterations in peritoneal cell composition, and quantifies bacterial burden in different organs.
Protocol
All experiments using Salmonella Typhimurium were conducted in Bio Safety Level 2 (BSL-2) facilities. Care must be taken to use proper personal protective equipment (PPE), ensure safety, and follow standard BSL-2 biohazard disposal methods. All mice experiments were conducted following guidelines stated by the Institutional Animal Ethics Committee, IISc. Mice were bred and maintained at the Central Animal Facility of IISc (Registration number: 48/1999/CPCSEA, dated 1/3/1999), approved by the Ministry of Environment and Forest, Government of India. The experimental protocols were approved by the Committee for Purpose and Control and Supervision of Experiments on Animals with the approved permit number CAF/Ethics/797/2020.
BSL2 definition: A BSL2 rating represents that the biohazardous agents pose a moderate threat to the environment and laboratory staff5.
1. Culture preparation of Salmonella Typhimurium
- Add 100 µL of Salmonella Typhimurium NCTC 12023 glycerol stock to 3 mL of Luria Bertani (LB) broth. Incubate the culture at 160 rpm at 37 °C overnight.
- Streak 50 µL of the overnight grown culture in LB broth onto a Salmonella Shigella (SS) agar plate and incubate at 37 °C for ~12 h. Store the SS agar plate with the bacterial colonies at 4 °C for several days before the in vivo infection experiment.
- Pick a single colony from the streaked SS agar plate using a microtip. Eject the microtip into 3 mL of LB broth and culture at 160 rpm at 37 °C overnight.
- Add 0.1 mL of the bacterial culture to 50 mL of LB broth and incubate at 37 °C in a shaker incubator at 160 rpm for 3-4 h to reach the logarithmic phase of growth. Dilute the culture by a factor of 2 using LB broth.
NOTE: During the logarithmic phase, bacterial cells are at their best health and are actively dividing. - Measure the optical density (OD) of the culture at 600 nm wavelength of light in a spectrophotometer or microplate reader. Once the OD reaches 1.0, make two aliquots of 1 mL of culture in 1.5 mL microfuge tubes.
- Centrifuge the tubes at 7,750 × g for 15 min. Discard the supernatant and wash the pellet with 1 mL of 1x PBS 2x. Centrifuge the tubes at 7,750 × g for 15 min.
- Resuspend the pellet in 0.5 mL of 1x PBS in two different 1.5 mL microfuge tubes. Combine the suspensions from both the tubes into one 1.5 mL tube now containing ~2 × 108 colony-forming units (CFU)/mL.
- Prepare a bacterial cell suspension of 1 × 106 CFU/mL by diluting this stock solution.
CAUTION: Optimize the CFU corresponding to OD under specific laboratory conditions to determine the CFU for OD 1.0 before initiating the experiments.
2. Mice and infections
- House 8-10-week-old male C57BL/6 mice weighing ~20 g in the clean-air room of the animal facility for several days for acclimation.
- On the day of infection, hold the mouse with one hand, wipe the abdominal skin with 70% ethanol, and spread the hind legs for better accessibility of the abdominal wall.
- Inject 0.5 mL of 1 × 106 CFU/mL bacterial suspension intraperitoneally with the help of a 1 mL syringe. Therefore, each mouse receives 5 × 105 CFU. Control, uninfected mice receive 0.5 mL of PBS alone. Post infection, plate the culture to check the actual CFU injected, which may vary from 0.2-0.8 million CFU/0.5 mL.
- Put the mice back in the cages as assigned.
- Sacrifice the mice using CO2 asphyxiation ~12-18 h post infection for the best response. Usually, all infected mice die within 96 h. Under some experimental interventions, some mice may survive. Euthanize these mice after 96 h. Also, euthanize any mouse with a body temperature below 33.2 °C and acute distress at 96 h as a humane endpoint.
NOTE: In this model, some mice may start dying after 12 h of Salmonella injection. Therefore, plan experiments properly involving multiple time points.
3. CFU assessment of organs
- Sacrifice the infected mouse by CO2 asphyxiation, and wipe the abdomen with a piece of cotton dipped in 70% ethanol. Cut open the abdominal skin. Refer to the article by Ray and Dittel for a video protocol on how to collect peritoneal lavage fluid6. Cut open the peritoneal cavity and collect the organs of interest in microfuge tubes. Additionally, as the blood in mice with sepsis gets coagulated fast and the amounts are low, collect it quickly after sacrificing.
NOTE: This video demonstrates the enumeration of organ CFU from the liver as the liver undergoes extensive histopathological damage in this model of sepsis. - Cut a small piece of the liver and place it in a microfuge tube.
NOTE: This can be stored on ice for a maximum of 2-3 h before proceeding to the next step. - Weigh and transfer the pieces to microcentrifuge tubes. Preferably, cut pieces weighing ~10-15 mg for proper homogenization. Use entire organs in the case of smaller ones such as the mesenteric lymph nodes (MLNs) or thymus.
- Add 0.5 mL of 1x PBS to the tube and homogenize the organs using a hand homogenizer. Make sure that the organs are completely homogenized. Make up the volume to 1 mL by adding 0.5 mL of 1x PBS.
- Centrifuge the tubes at 200 × g for 5 min at 4 °C.
- Collect the supernatant into fresh microfuge tubes and prepare dilutions of 1 × 10−1 and 10−2 in a 96-well plate.
- Spread 50 µL of the diluent onto fresh SS agar plates and incubate the plates at 37 °C for 12 h.
- Count the number of colonies that appear in each condition and normalize the data with the organ weight using Equation (1):
CFU/mg =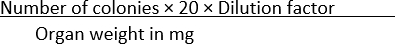 (1)
(1)
NOTE: The number 20 is used in the formula to convert the colonies per plate to CFU/mL. This number is arrived at by dividing 1 mL by the amount of a given volume of the culture plated-in this case, 50 µL.
For example, if 100 colonies are found in an SS agar plate, where 50 µL of a 1 × 10-1 dilution of a homogenized organ weighing 10 mg is spread, then
CFU/mg =
4. Flow cytometric analysis of various immune cell populations in peritoneal exudate
- Collect the peritoneal cells as described previously by Ray and Dittel6.
- Resuspend the cell pellet from peritoneal lavage fluid in 1 mL of RPMI supplemented with 10% fetal bovine serum (FBS). Enumerate the total cell numbers in the peritoneal lavage using a hemocytometer. Adjust the cell number so that every tube receives 0.2-0.5 million cells.
NOTE: Peritoneal lavage in mice with sepsis may contain RBCs, which probably appear due to hemorrhage. Be careful to exclude RBCs while counting peritoneal cells. In the brightfield microscope, RBCs appear much smaller than the immune cells. These appear as flat disks or doughnuts, round, with an indentation in the center, but not hollow. A step to lyse RBCs may be added7. - Spin the cells down at 200 × g at 4 °C for 10 min, discard the supernatant, and wash the cells 1x with 1x cold PBS. Centrifuge the cells at 200 × g at 4 °C for 10 min.
- Block the Fc receptors on PECs using FcR blocker (1:400 dilution), prepared in blocking buffer consisting of 5% FBS and 0.02% sodium azide in PBS. Incubate on ice for 15 min.
- Centrifuge the cells at 200 × g at 4 °C for 10 min. Discard the supernatant. Dilute the fluorochrome-conjugated antibodies of interest in blocking buffer. Use a 1:500 dilution of anti-mouse LY6G to stain the neutrophils.
NOTE: Other immune cell populations can also be detected using, for example, anti-mouse B220 for B cells, anti-mouse CD3 for T cells, and anti-mouse F4/80 for macrophages. - Incubate ~0.2 million cells in 200 µL of the diluted solutions of antibodies in separate tubes. As a negative control, set aside one tube in each fluorochrome type for the unstained control to incubate the cells with 200 µL of blocking buffer without antibody.
- Incubate the samples on ice for 45 min with intermittent tapping every 15 min.
- Centrifuge the cells at 200 × g for 10 min at 4 °C. Discard the supernatant. Fix the cells with 4% paraformaldehyde for ~15 min at room temperature if they need to be be stored for several days. However, it is best to acquire data from freshly stained samples in a flow cytometer.
- Resuspend the cells in 200 µL of FACS staining buffer (2% FBS in PBS). Acquire the data in a flow cytometer.
Results
A detailed characterization of the host immune response using this particular model is shown in previous publications8,9. A few representative results of the described protocol are depicted in this section. This model aims to induce systemic infection of S. Typhimurium by intraperitoneal injection of the bacterial culture to induce sepsis. To confirm the infection, the lysates of the liver and spleen from septic mice were spread on SS agar plates, and th...
Discussion
This article describes a method of inducing a severe form of bacterial sepsis by intraperitoneal injection of Salmonella Typhimurium. This model is advantageous over others as Salmonella Typhimurium is an intracellular pathogen and, hence, highly pathogenic, mimicking the clinically relevant condition of Gram-negative sepsis. The outcome of peritonitis sepsis in this model is systemic, with 100% mortality within 96 h post infection. Therefore, this model is instrumental in studying the inflammatory and ...
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
We thank the Central Animal Facility, IISc for supplying us with mice for research. This study was funded by grants to DpN from the Department of Biotechnology and Science and Engineering Research Board, Government of India. The infrastructural support from the DBT-IISc program and DST-FIST grants are greatly acknowledged. We thank all previous and current members of the DpN lab for their support.
Materials
| Name | Company | Catalog Number | Comments |
| Consumables | |||
| 1 mL Sterile Syringe with 26 G needle | Beckton Dickinson, Singapore | 303060 | |
| 1.5 mL Microcentrifuge Tube | Tarsons, USA | 500010 | |
| 10 mL Sterile Syringe with 21 G needle | Beckton Dickinson, Spain | 307758 | |
| 50 mL Conical Flask | Tarsons, USA | 441150 | |
| 50 mL Graduated Centrifuge Tube | Tarsons, USA | 546041 | |
| 50 mL Graduated Centrifuge Tube | Tarsons, USA | 546021 | |
| Cell spreader | VWR, USA | VWRU60828-680 | |
| Dulbecco’s Phosphate Buffered Saline | HiMedia, Mumbai, India | TS1006 | |
| Ethanol | Merck | 100983 | |
| FcR blocker | BD Biosciences | 553142 | |
| Fetal Bovine Serum | Gibco | 10270-106 | |
| FITC Rat anti-mouse Ly6G (Clone 1A8) | BD Pharmingen | 551460 | |
| Glycerol | Sigma-Aldrich | G9012 | |
| Hand based Homogenizer | - | - | |
| Hemocytometer (Neubauer counting chamber) | Rohem, India | I.S. 10269 | |
| Luria Bertani Broth | HiMedia, Mumbai, India | M1245 | |
| Paraformaldehyde | Sigma-Aldrich | 158127 | |
| Petriplates | Tarsons, USA | 460091 | |
| RPMI | Himedia, Mumbai, India | AT060-10X1L | |
| Salmonella-Shigella Agar | HiMedia, Mumbai, India | M108 | |
| Sodium azide | Sigma-Aldrich | S2002 | |
| Equipments | |||
| Centrifuge | Kubota | ||
| Flow cytometer | BD FACSverse | ||
| Incubator | N-biotek | ||
| Spectrophotometer | Shimadzu | ||
| Weighing machine | Sartorius |
References
- Hotchkiss, R. S., et al. Sepsis and septic shock. Nature Reviews Disease Primers. 2 (1), 1-21 (2016).
- Rudd, K. E., et al. regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. The Lancet. 395 (10219), 200-211 (2020).
- Vincent, J. L., et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 302 (21), 2323-2329 (2009).
- Lewis, A. J., Seymour, C. W., Rosengart, M. R. Current murine models of sepsis. Surgical Infections. 17 (4), 385-393 (2016).
- Ta, L., Gosa, L., Nathanson, D. A. Biosafety and biohazards: Understanding biosafety levels and meeting safety requirements of a biobank. Biobanking. 1897, 213-225 (2019).
- Ray, A., Dittel, D. N. Isolation of Mouse Peritoneal Cavity Cells. Journal of Visualized Experiments: JoVE. (35), e1488 (2010).
- Liu, X., Quan, N. Immune cell isolation from mouse femur bone marrow. Bio-protocol. 5 (20), 1631 (2015).
- Yadav, S., et al. Nitric oxide synthase 2 enhances the survival of mice during Salmonella Typhimurium infection-induced sepsis by increasing reactive oxygen species, inflammatory cytokines and recruitment of neutrophils to the peritoneal cavity. Free Radical Biology & Medicine. 116, 73-87 (2018).
- Verma, T., et al. Cell-free hemoglobin is a marker of systemic inflammation in mouse models of sepsis: A Raman spectroscopic study. Analyst. 146 (12), 4022-4032 (2021).
- Cassado, A. D. A., Lima, M. R. D., Bortoluci, K. R. Revisiting mouse peritoneal macrophages: Heterogeneity, development, and function. Frontiers in Immunology. 6, 225 (2015).
- Yadav, S., Verma, T., Chattopadhyay, A., Nandi, D. Factors affecting the pathophysiology of sepsis, an inflammatory disorder: Key roles of oxidative and nitrosative stress. Indian Journal of Inflammation Research. 3 (1), 2 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved